Abstract
1. End-plate potentials (e.p.p.s) and miniature end-plate potentials (min.e.p.p.s) were recorded intracellularly from the cutaneous pectoris nerve-muscle preparation of the frog during prolonged stimulation at low frequencies (5/sec—50/sec).
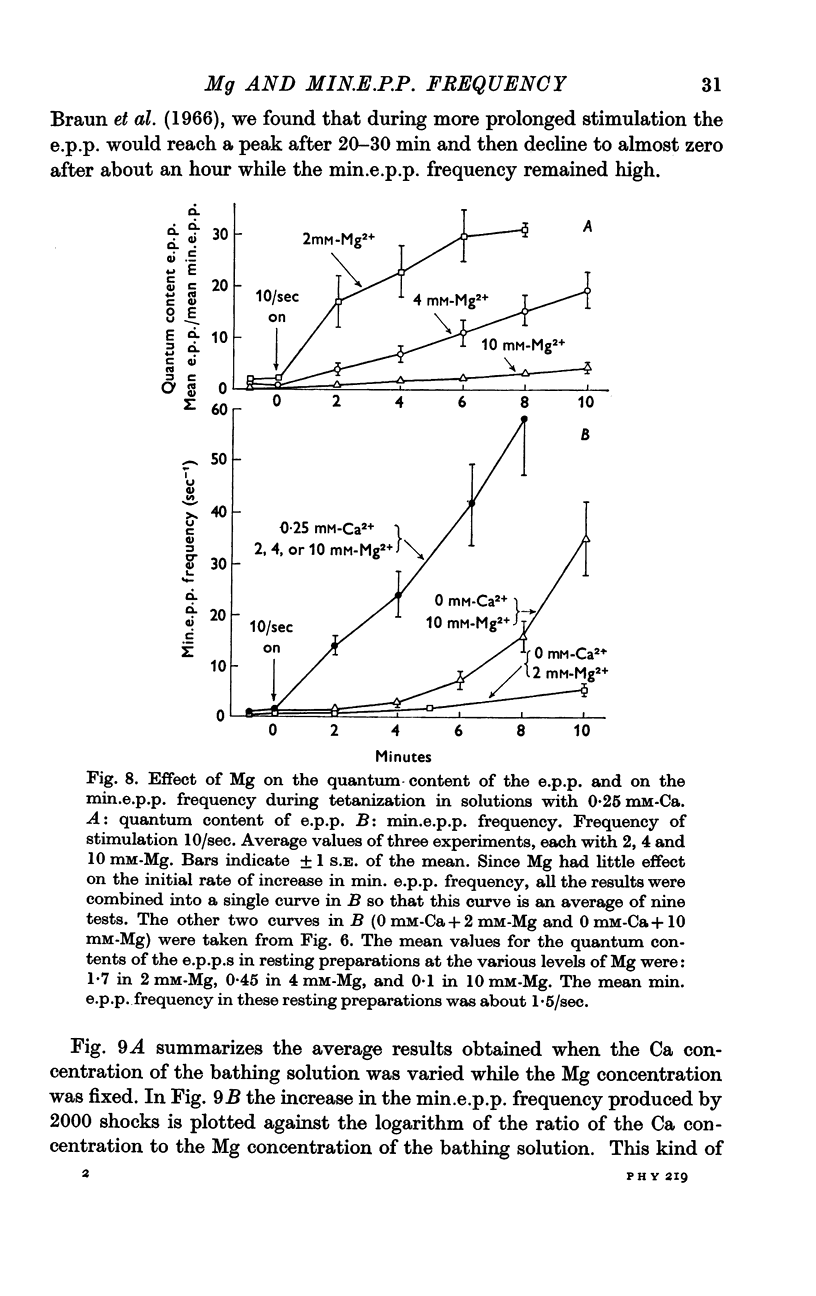
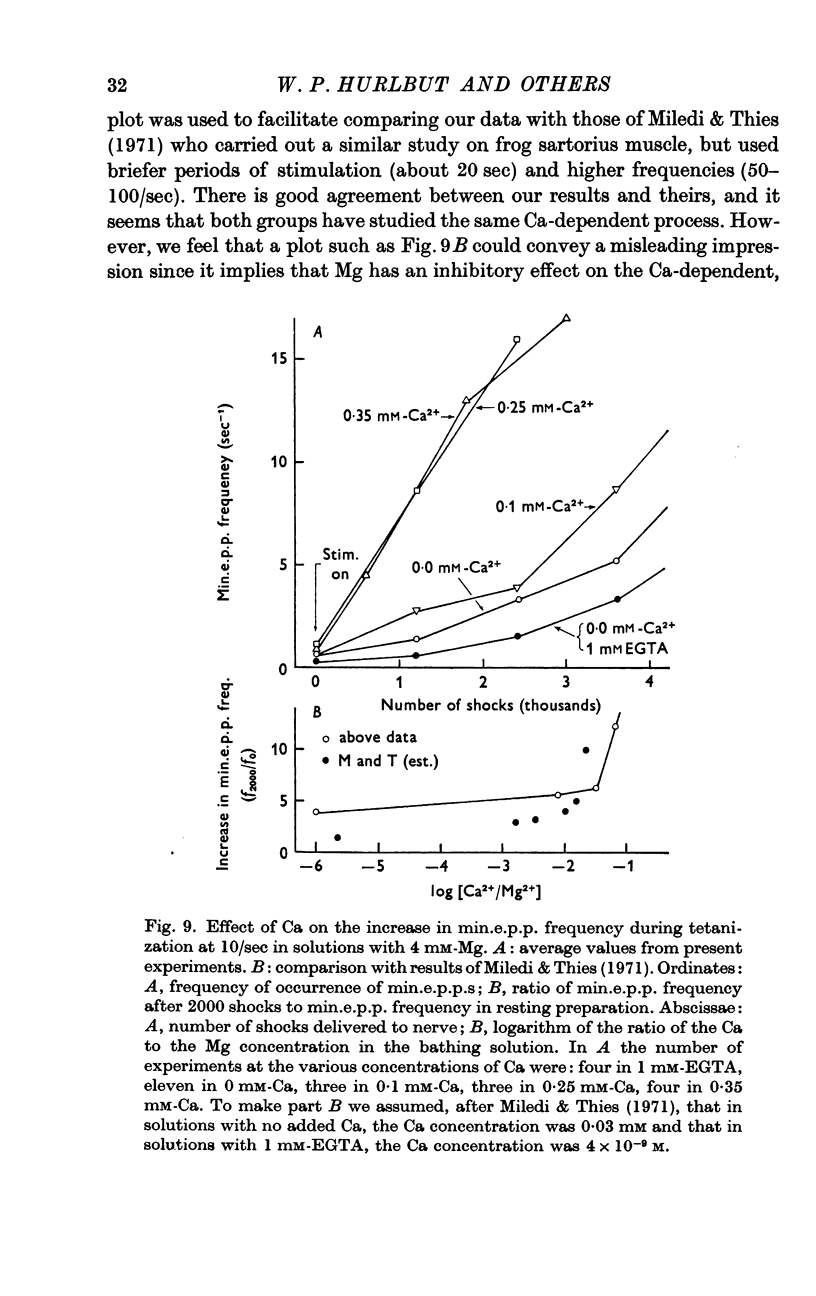
2. When Ca was present in the bathing solution, the quantum content of the e.p.p. and the frequency of occurrence of the min.e.p.p.s gradually increased during the period of stimulation. During the first few minutes of stimulation, the min.e.p.p. frequency increased linearly with time, and the rate of increase was dependent on the Ca concentration of the bathing solution. However, Mg had no effect on this Ca-dependent increase in min.e.p.p. frequency.
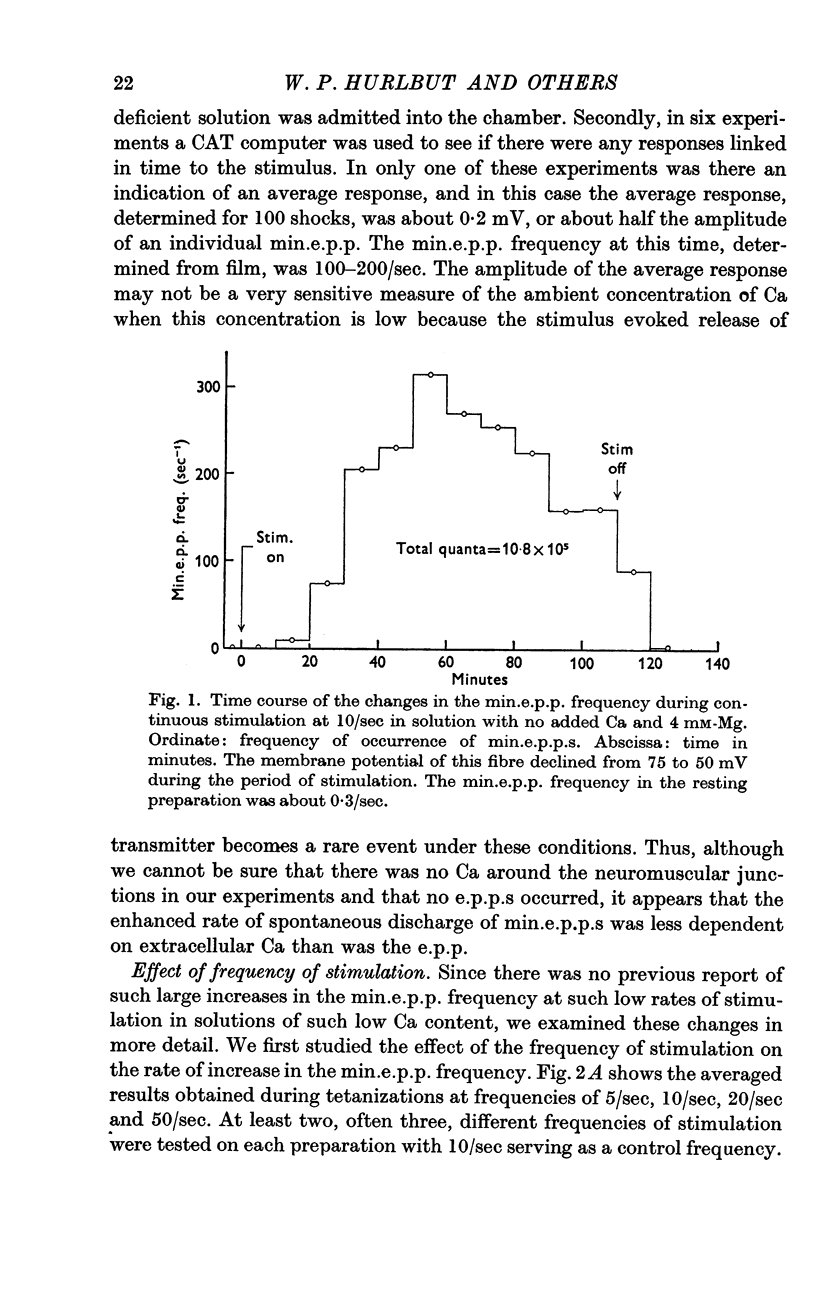
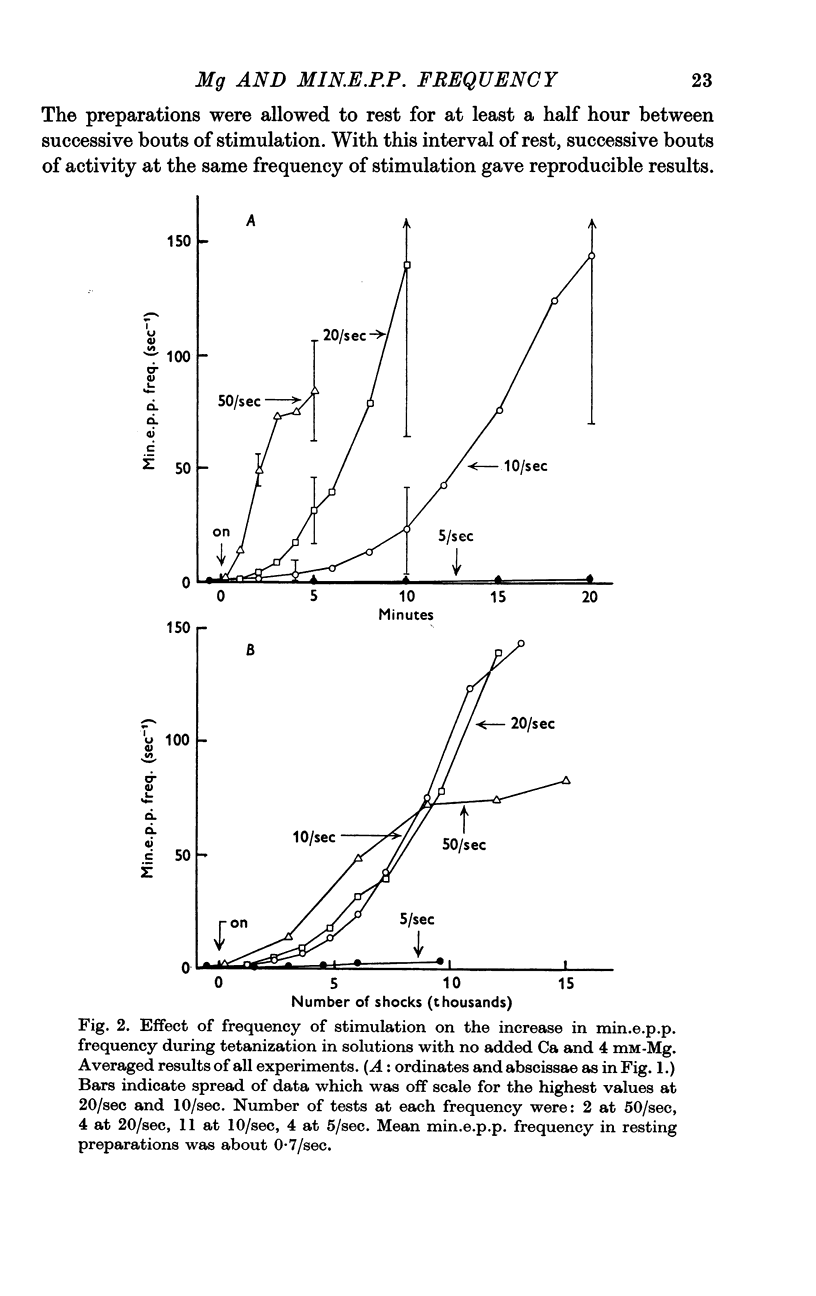
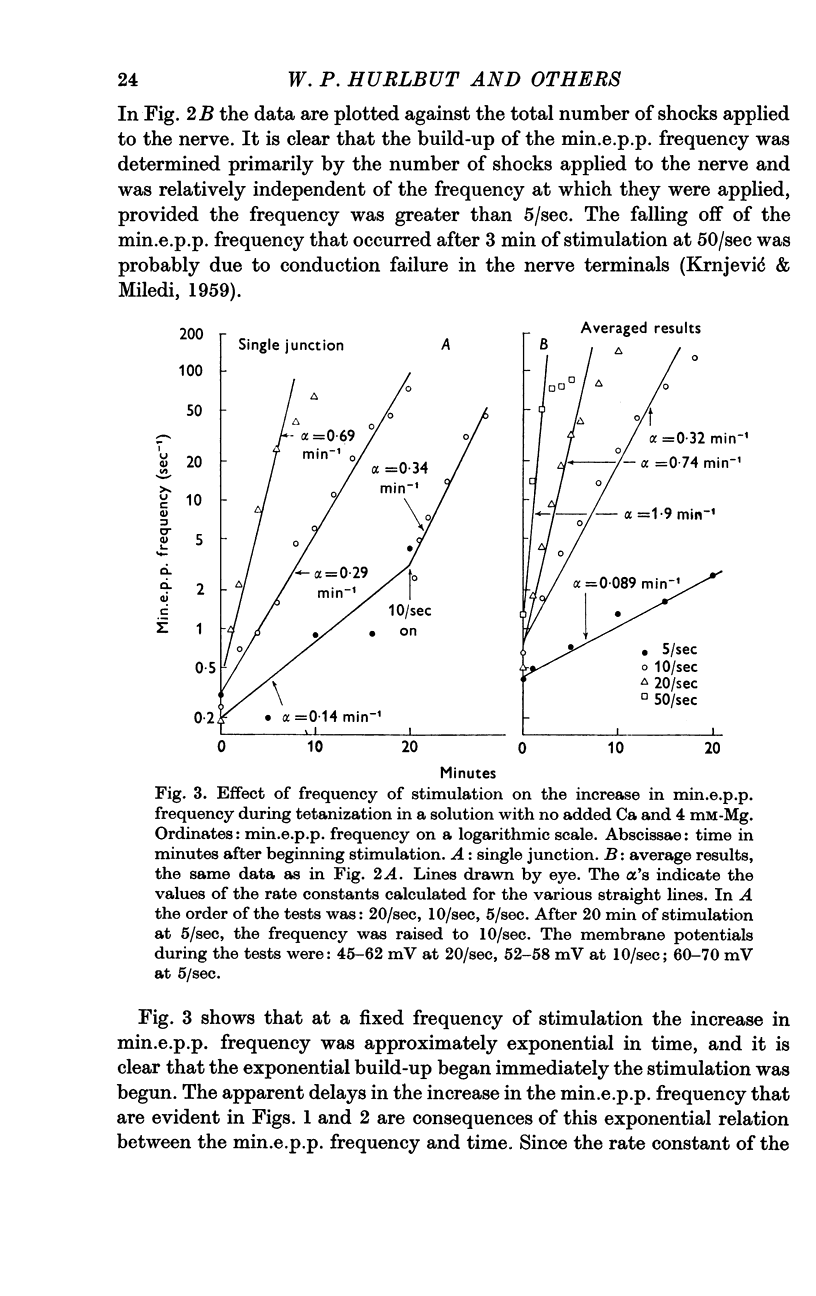
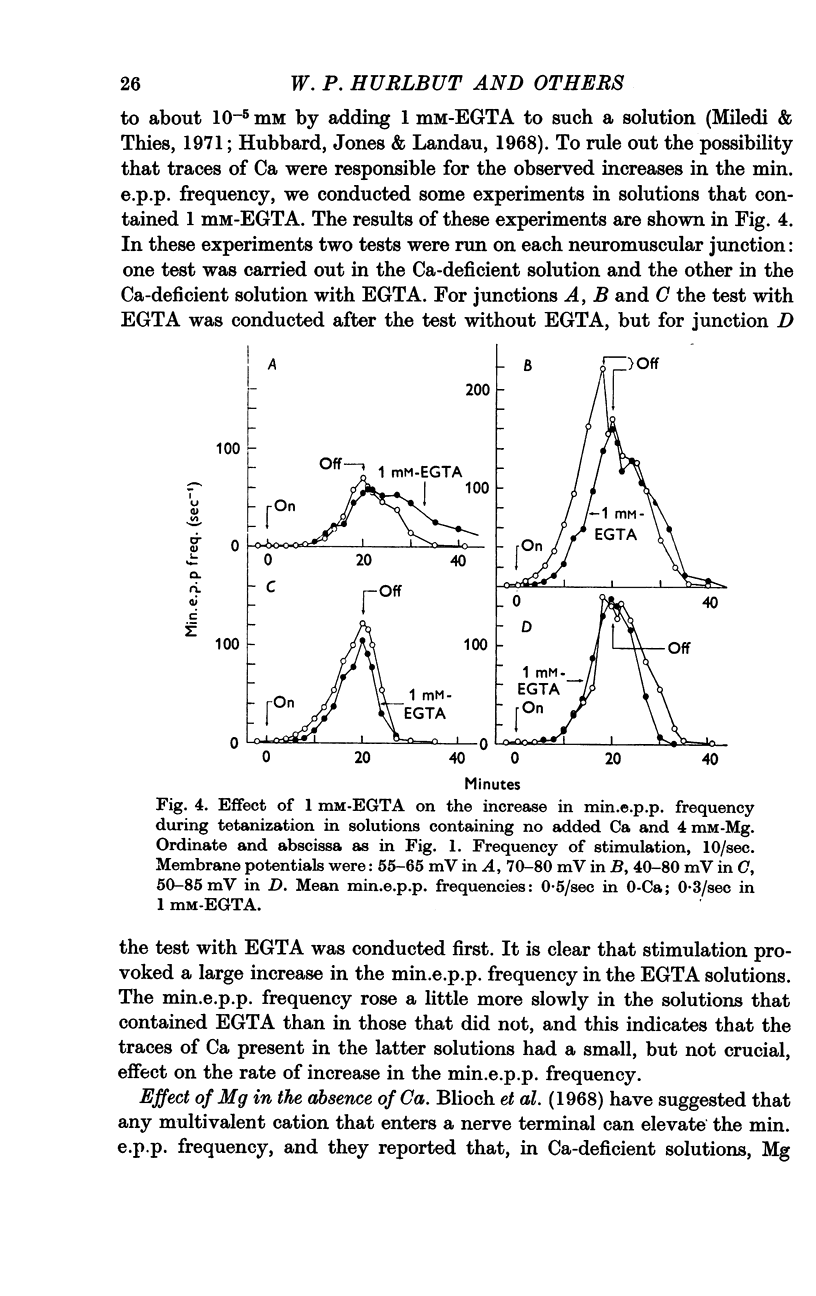
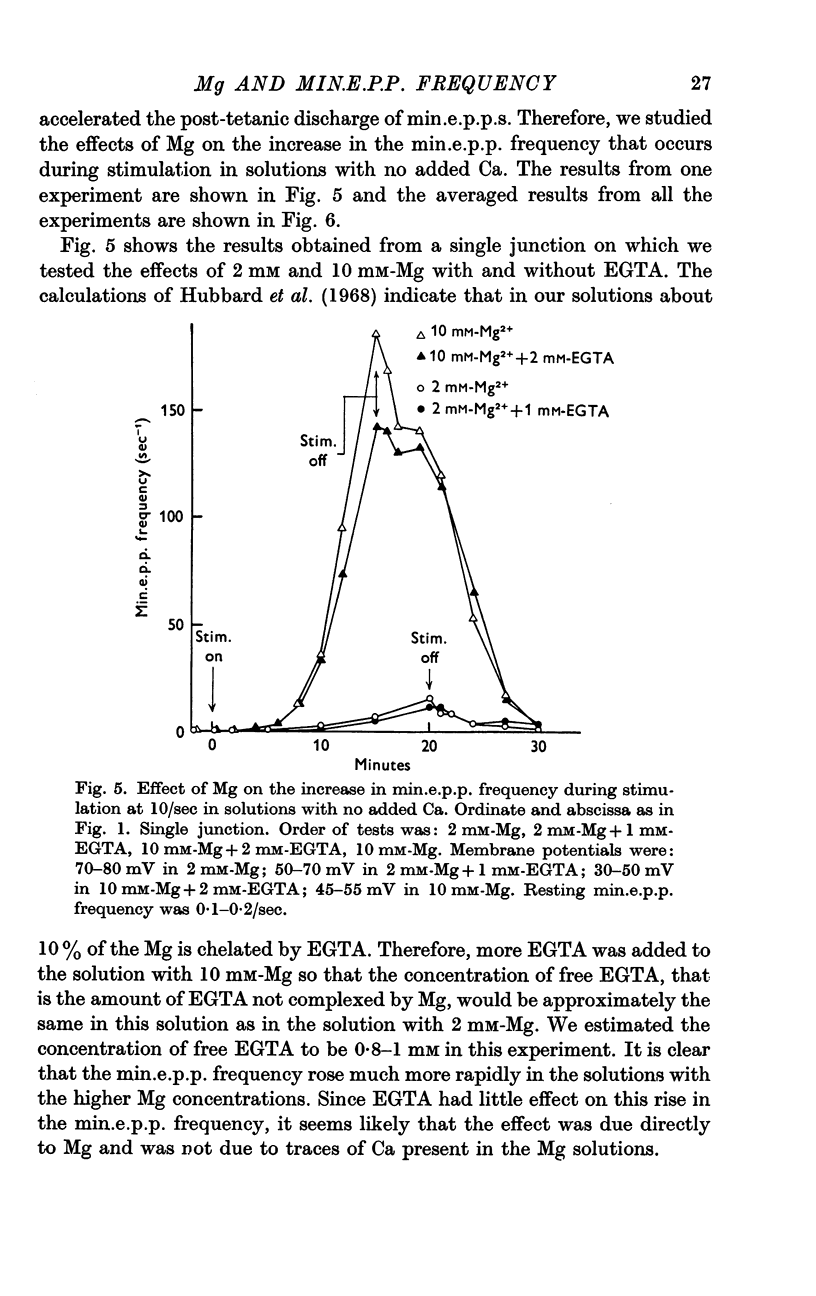
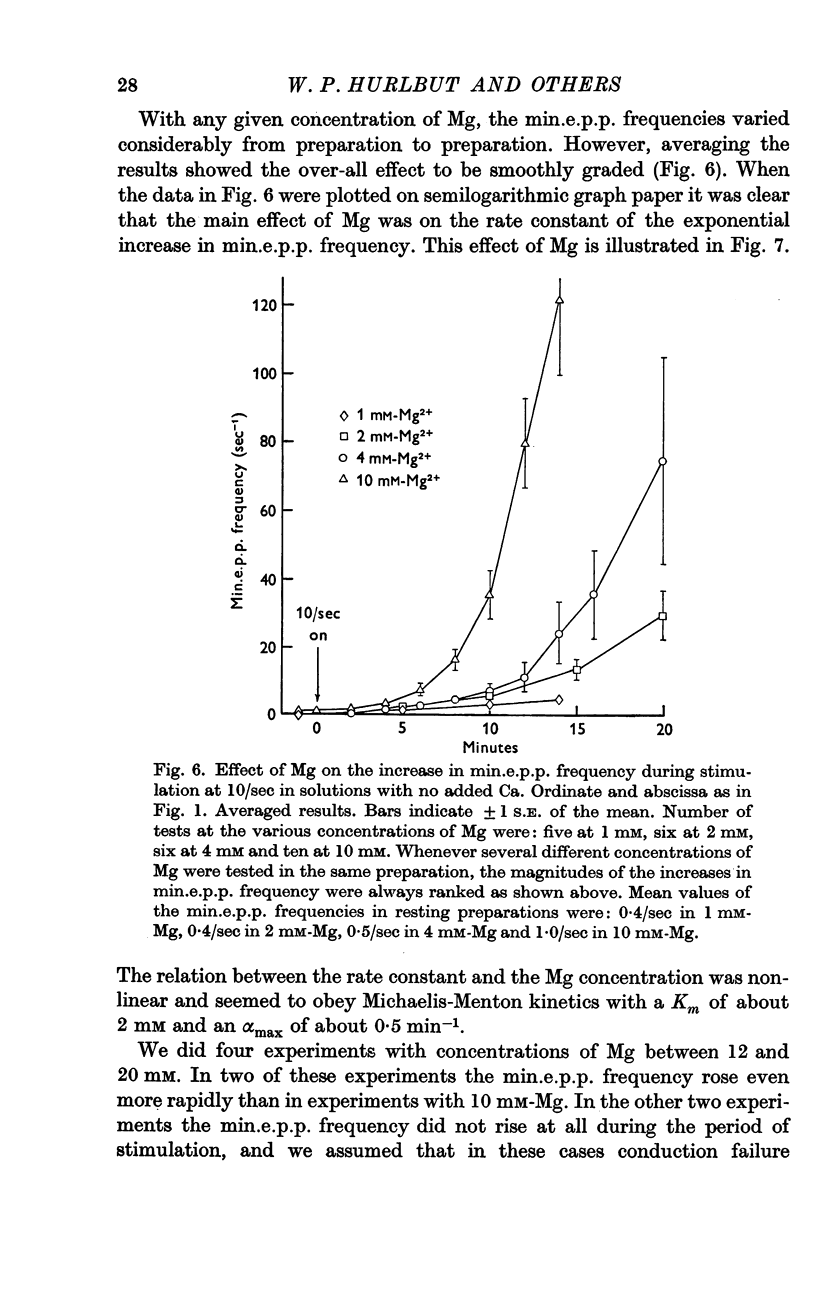
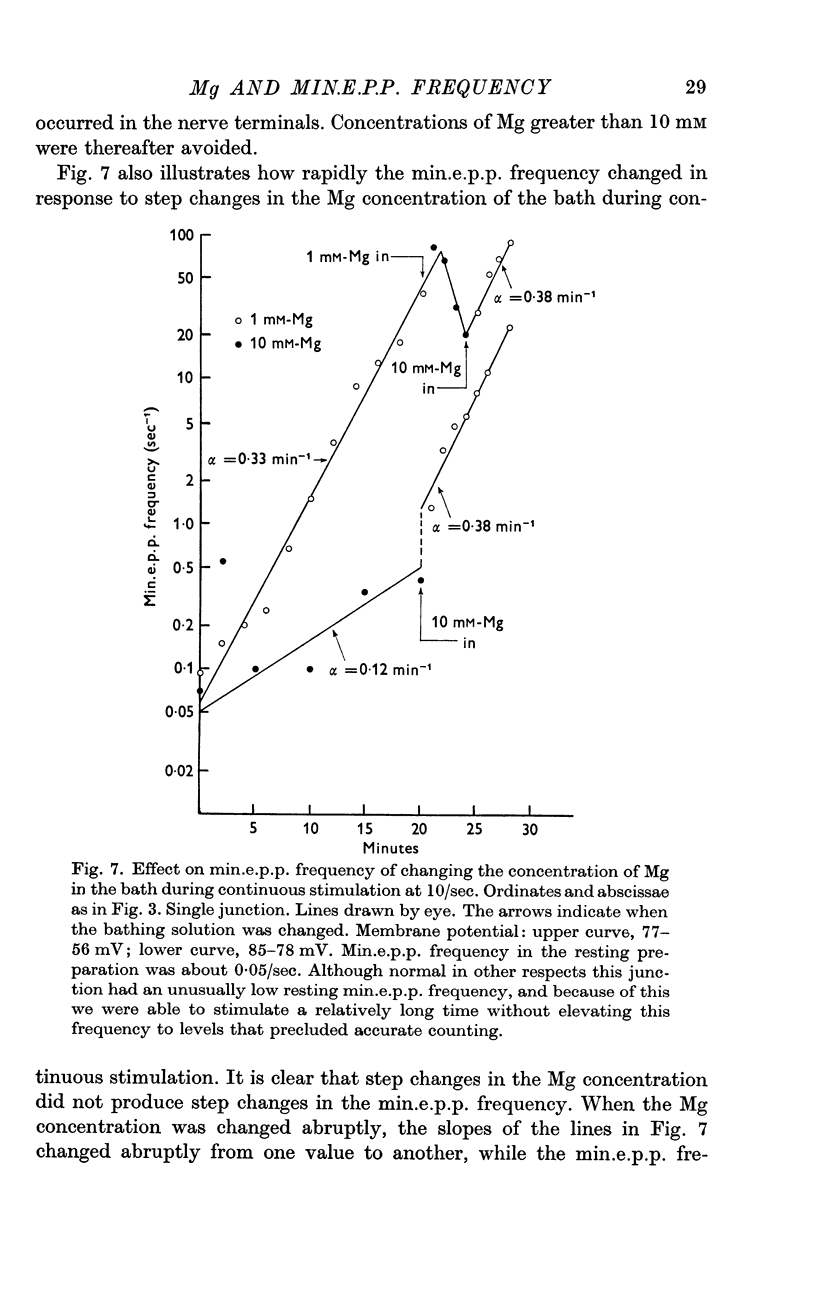
3. A large maintained increase in min.e.p.p. frequency also occurred during prolonged stimulation in solutions that contained no added Ca and 1-2 mM-EGTA. Under these conditions the increase in min.e.p.p. frequency was dependent on the Mg concentration of the bathing solution and was exponential in time.
4. It is suggested that the rise in min.e.p.p. frequency is caused by an accumulation of Ca or Mg ions in the nerve terminal, and it is suggested that these ions enter the terminal at relatively non-specific sites distinct from the Ca-specific sites that trigger the `phasic' release of transmitter.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKS V. B. An intracellular study of the action of repetitive nerve volleys and of botulinum toxin on miniature end-plate potentials. J Physiol. 1956 Nov 28;134(2):264–277. doi: 10.1113/jphysiol.1956.sp005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., HOLMAN M., LULLMANN H. Effects of calcium deficiency on striated muscle of the frog. J Physiol. 1956 Jul 27;133(1):101–117. doi: 10.1113/jphysiol.1956.sp005569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. Sodium-dependent transport of magnesium ions in giant axons of Loligo forbesi. J Physiol. 1971 Jul;216(1):38P–40P. [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The influence of internal sodium on the behaviour of motor nerve endings. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):401–421. doi: 10.1098/rspb.1968.0047. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Preganglionic stimulation increases calcium uptake by sympathetic ganglia. Science. 1971 Apr 23;172(3981):391–393. doi: 10.1126/science.172.3981.391. [DOI] [PubMed] [Google Scholar]
- Blioch Z. L., Glagoleva I. M., Liberman E. A., Nenashev V. A. A study of the mechanism of quantal transmitter release at a chemical synapse. J Physiol. 1968 Nov;199(1):11–35. doi: 10.1113/jphysiol.1968.sp008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M., Schmidt R. F., Zimmermann M. Facilitation at the frog neuromuscular junction during and after repetitive stimulation. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;287(1):41–55. doi: 10.1007/BF00362453. [DOI] [PubMed] [Google Scholar]
- CURTIS B. A. Some effects of Ca-free choline-Ringer solution on frog skeletal muscle. J Physiol. 1963 Apr;166:75–86. doi: 10.1113/jphysiol.1963.sp007091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. W., Mauro A., Longenecker H. E., Jr, Hurlbut W. P. Effects of black widow spider venom on the frog neuromuscular junction. Effects on the fine structure of the frog neuromuscular junction. Nature. 1970 Feb 21;225(5234):703–705. doi: 10.1038/225703a0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., MEVES H. The effect of magnesium and calcium on the frog myelinated nerve fibre. J Physiol. 1958 Jul 14;142(2):360–365. doi: 10.1113/jphysiol.1958.sp006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. The effect of calcium on the myelinated nerve fibre. J Physiol. 1957 Jul 11;137(2):245–260. doi: 10.1113/jphysiol.1957.sp005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. REPETITIVE STIMULATION AT THE MAMMALIAN NEUROMUSCULAR JUNCTION, AND THE MOBILIZATION OF TRANSMITTER. J Physiol. 1963 Dec;169:641–662. doi: 10.1113/jphysiol.1963.sp007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. The effect of calcium and magnesium on the spontaneous release of transmitter from mammalian motor nerve endings. J Physiol. 1961 Dec;159:507–517. doi: 10.1113/jphysiol.1961.sp006824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W. W. Caffeine effects on transmitter depletion and mobilization at motor nerve terminals. Am J Physiol. 1969 Mar;216(3):621–629. doi: 10.1152/ajplegacy.1969.216.3.621. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENDEN D. J., REGER J. F. THE ROLE OF RESTING POTENTIAL CHANGES IN THE CONTRACTILE FAILURE OF FROG SARTORIUS MUSCLES DURING CALCIUM DEPRIVATION. J Physiol. 1963 Dec;169:889–901. doi: 10.1113/jphysiol.1963.sp007302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Presynaptic failure of neuromuscular propagation in rats. J Physiol. 1959 Dec;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970 May;207(3):789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W., NORTH K. A. An electrical investigation of effects of repetitive stimulation on mammalian neuromuscular junction. J Neurophysiol. 1953 Sep;16(5):509–527. doi: 10.1152/jn.1953.16.5.509. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The quantal components of the mammalian end-plate potential. J Physiol. 1956 Sep 27;133(3):571–587. doi: 10.1113/jphysiol.1956.sp005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau E. M. The interaction of presynaptic polarization with calcium and magnesium in modifying spontaneous transmitter release from mammalian motor nerve terminals. J Physiol. 1969 Aug;203(2):281–299. doi: 10.1113/jphysiol.1969.sp008864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. The action of calcium on neuronal synapses in the squid. J Physiol. 1966 May;184(2):473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Spontaneous synaptic potentials and quantal release of transmitter in the stellate ganglion of the squid. J Physiol. 1967 Sep;192(2):379–406. doi: 10.1113/jphysiol.1967.sp008306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel D. M., Hackett J. T., Cooke J. D. Calcium: is it required for transmitter secretion? Science. 1971 Jun 4;172(3987):1034–1036. doi: 10.1126/science.172.3987.1034. [DOI] [PubMed] [Google Scholar]
- SPYROPOULOS C. S., BRADY R. O. Prolongation of response of node of Ranvier by metal ions. Science. 1959 May 15;129(3359):1366–1367. doi: 10.1126/science.129.3359.1366. [DOI] [PubMed] [Google Scholar]
- SPYROPOULOS C. S. Changes in the duration of the electric response of single nerve fibers following repetitive stimulation. J Gen Physiol. 1956 Sep 20;40(1):19–25. doi: 10.1085/jgp.40.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]


