Abstract
1. The neural response of semicircular canal-dependent units in the vestibular nuclei of cats has been examined over a frequency range of sinusoidal rotation extending from 0·004 to 0·9 Hz.
2. Frequency—response analysis indicates that, over the range examined, the information contained in the neural signal received by the brain stem was similar to that expected from the mechanical end-organ.
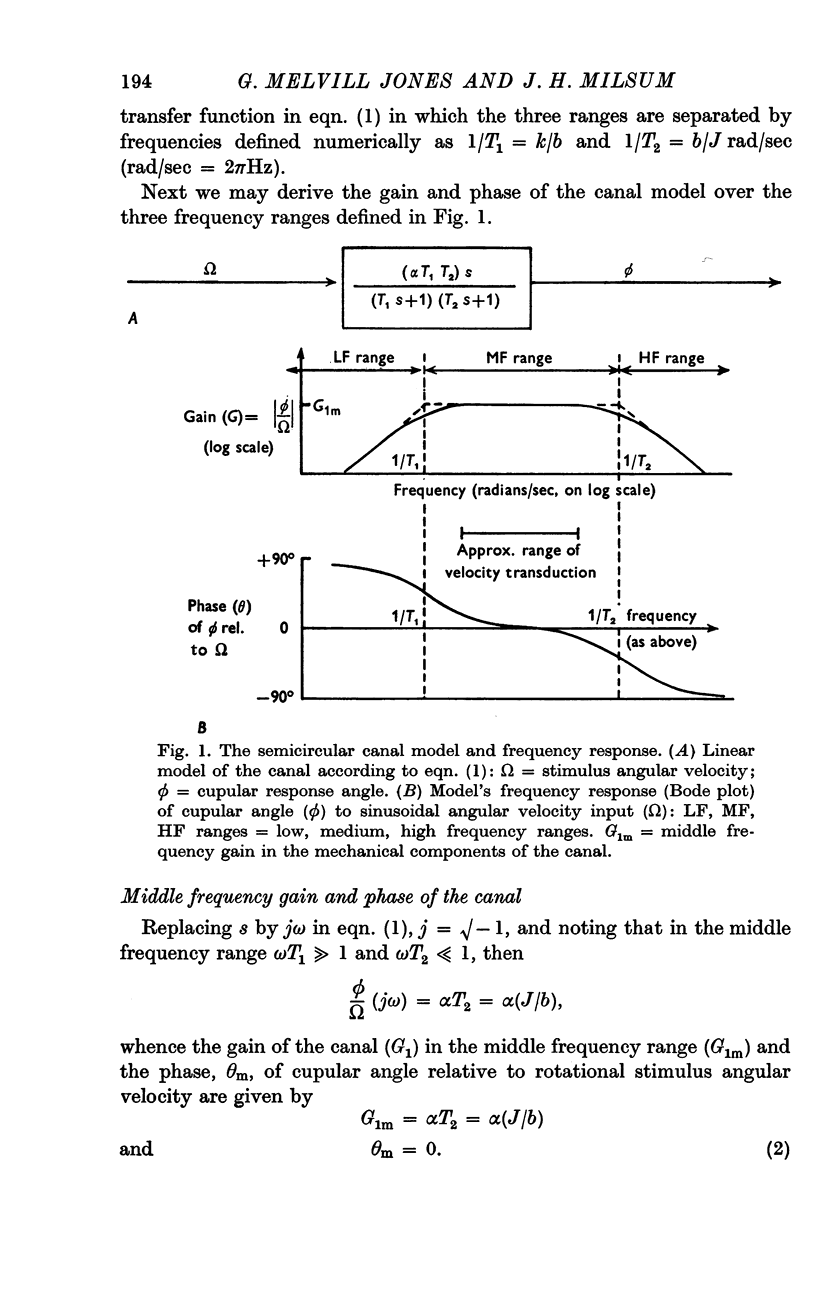
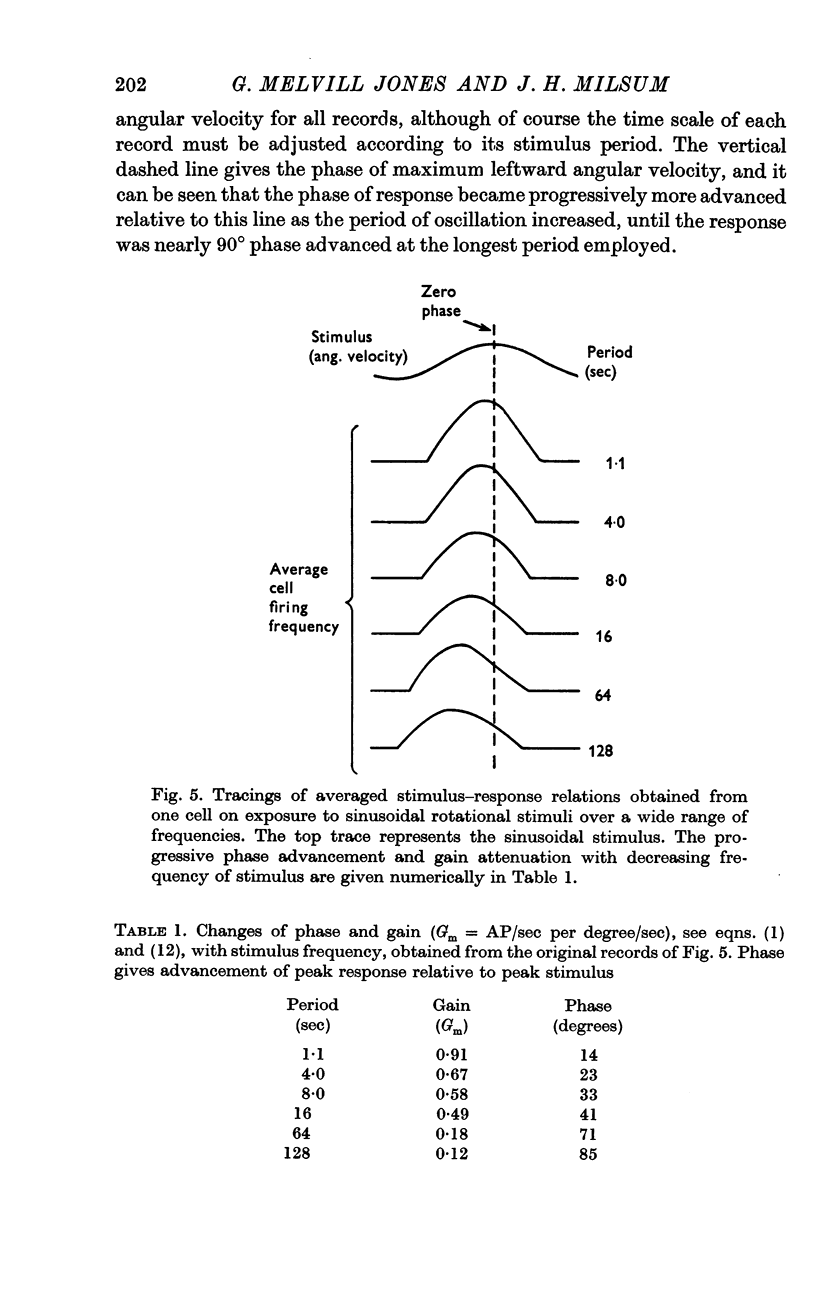
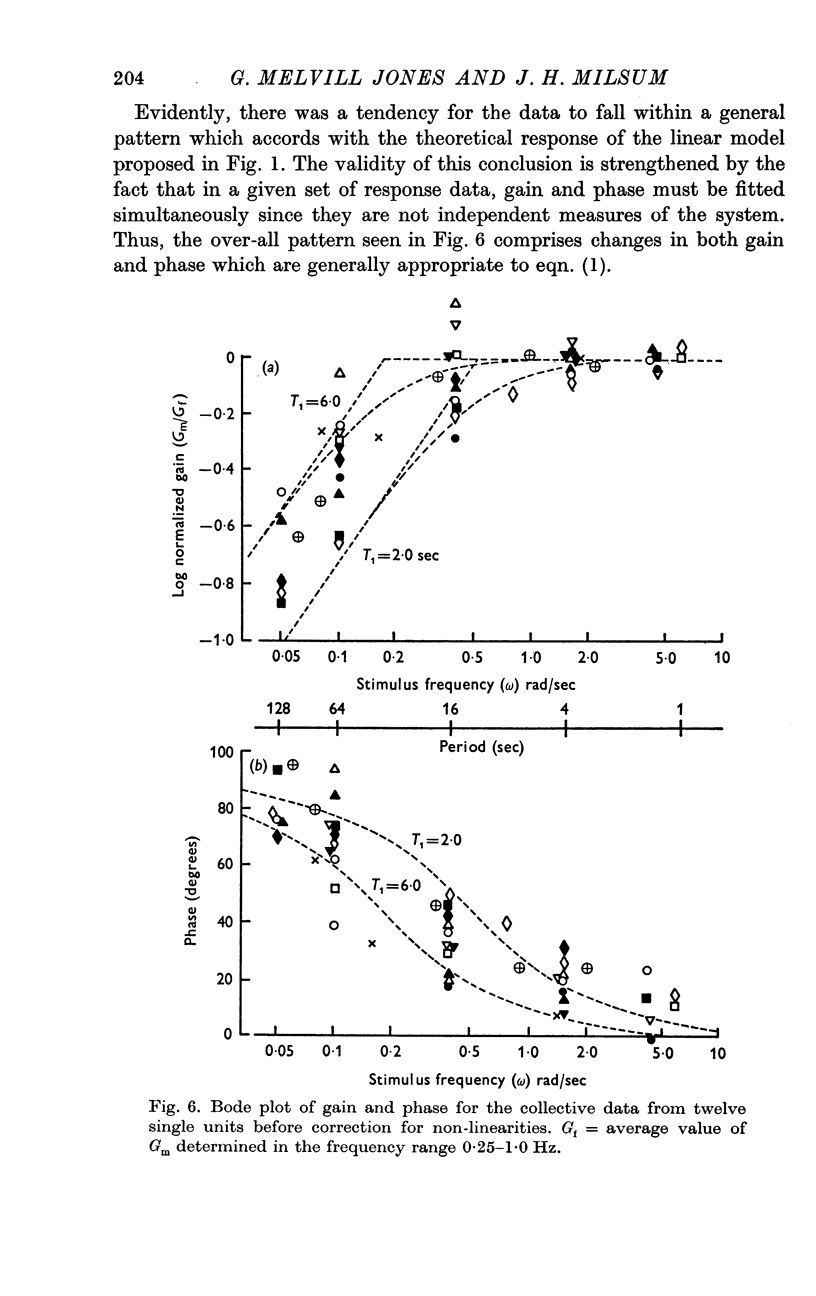
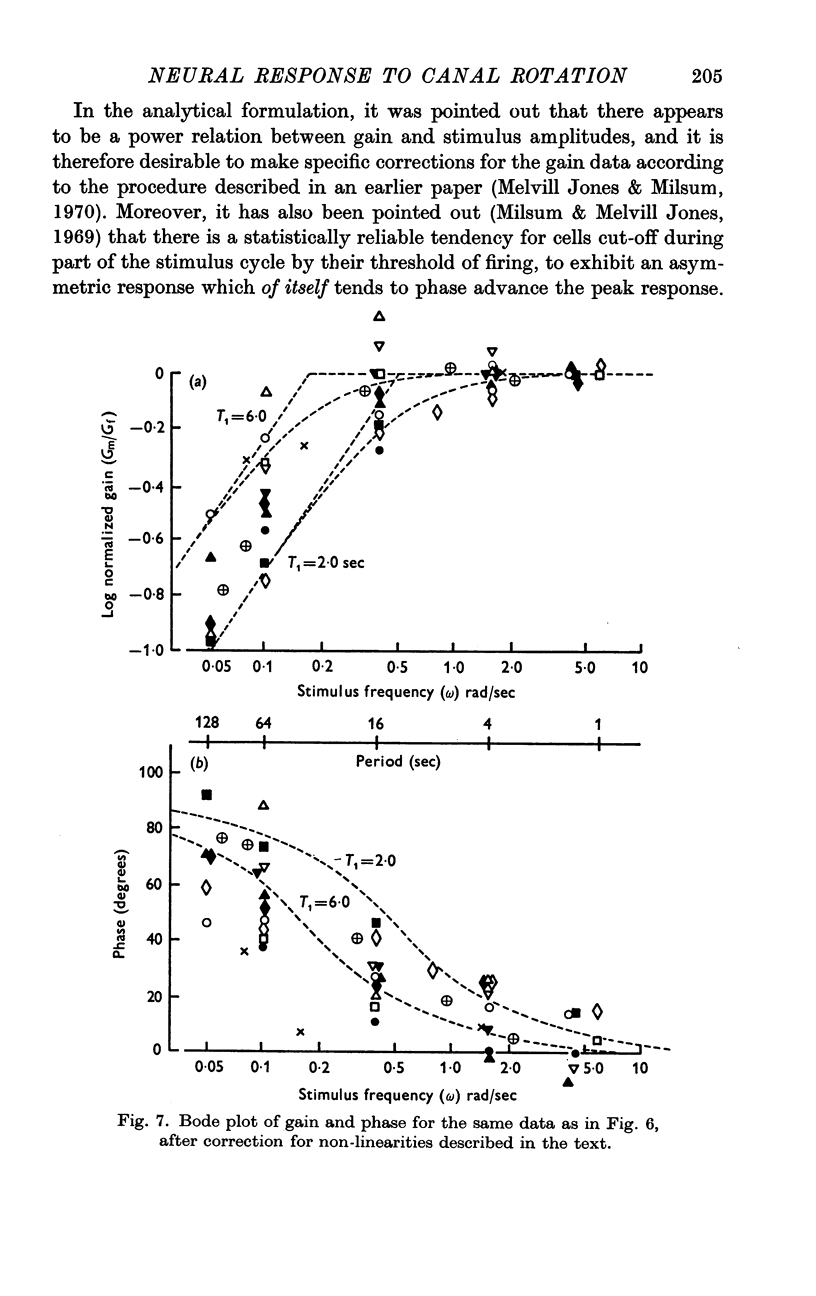
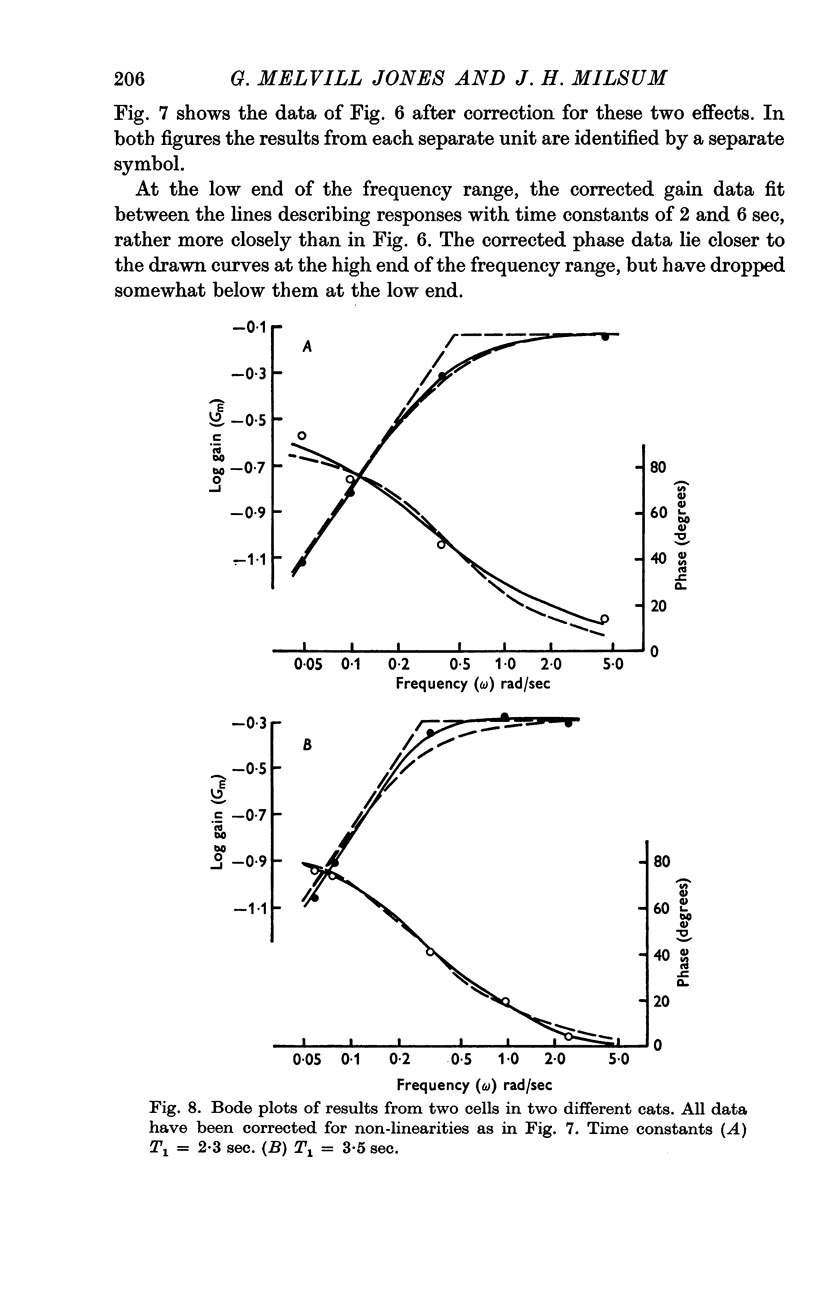
3. Over the experimental frequency range, the relation between neural response and mechanical stimulus was found to be dominated by a single time constant of about 4 sec, such that two response regions can be defined above and below a stimulus frequency of ¼ rad/sec (≃ ≃ Hz).
4. Above this frequency the information content of the neural signal tends towards that of angular velocity and below that frequency it tends towards that of angular acceleration.
5. It is inferred (a) that the so-called `long' time constant of the cat's horizontal canal is about 4 sec and (b) that during most normal head movements containing frequencies below about 1 Hz the informational mode of neural signals generated in the canal and received in the brain stem probably tends towards that of head angular velocity.
6. This seems appropriate for the generation of vestibulo-ocular reflex compensation for head movement and for reflex damping (negative velocity feed-back) of unintended head and body movements.
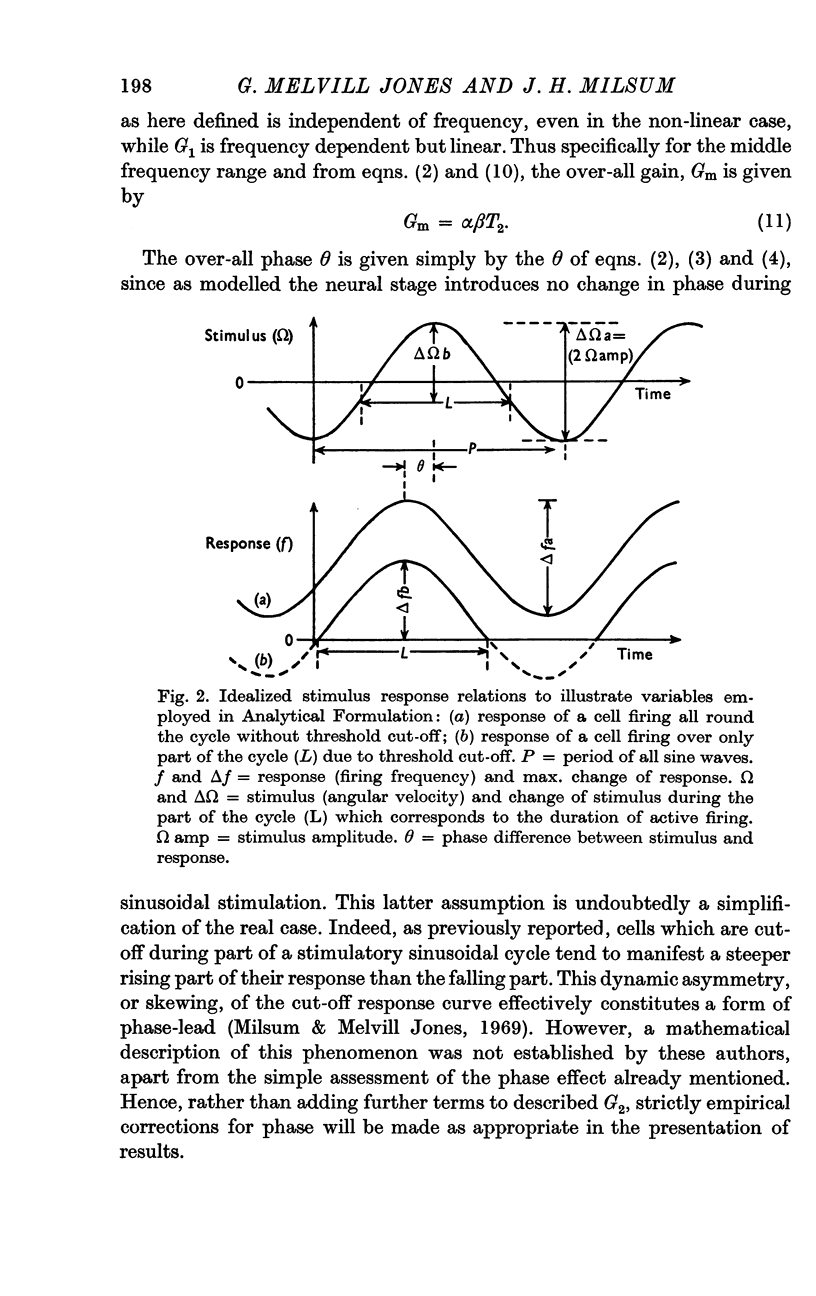
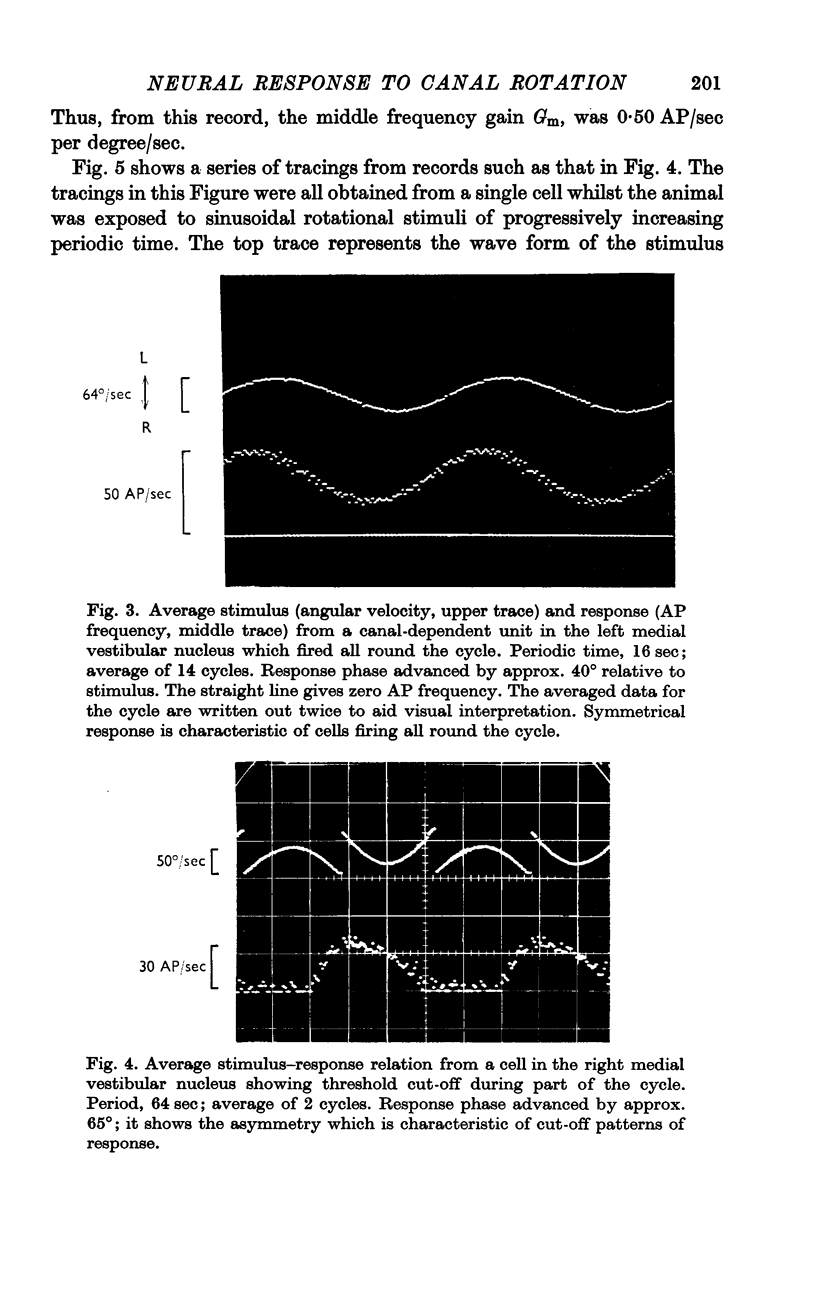
7. The average neural gain of central unit responses is estimated at 1264 action potentials/sec, per degree of cupular deflexion. This high value reflects the very small angles of cupular deflexion assessed on the basis of physical characteristics of the canal.
8. The results permit a rough estimate of the elastic restoring coefficient of the cupula in the horizontal canal as 2·05 × 10-3 dyne. cm.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D. Discharges from vestibular receptors in the cat. J Physiol. 1943 Mar 25;101(4):389–407. doi: 10.1113/jphysiol.1943.sp003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUENSING F., SCHAEFER K. P. Die Aktivität einzelner Neurone im Bereich der Vestibulariskerne bei Horizontalbeschleunigungen unter besonderer Berücksichtigung des vestibulären Nystagmus. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1958;198(2):225–252. doi: 10.1007/BF00941383. [DOI] [PubMed] [Google Scholar]
- Erulkar S. D., Sprague J. M., Whitsel B. L., Dogan S., Jannetta P. J. Organization of the vestibular projection to the spinal cord of the cat. J Neurophysiol. 1966 Jul;29(4):626–664. doi: 10.1152/jn.1966.29.4.626. [DOI] [PubMed] [Google Scholar]
- Fernandez C., Goldberg J. M. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971 Jul;34(4):661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- Fernández C., Valentinuzzi M. A study on the biophysical characteristics of the cat labyrinth. Acta Otolaryngol. 1968 Mar;65(3):293–310. doi: 10.3109/00016486809120969. [DOI] [PubMed] [Google Scholar]
- Goldberg J. M., Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol. 1971 Jul;34(4):635–660. doi: 10.1152/jn.1971.34.4.635. [DOI] [PubMed] [Google Scholar]
- JONES G. M., SPELLS K. E. A theoretical and comparative study of the functional dependence of the semicircular canal upon its physical dimensions. Proc R Soc Lond B Biol Sci. 1963 Mar 26;157:403–419. doi: 10.1098/rspb.1963.0019. [DOI] [PubMed] [Google Scholar]
- Johnstone J. R., Johnstone B. M. Origin of summating potential. J Acoust Soc Am. 1966 Dec;40(6):1405–1413. doi: 10.1121/1.1910240. [DOI] [PubMed] [Google Scholar]
- Jones G. M., Milsum J. H. Characteristics of neural transmission from the semicircular canal to the vestibular nuclei of cats. J Physiol. 1970 Aug;209(2):295–316. doi: 10.1113/jphysiol.1970.sp009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. M., Milsum J. H. Spatial and dynamic aspects of visual fixation. IEEE Trans Biomed Eng. 1965 Apr;12(2):54–62. doi: 10.1109/tbme.1965.4502350. [DOI] [PubMed] [Google Scholar]
- Klinke R. Efferent influence on the vestibular organ during active movements of the body. Pflugers Arch. 1970;318(4):325–332. doi: 10.1007/BF00586972. [DOI] [PubMed] [Google Scholar]
- Lund S., Pompeiano O. Monosynaptic excitation of alpha motoneurones from supraspinal structures in the cat. Acta Physiol Scand. 1968 May-Jun;73(1):1–21. doi: 10.1111/j.1748-1716.1968.tb04075.x. [DOI] [PubMed] [Google Scholar]
- MAYNE R. The dynamic characteristics of the semicircular canals. J Comp Physiol Psychol. 1950 Aug;43(4):309–319. doi: 10.1037/h0054827. [DOI] [PubMed] [Google Scholar]
- Malcolm R., Jones G. M. A quantitative study of vestibular adaptation in humans. Acta Otolaryngol. 1970 Aug;70(2):126–135. doi: 10.3109/00016487009181867. [DOI] [PubMed] [Google Scholar]
- Milsum J. H., Jones G. M. Dynamic asymmetry in neural components of the vestibular system. Ann N Y Acad Sci. 1969 Apr 21;156(2):851–871. doi: 10.1111/j.1749-6632.1969.tb14018.x. [DOI] [PubMed] [Google Scholar]
- NYBERG-HANSEN R. ORIGIN AND TERMINATION OF FIBERS FROM THE VESTIBULAR NUCLEI DESCENDING IN THE MEDIAL LONGITUDINAL FASCICULUS. AN EXPERIMENTAL STUDY WITH SILVER IMPREGNATION METHODS IN THE CAT. J Comp Neurol. 1964 Jun;122:355–367. doi: 10.1002/cne.901220306. [DOI] [PubMed] [Google Scholar]
- RUPERT A., MOUSHEGIAN G., GALAMBOS R. Microelectrode studies of primary vestibular neurons in cat. Exp Neurol. 1962 Feb;5:100–109. doi: 10.1016/0014-4886(62)90026-2. [DOI] [PubMed] [Google Scholar]
- Ross D. A. Electrical studies on the frog's labyrinth. J Physiol. 1936 Feb 8;86(2):117–146. doi: 10.1113/jphysiol.1936.sp003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J. H., McCabe B. F., Funasaka S. Types of neuronal activity in the medial vestibular nucleus. Acta Otolaryngol. 1969 Jul-Aug;68(1):137–141. doi: 10.3109/00016486909121551. [DOI] [PubMed] [Google Scholar]
- Shimazu H., Precht W. Inhibition of central vestibular neurons from the contralateral labyrinth and its mediating pathway. J Neurophysiol. 1966 May;29(3):467–492. doi: 10.1152/jn.1966.29.3.467. [DOI] [PubMed] [Google Scholar]
- VAN EGMOND A. A. J., GROEN J. J., JONGKEES L. B. W. The mechanics of the semicircular canal. J Physiol. 1949 Dec 15;110(1-2):1–17. doi: 10.1113/jphysiol.1949.sp004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V. J., Kato M., Peterson B. W., Wylie R. M. A single-unit analysis of the organization of Deiters' nucleus. J Neurophysiol. 1967 May;30(3):603–619. doi: 10.1152/jn.1967.30.3.603. [DOI] [PubMed] [Google Scholar]
- Wilson V. J., Wylie R. M., Marco L. A. Synaptic inputs to cells in the medial vestibular nucleus. J Neurophysiol. 1968 Mar;31(2):176–185. doi: 10.1152/jn.1968.31.2.176. [DOI] [PubMed] [Google Scholar]
- Wilson V. J., Yoshida M. Comparison of effects of stimulation of Deiters' nucleus and medial longitudinal fasciculus on neck, forelimb, and hindlimb motoneurons. J Neurophysiol. 1969 Sep;32(5):743–758. doi: 10.1152/jn.1969.32.5.743. [DOI] [PubMed] [Google Scholar]
- Wilson V. J., Yoshida M. Monosynaptic inhibition of neck motoneurons by the medial vestibular nucleus. Exp Brain Res. 1969;9(4):365–380. doi: 10.1007/BF00235245. [DOI] [PubMed] [Google Scholar]


