Abstract
1. Transmission in the lemniscal afferent pathway was studied in thirteen decerebrate, unanaesthetized cats while changing the concentration of inspired PCO2.
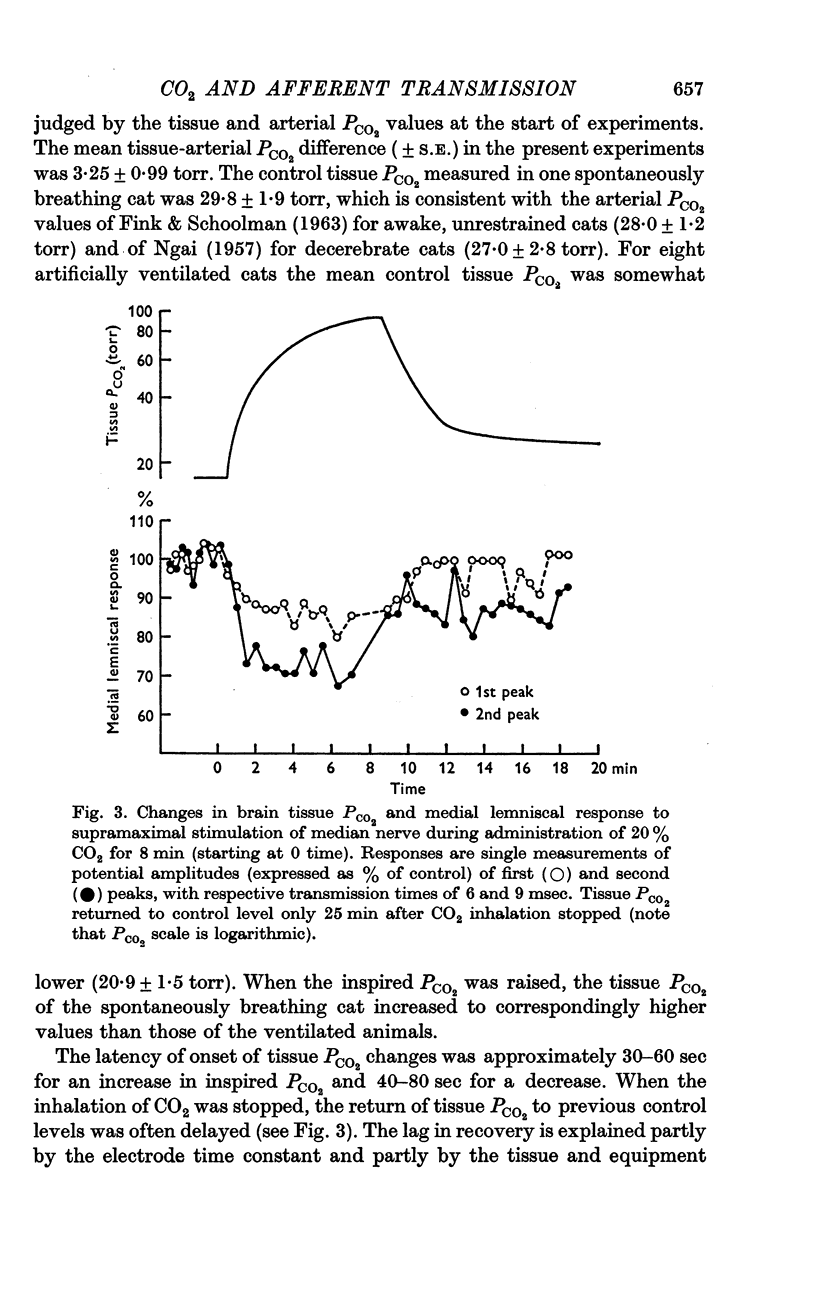
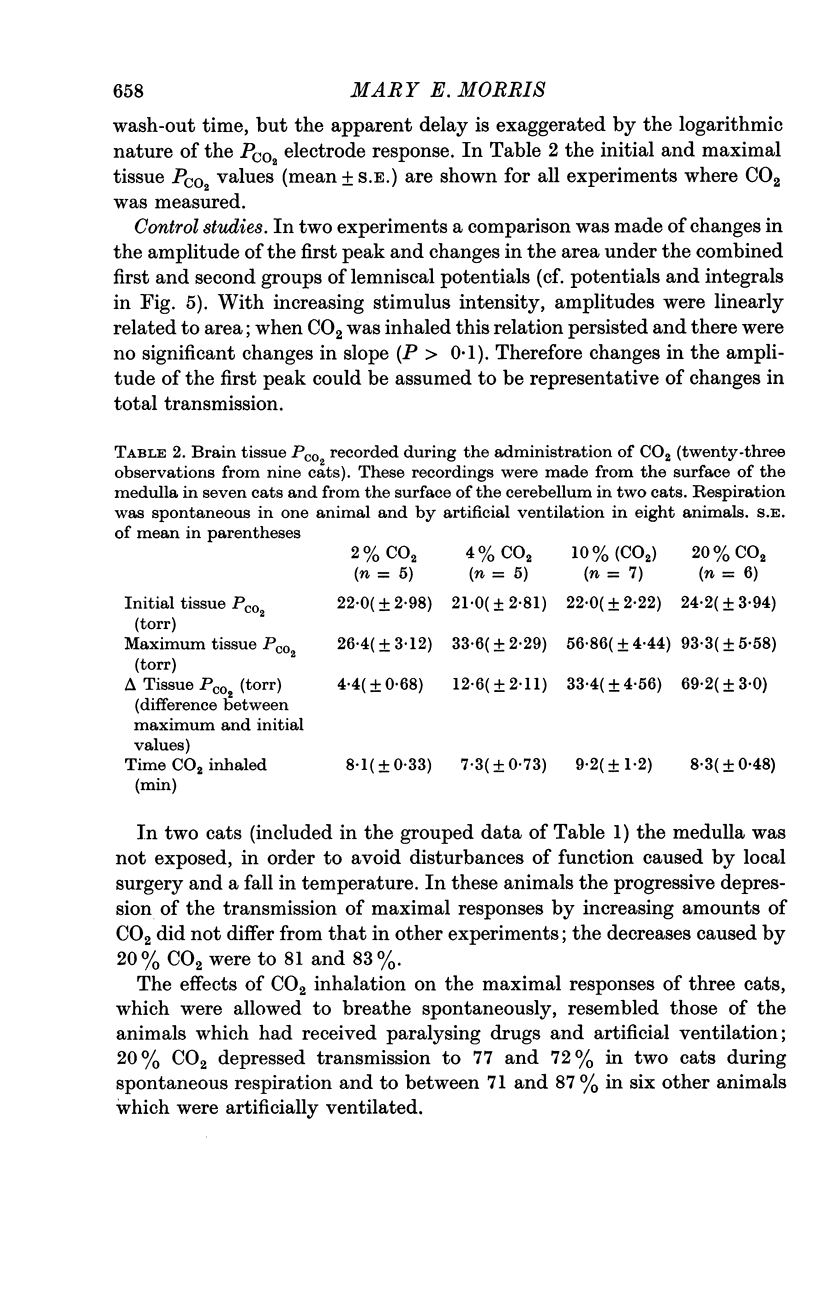
2. 2-20% CO2, when inhaled for ≤ 10 min, raised the mean tissue PCO2, recorded from the surface of the medulla or cerebellum, from 22 torr to between 26 and 93 torr.
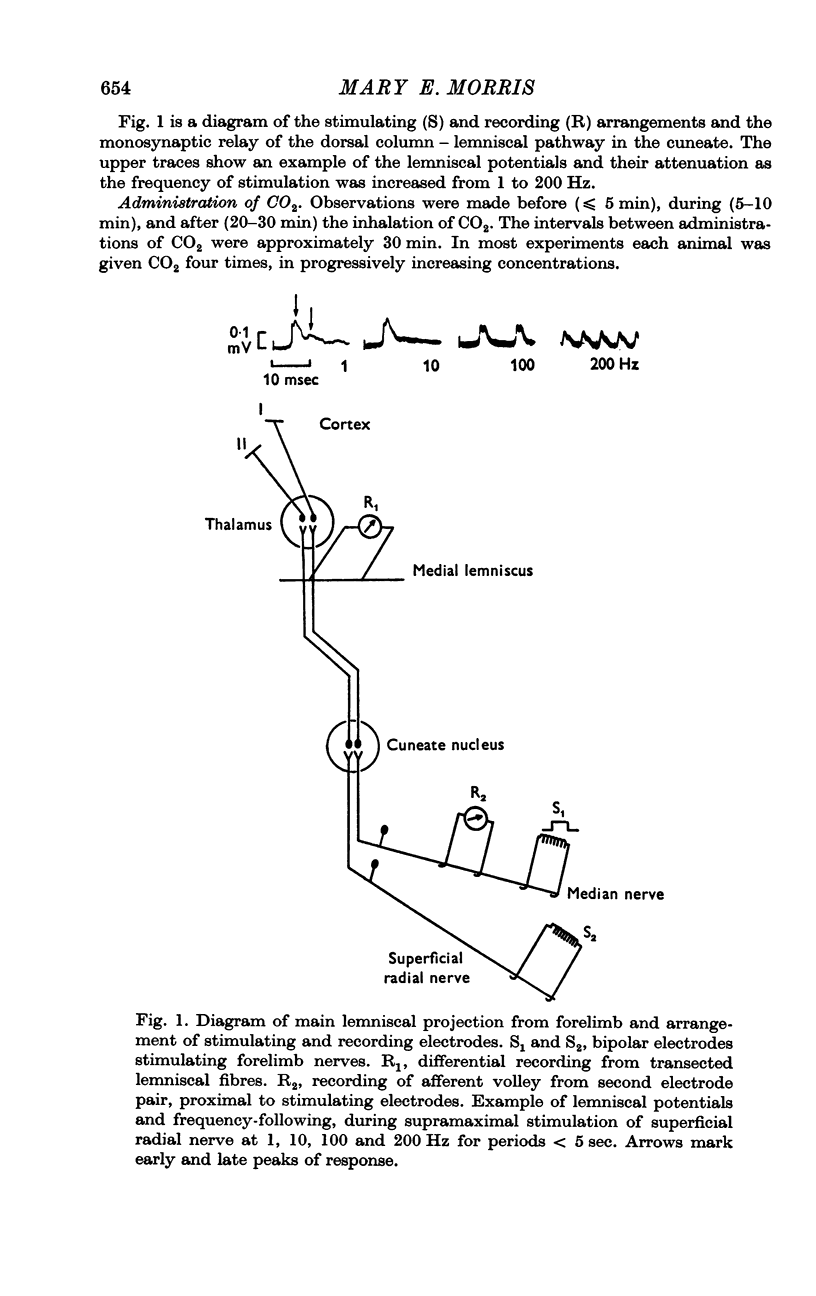
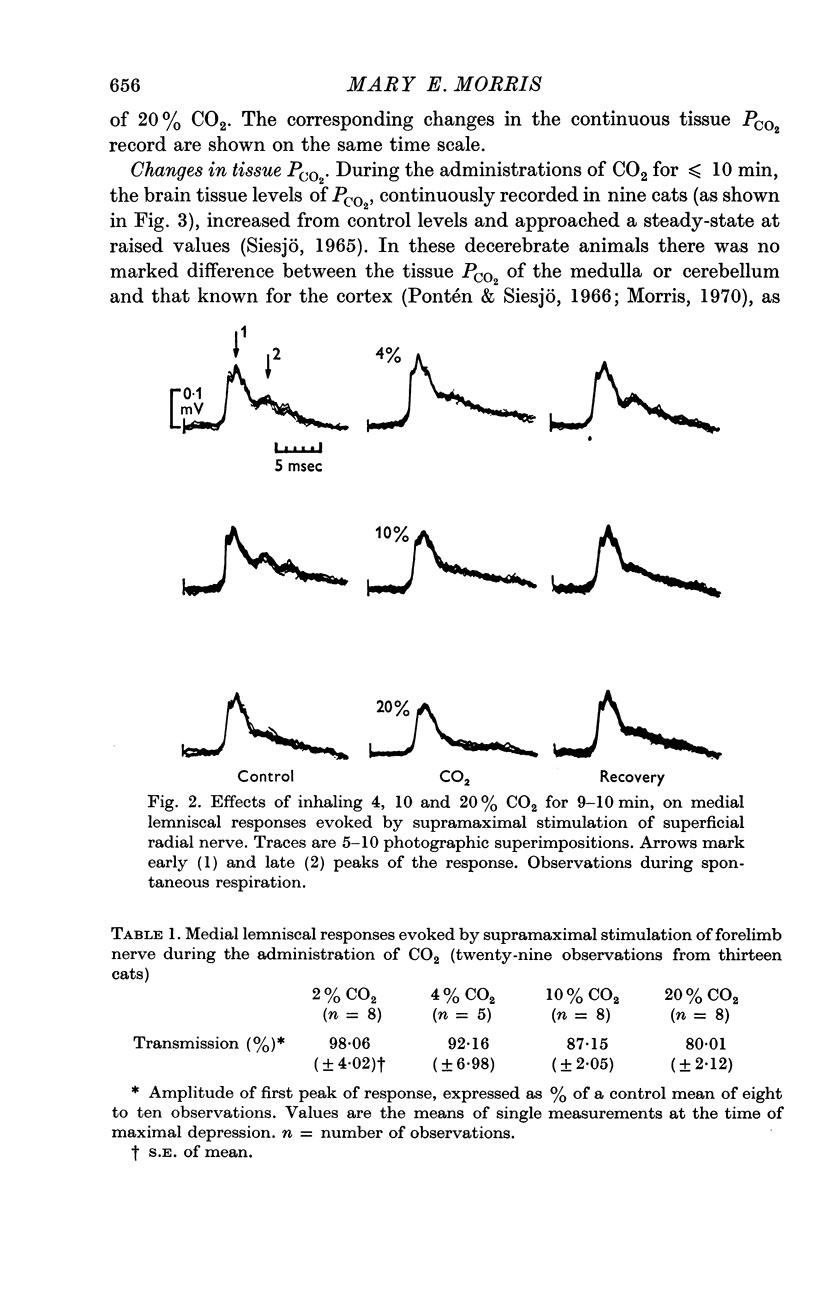
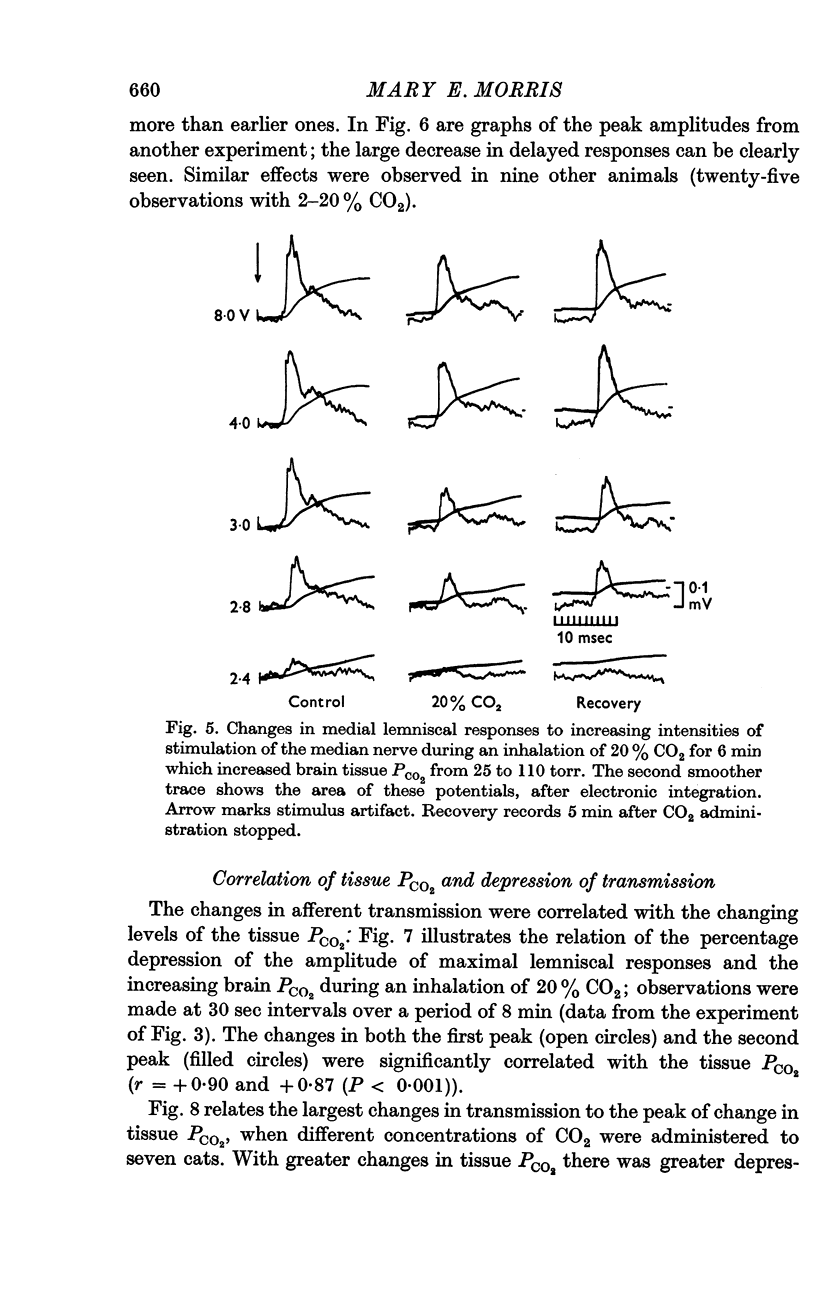
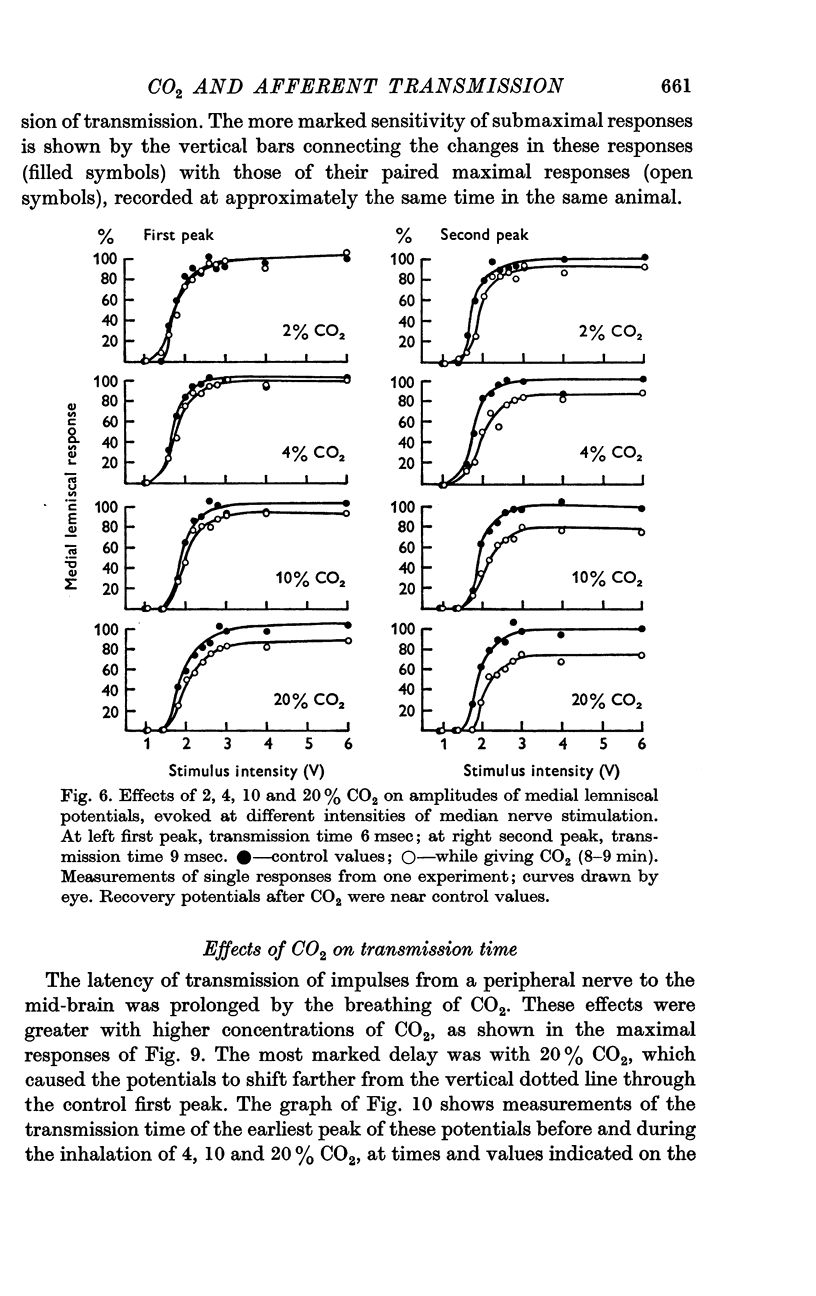
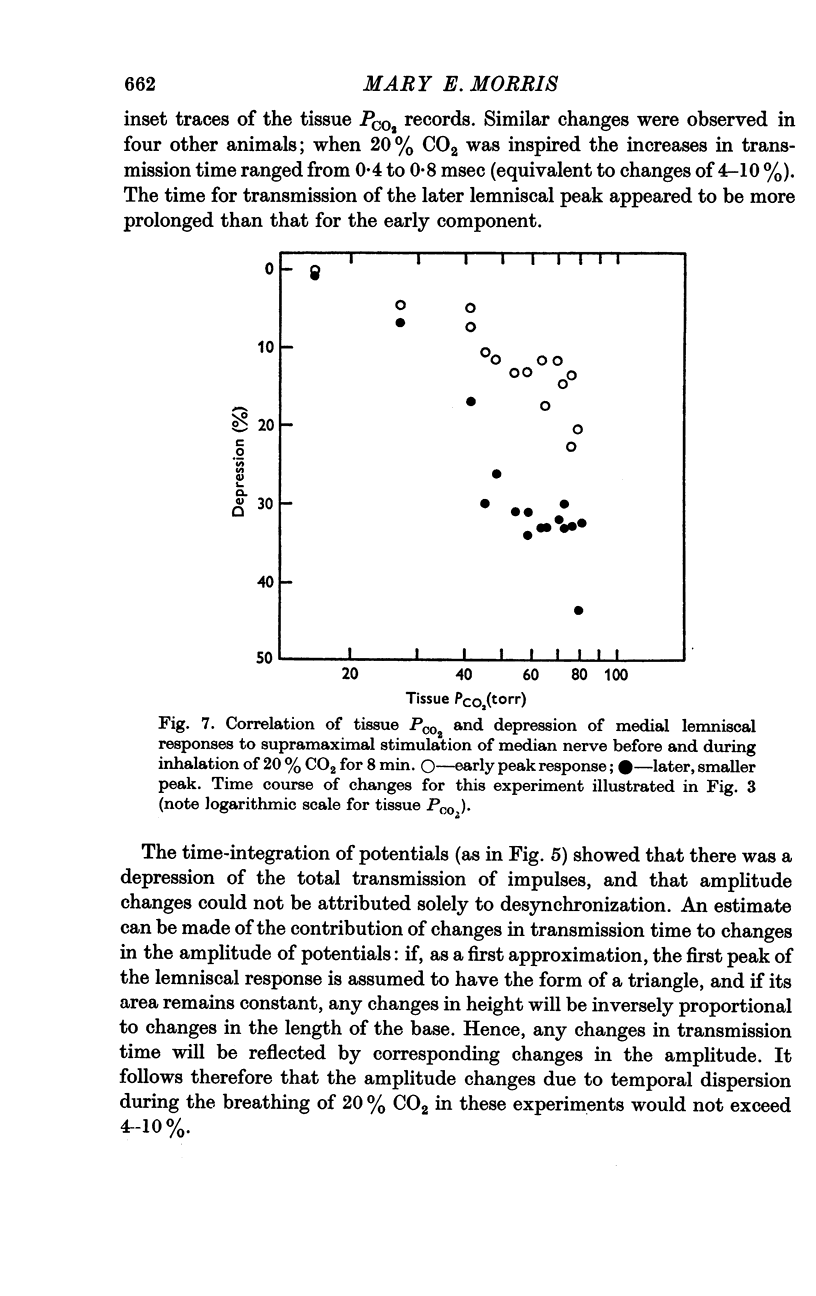
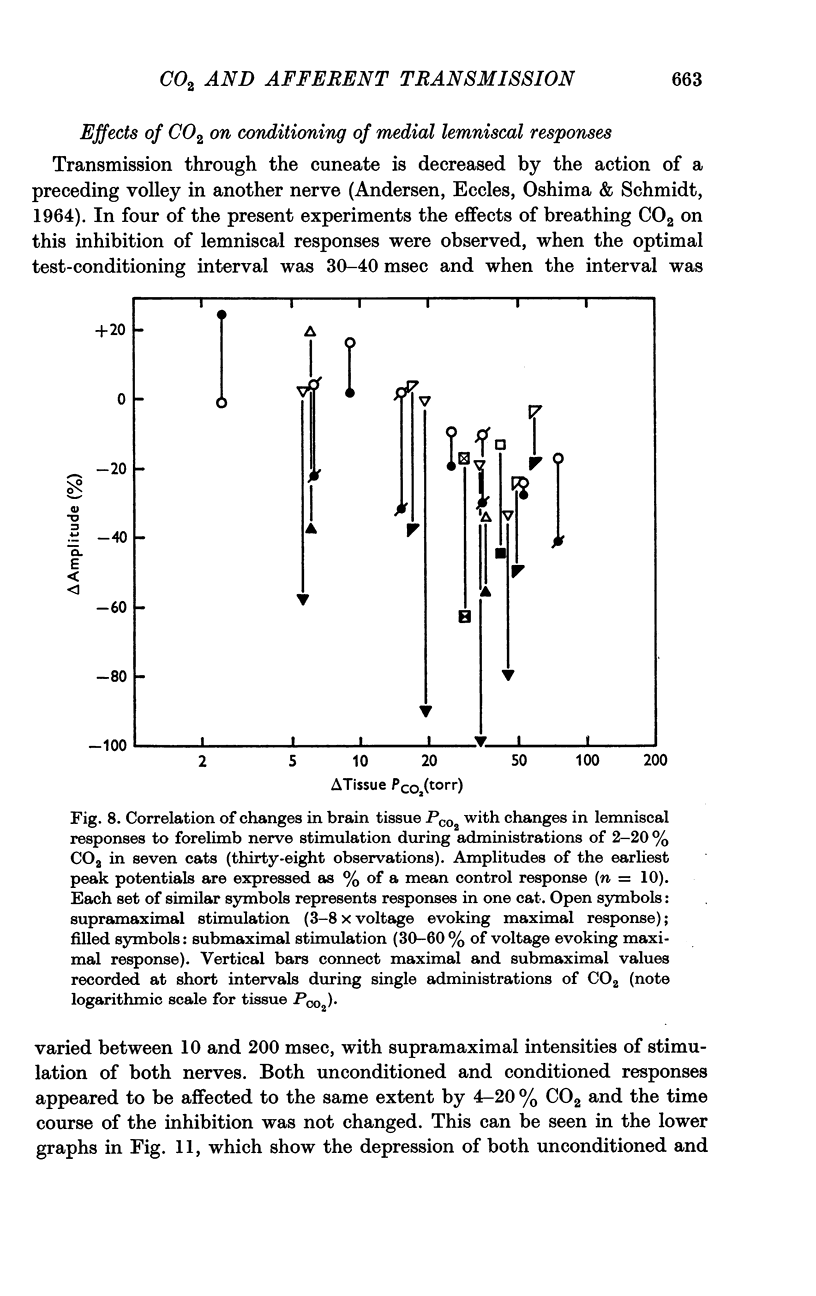
3. Medial lemniscal potentials were evoked by stimulation of a forelimb nerve and recorded from the transected surface of the contralateral mid-brain. The transmission of supramaximal responses was progressively and reversibly depressed as tissue levels of PCO2 were raised and lowered. The time course of the changes in transmission corresponded closely to changes in the tissue PCO2.
4. The amplitude of the main early lemniscal peak was decreased to 80% by the inhalation of 20% CO2. A late component of the lemniscal response, presumably due to repetitive firing and conduction in smaller fibres and polysynaptic pathways, was more affected than the early main response.
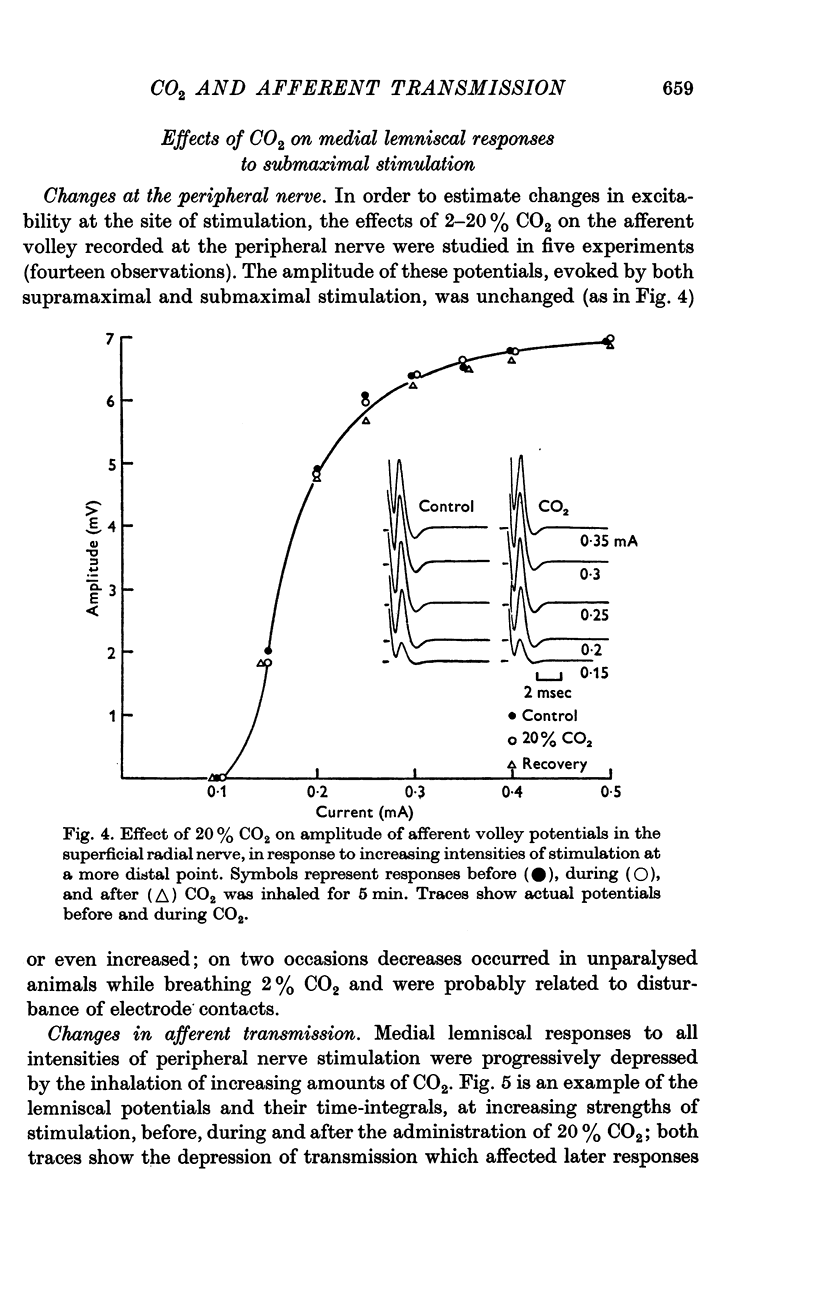
5. The failure of transmission was unrelated to threshold changes in the peripheral nerve, since potentials recorded close to the site of stimulation showed no changes.
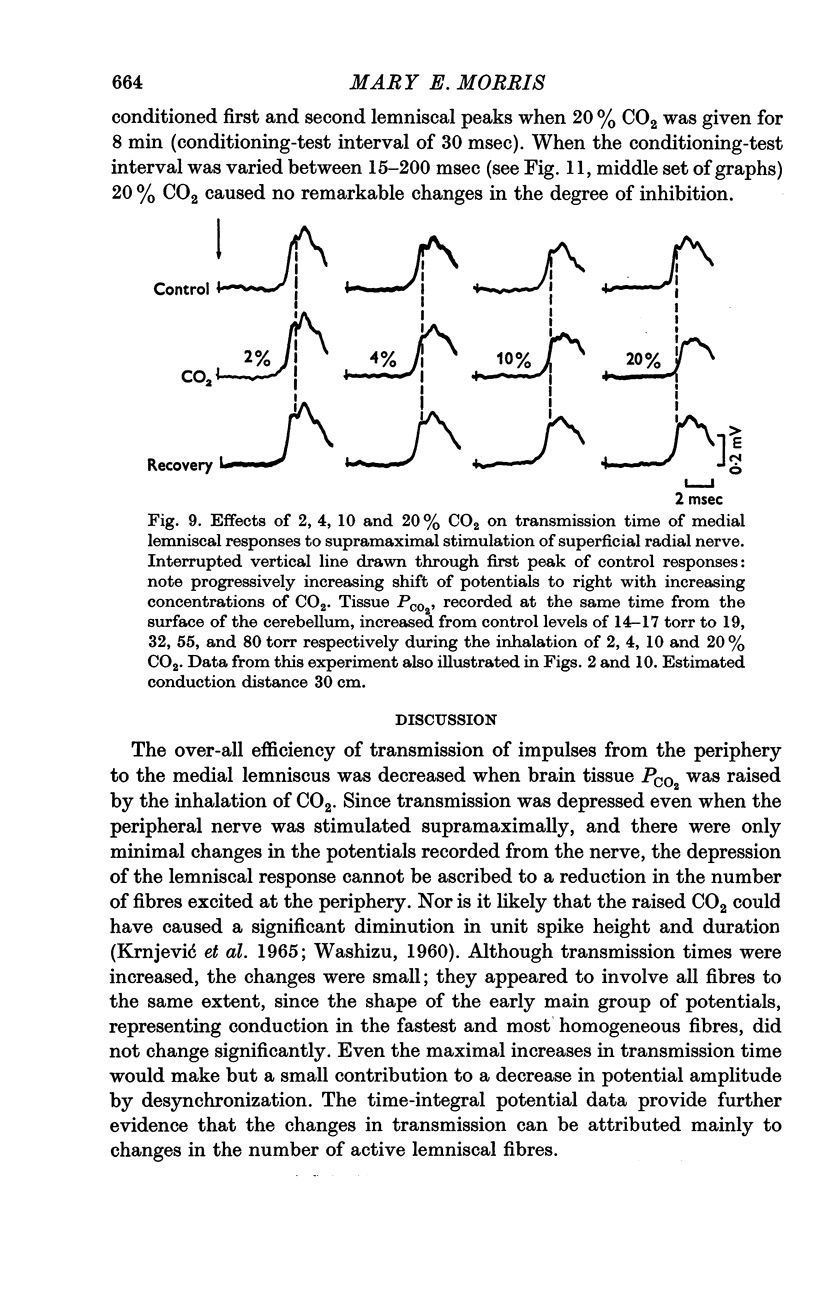
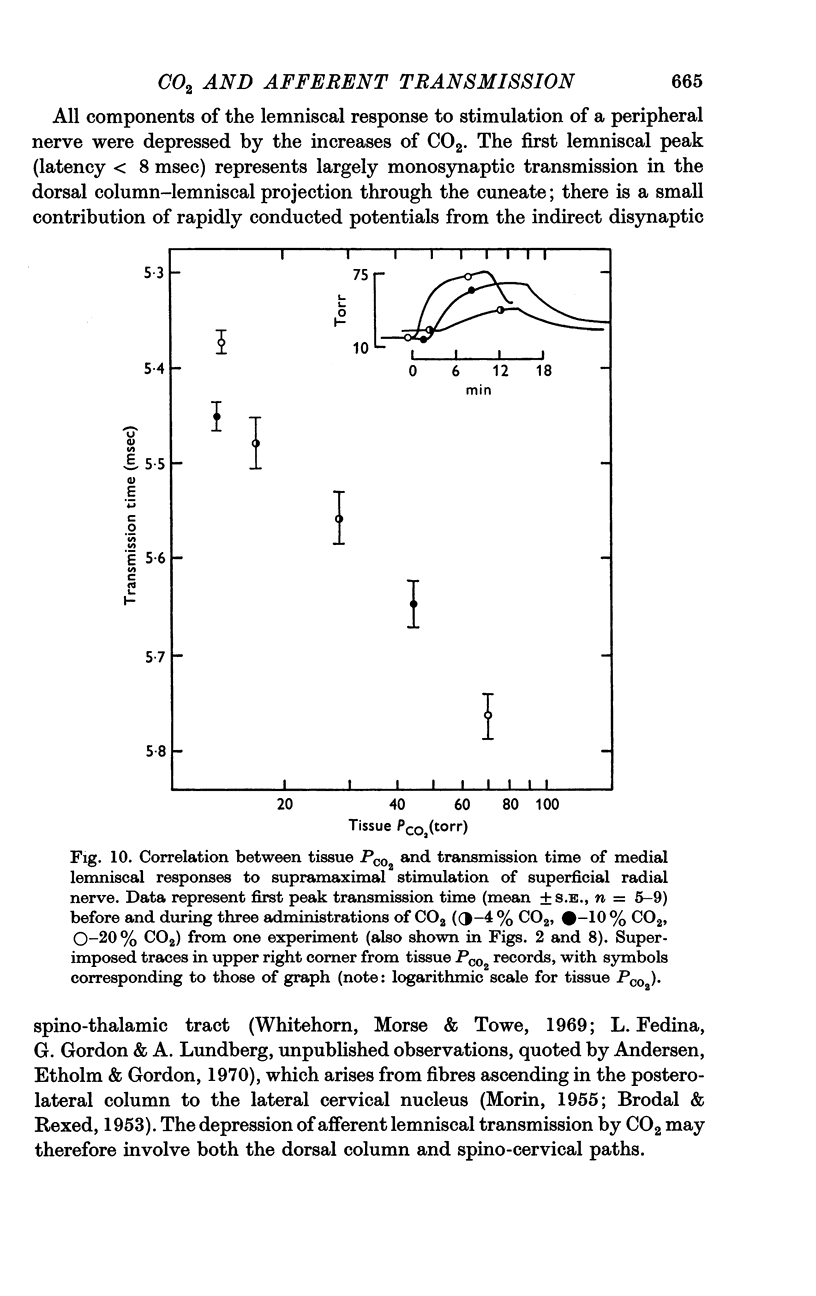
6. Increases in the transmission time of the main lemniscal potentials were uniform and < 10% during the administration of CO2 and did not appear to contribute to amplitude changes.
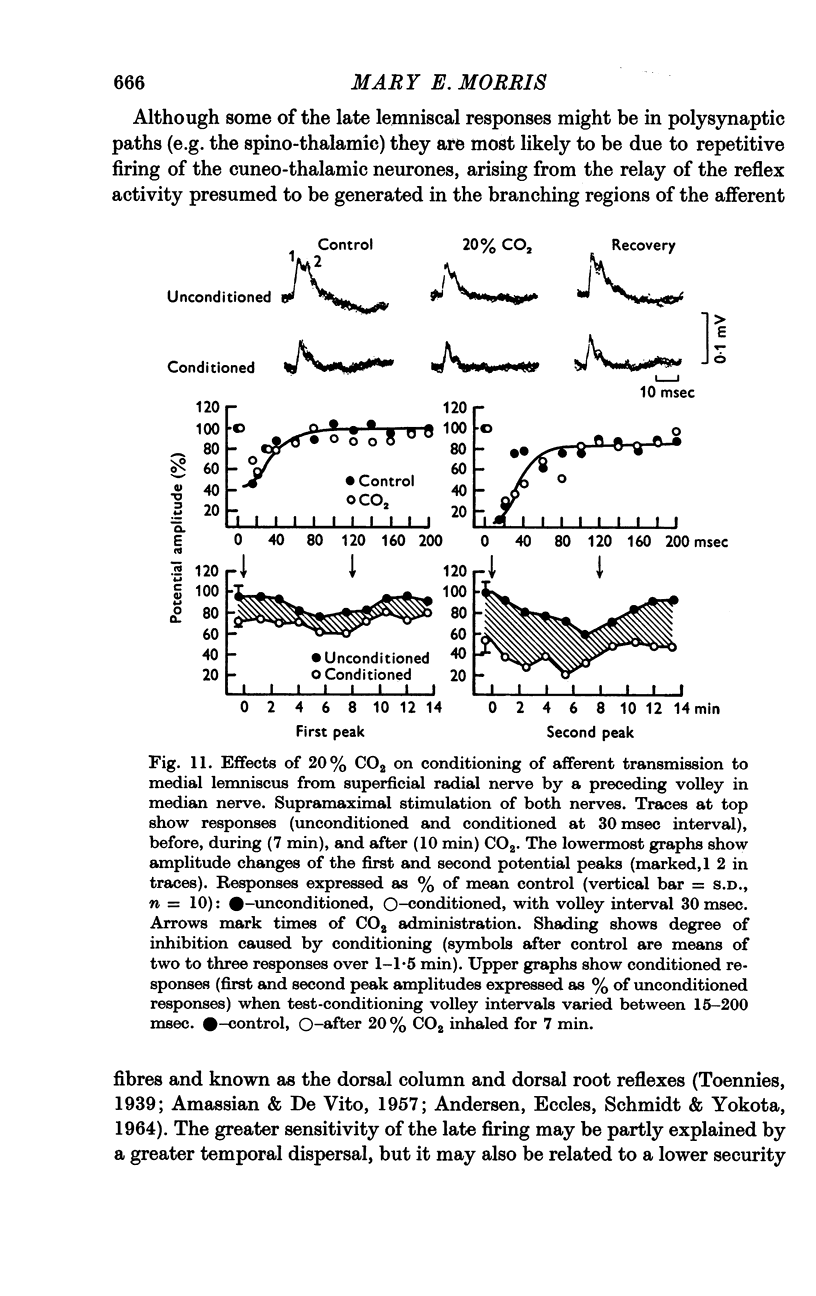
7. The inhibition of afferent transmission from one nerve by a preceding conditioning volley in a second nerve was not altered by hypercarbia.
8. It is concluded that CO2 has a blocking action on afferent transmission in the pre-thalamic lemniscal system. The site of this block may be at the synapses and/or other regions of low safety factor in the afferent fibres.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. DEPOLARIZATION OF PRESYNAPTIC FIBERS IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Jan;27:92–106. doi: 10.1152/jn.1964.27.1.92. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. IDENTIFICATION OF RELAY CELLS AND INTERNEURONS IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1080–1095. doi: 10.1152/jn.1964.27.6.1080. [DOI] [PubMed] [Google Scholar]
- Andersen P., Etholm B., Gordon G. Presynaptic and post-synaptic inhibition elicited in the cat's dorsal column nuclei by mechanical stimulation of skin. J Physiol. 1970 Sep;210(2):433–455. doi: 10.1113/jphysiol.1970.sp009219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOISTEL J., CORABOEUF E. Action de l'anhydride carbonique sur l'activité électrique du nerf isolé d'insecte. J Physiol (Paris) 1954;46(1):258–261. [PubMed] [Google Scholar]
- BRODAL A., REXED B. Spinal afferents to the lateral cervical nucleus in the cat; an experimental study. J Comp Neurol. 1953 Apr;98(2):179–211. doi: 10.1002/cne.900980202. [DOI] [PubMed] [Google Scholar]
- DELL P., BONVALLET M. Contrôle direct et réflexe de l'activité du système réticulé activateur ascendant du tronc cérébral par l'oxygène et le gaz carbonique du sang. C R Seances Soc Biol Fil. 1954 May;148(9-10):855–858. [PubMed] [Google Scholar]
- FRIEDLANDER W. J., HILL T. E. E. G. changes during administration of carbon dioxide. Dis Nerv Syst. 1954 Mar;15(3):71–75. [PubMed] [Google Scholar]
- GELLHORN E. On the physiological action of carbon dioxide on cortex and hypothalamus. Electroencephalogr Clin Neurophysiol. 1953 Aug;5(3):401–413. doi: 10.1016/0013-4694(53)90083-2. [DOI] [PubMed] [Google Scholar]
- GELLHORN F. Experimental contribution to the duplicity theory of consciousness and perception. Pflugers Arch. 1952 Apr;255(1):75–92. doi: 10.1007/BF00371776. [DOI] [PubMed] [Google Scholar]
- GILL P. K., KUNO M. PROPERTIES OF PHRENIC MOTONEURONES. J Physiol. 1963 Sep;168:258–273. doi: 10.1113/jphysiol.1963.sp007191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSTEIN L. Early effects of oxygen lack and carbon dioxide excess on spinal reflexes. Acta Physiol Scand Suppl. 1951;23(80):1–54. [PubMed] [Google Scholar]
- MORIN F. A new spinal pathway for cutaneous impulses. Am J Physiol. 1955 Nov;183(2):245–252. doi: 10.1152/ajplegacy.1955.183.2.245. [DOI] [PubMed] [Google Scholar]
- Morris M. E. The action of carbon dioxide on synaptic transmission in the cuneate nucleus. J Physiol. 1971 Nov;218(3):671–689. doi: 10.1113/jphysiol.1971.sp009639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. E. The effects of respiratory acidosis on a sensory relay system. Can Anaesth Soc J. 1969 Nov;16(6):494–507. doi: 10.1007/BF03004543. [DOI] [PubMed] [Google Scholar]
- NGAI S. H. Pulmonary ventilation studies on pontile and medullary cats; changes on O2 consumption, in arterial blood pH, CO2 tension and O2 saturation, and in response to CO2 and cyanide. Am J Physiol. 1957 Aug;190(2):356–360. doi: 10.1152/ajplegacy.1957.190.2.356. [DOI] [PubMed] [Google Scholar]
- Stokes J., 3rd, Chapman W. P., Smith L. H. EFFECTS OF HYPOXIA AND HYPERCAPNIA ON PERCEPTION OF THERMAL CUTANEOUS PAIN. J Clin Invest. 1948 May;27(3 Pt 1):299–304. doi: 10.1172/JCI101958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehorn D., Morse R. W., Towe A. L. Role of the spinocervical tract in production of the primary cortical response evoked by forepaw stimulation. Exp Neurol. 1969 Nov;25(3):349–364. doi: 10.1016/0014-4886(69)90130-7. [DOI] [PubMed] [Google Scholar]


