Abstract
1. The effect of various intracellular Na concentrations ([Na]i) on the membrane potential after hypothermia was studied in guinea-pig auricles.
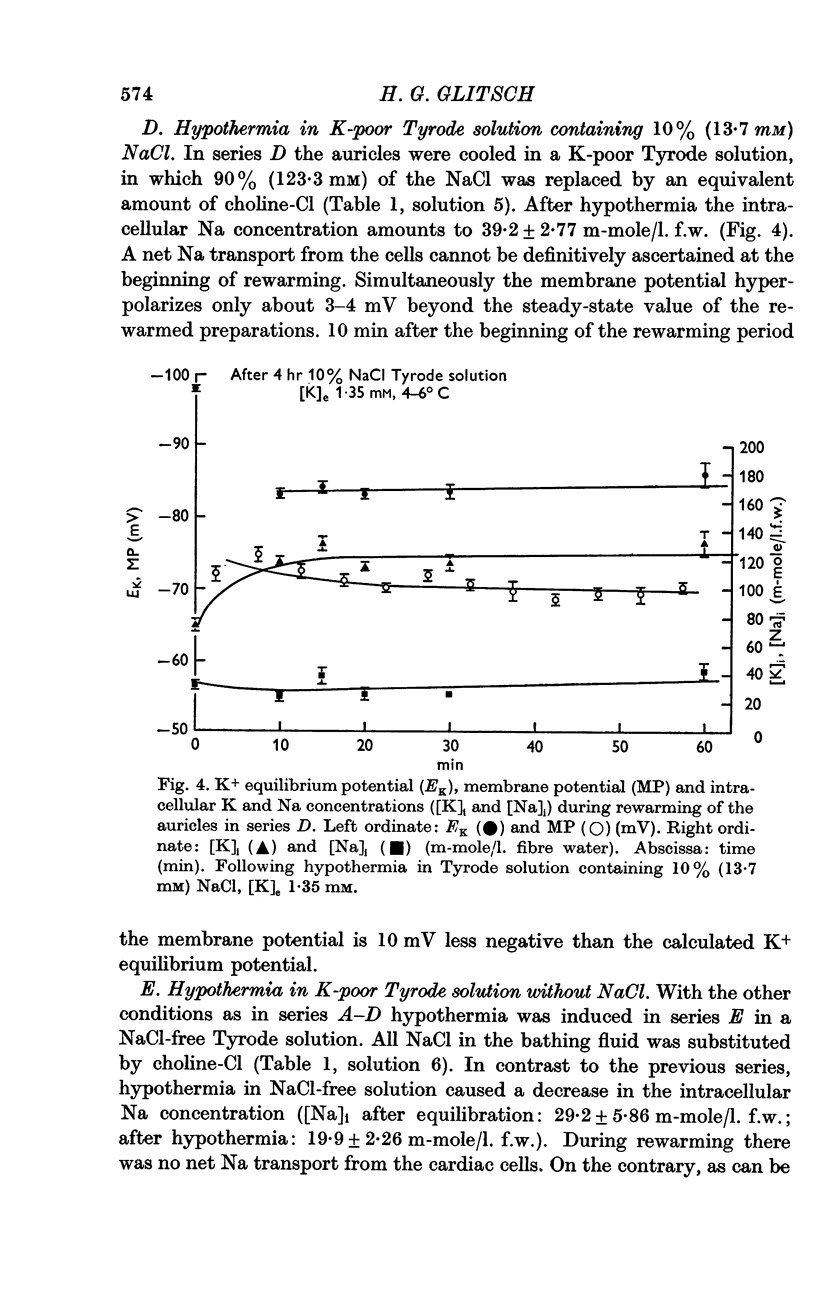
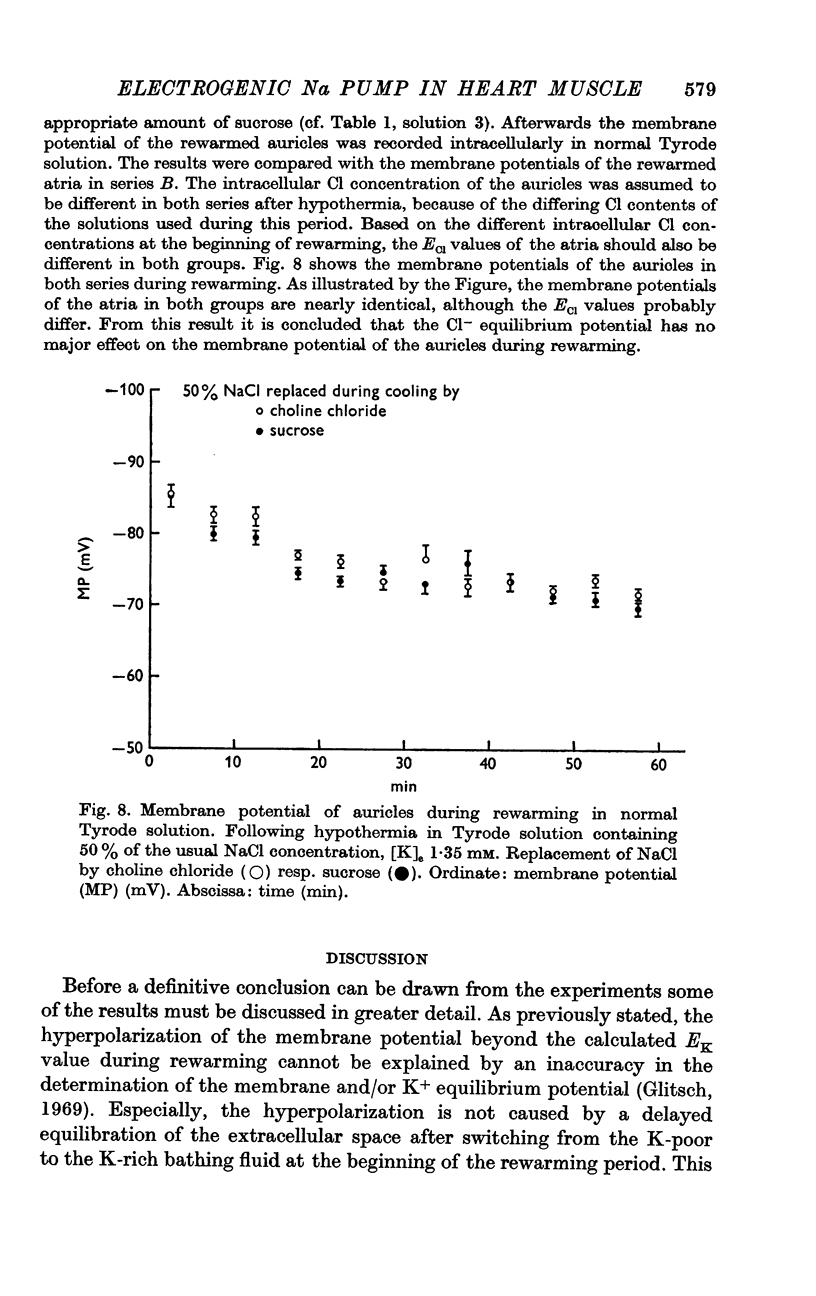
2. For varying [Na]i, the atria were cooled for 4 hr at 4-6° C in a K-poor solution with different concentrations of NaCl. The auricles were rewarmed in normal Tyrode solution at 35° C.
3. Extracellular space (ECS), intracellular Na and K concentrations ([Na]i and [K]i) and membrane potential of the atria were measured before and after hypothermia.
4. The ECS, measured as inulin space, amounted to 350 ml./kg wet wt. at 35° C and to 300 ml./kg wet wt. at 4-6° C.
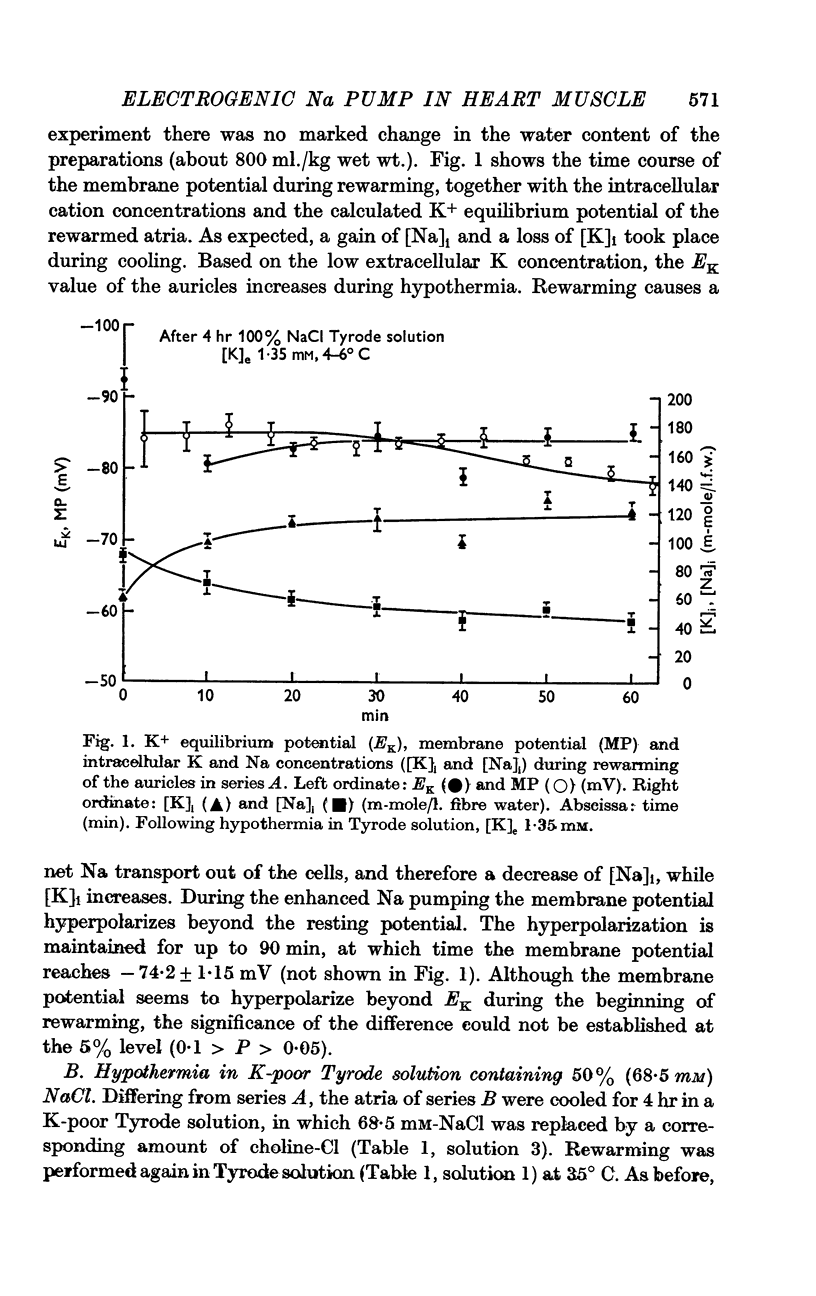
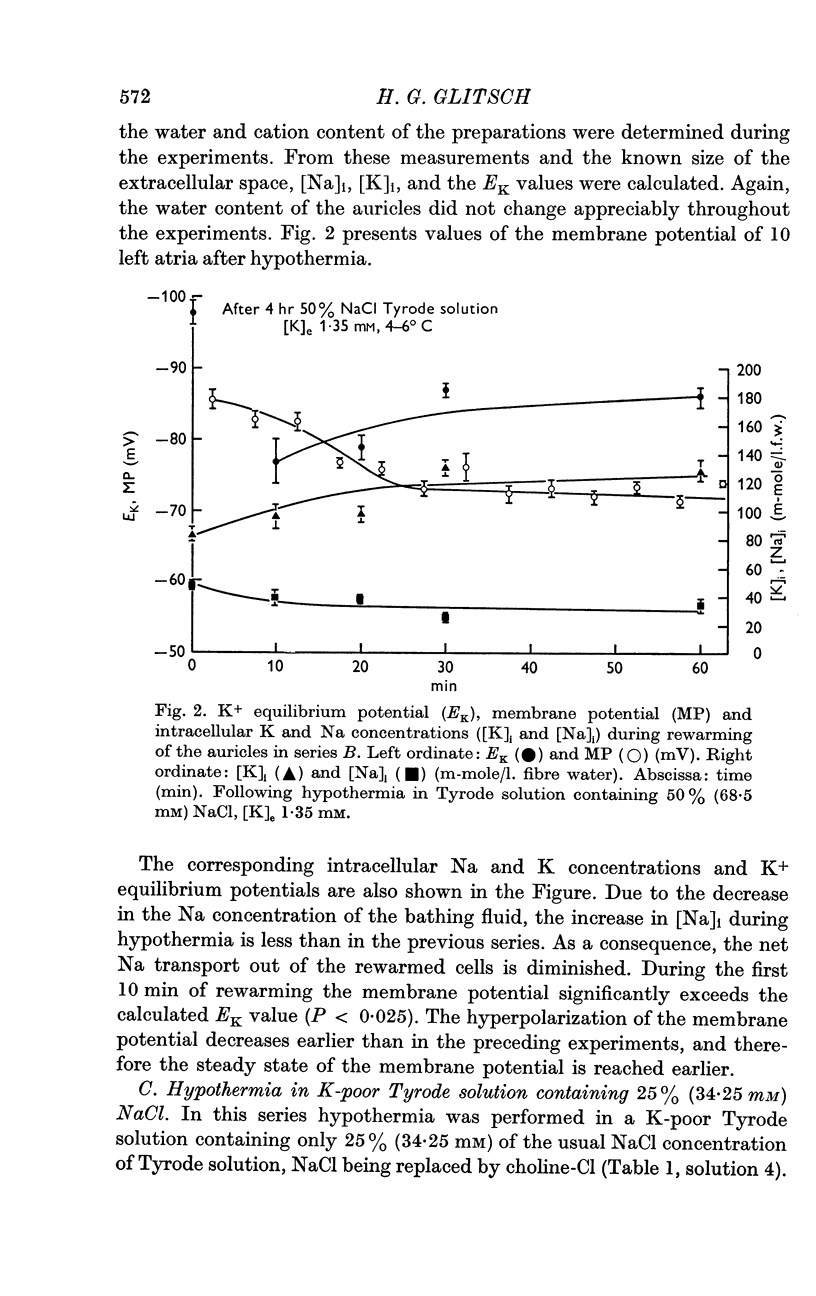
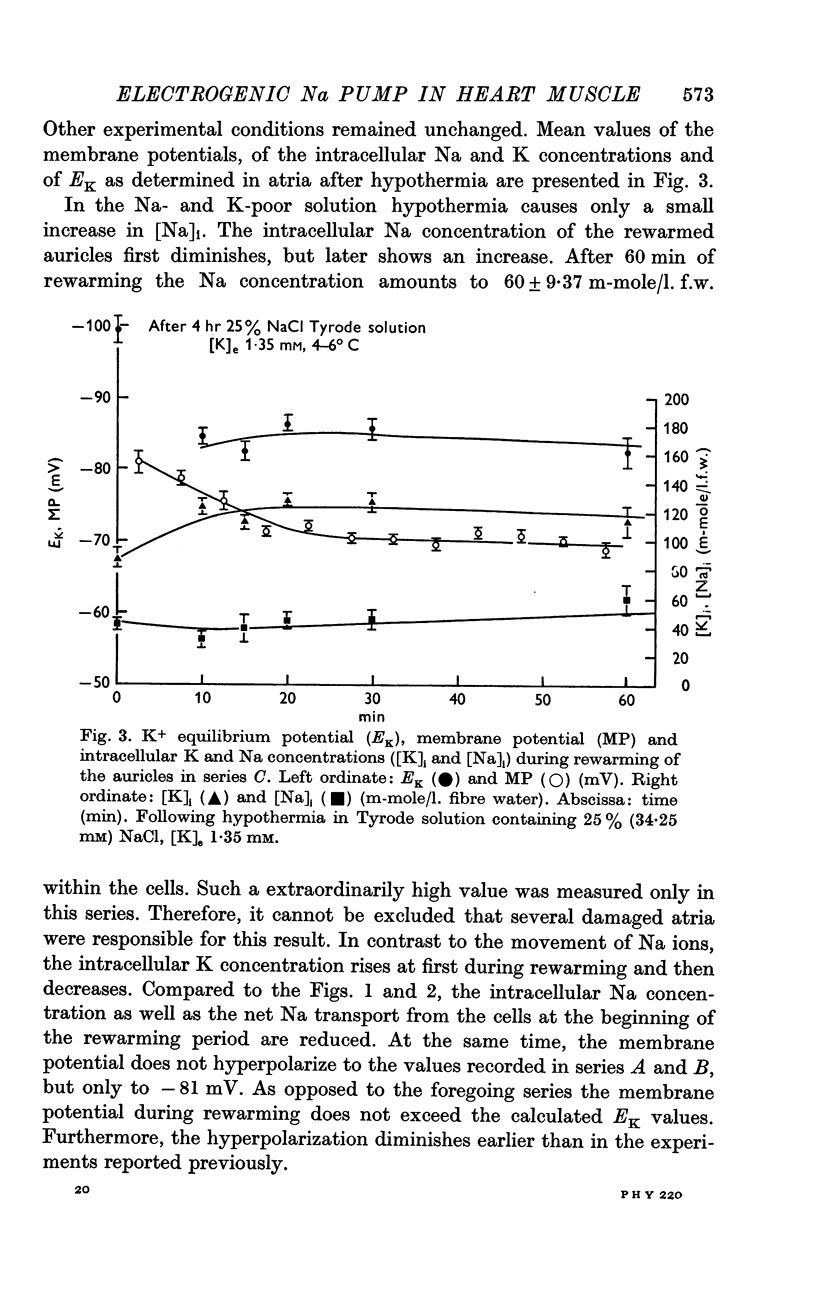
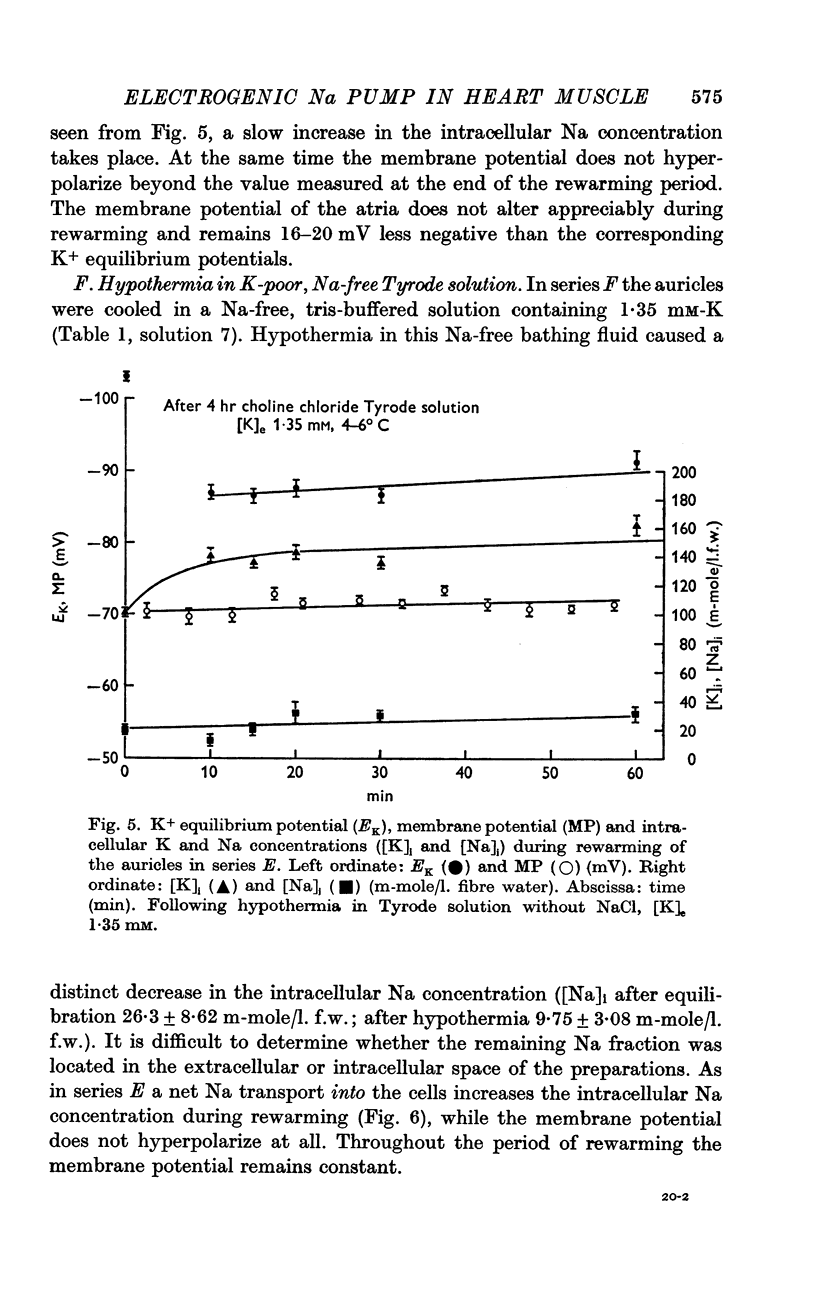
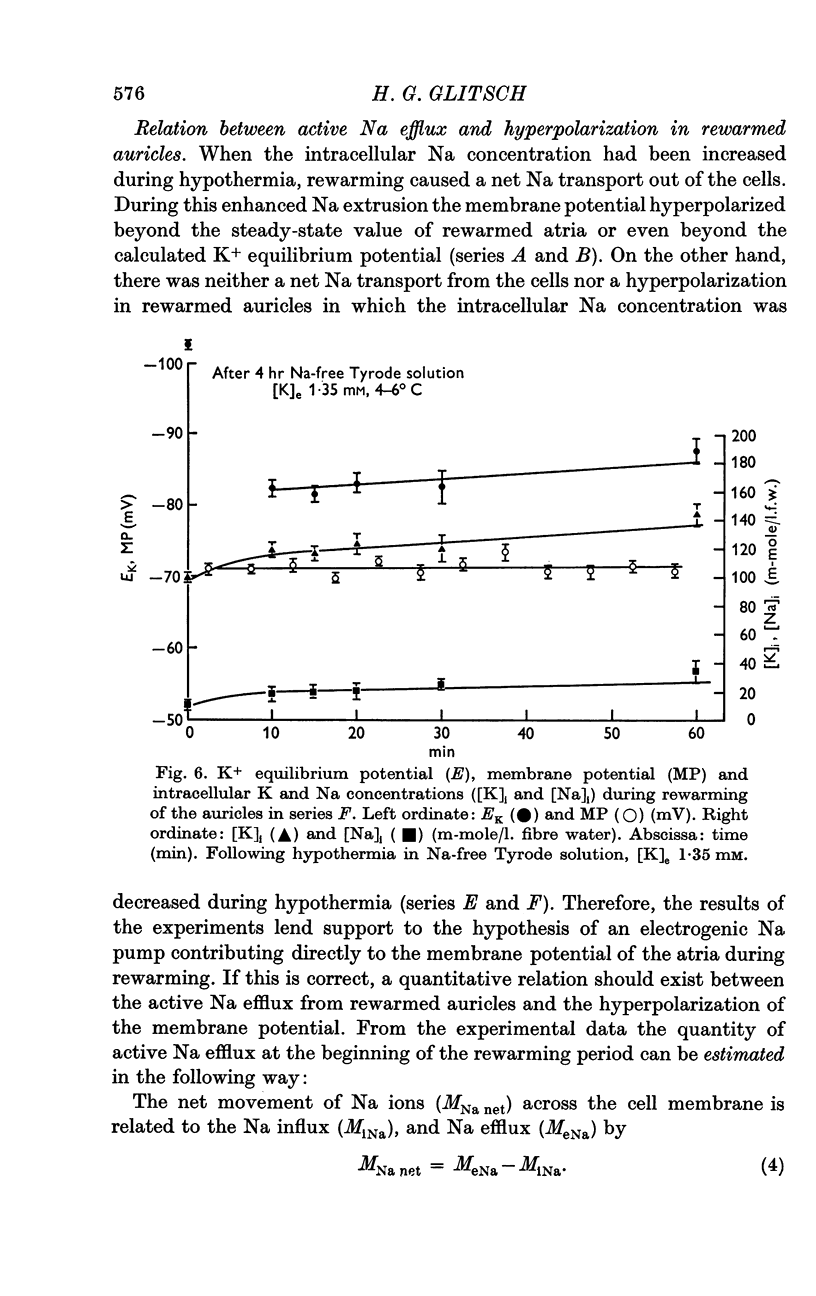
5. [K]i decreased during cooling and increased during rewarming the auricles. [Na]i increased during hypothermia in bathing fluids containing NaCl, but decreased in NaCl- and Na-free solutions. At the beginning of rewarming a net Na transport occurred from cells with high [Na]i, while a net Na uptake took place in atria with low [Na]i.
6. At the same time, the membrane potential of auricles with increased [Na]i hyperpolarized beyond the steady-state value recorded at the end of rewarming, or even beyond the calculated K+ equilibrium potential (EK). Afterwards, the hyperpolarization levelled off, while the EK values increased further. The membrane potential of atria with decreased [Na]i showed no transitory hyperpolarization during rewarming.
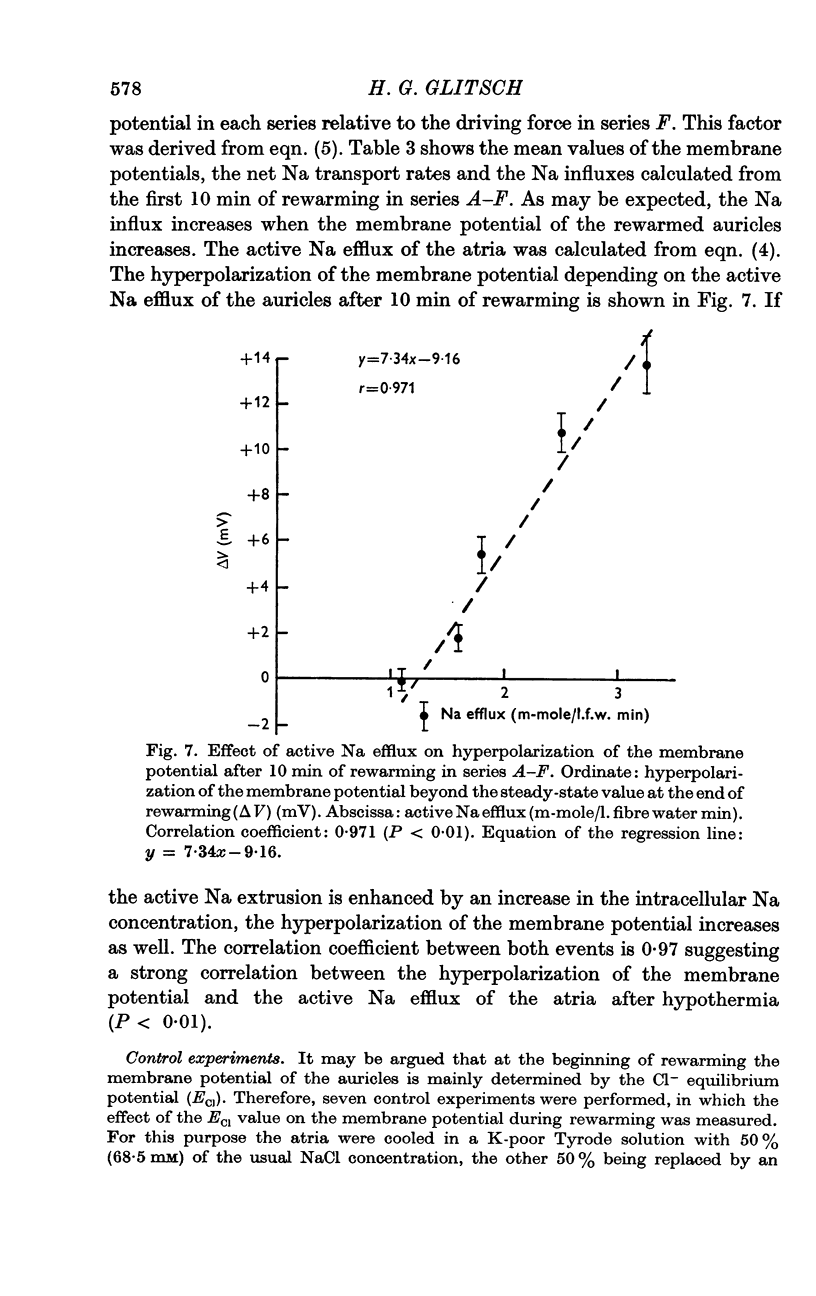
7. The hyperpolarization beyond the steady-state value of membrane potential in rewarmed auricles was significantly correlated to the active Na efflux.
8. From these results it is concluded that the membrane potential of guinea-pig atria after hypothermia is affected by an active, electrogenic Na pump activated by intracellular Na ions.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Slayman C. L. Membrane potential and conductance during transport of sodium, potassium and rubidium in frog muscle. J Physiol. 1966 Jun;184(4):970–1014. doi: 10.1113/jphysiol.1966.sp007961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosteels S., Vleugels A., Carmeliet E. Choline permeability in cardiac muscle cells of the cat. J Gen Physiol. 1970 May;55(5):602–619. doi: 10.1085/jgp.55.5.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter D. O., Alving B. O. A contribution of an electrogenic Na+ pump to membrane potential in Aplysia neurons. J Gen Physiol. 1968 Jul;52(1):1–21. doi: 10.1085/jgp.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. B., Keynes R. D., Rybová R. The coupling of sodium efflux and potassium influx in frog muscle. J Physiol. 1965 Dec;181(4):865–880. doi: 10.1113/jphysiol.1965.sp007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRUMENTO A. S. SODIUM PUMP: ITS ELECTRICAL EFFECTS IN SKELETAL MUSCLE. Science. 1965 Mar 19;147(3664):1442–1443. doi: 10.1126/science.147.3664.1442. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANDELSMAN M. B., DRABKIN J. Use of anthrone reagent to estimate inulin in the presence of glucose. Proc Soc Exp Biol Med. 1954 Jun;86(2):356–360. doi: 10.3181/00379727-86-21097. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., Ochs S. Effects of sodium extrusion and local anaesthetics on muscle membrane resistance and potential. J Physiol. 1966 Nov;187(1):5–21. doi: 10.1113/jphysiol.1966.sp008072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERKUT G. A., THOMAS R. C. AN ELECTROGENIC SODIUM PUMP IN SNAIL NERVE CELLS. Comp Biochem Physiol. 1965 Jan;14:167–183. doi: 10.1016/0010-406x(65)90017-4. [DOI] [PubMed] [Google Scholar]
- KERNAN R. P. Membrane potential changes during sodium transport in frog sartorius muscle. Nature. 1962 Mar 10;193:986–987. doi: 10.1038/193986a0. [DOI] [PubMed] [Google Scholar]
- MULLINS L. J., AWAD M. Z. THE CONTROL OF THE MEMBRANE POTENTIAL OF MUSCLE FIBERS BY THE SODIUM PUMP. J Gen Physiol. 1965 May;48:761–775. doi: 10.1085/jgp.48.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor M. F., Gorman A. L. Membrane potential as the sum of ionic and metabolic components. Science. 1970 Jan 2;167(3914):65–67. doi: 10.1126/science.167.3914.65. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Takahashi K. Post-tetanic hyperpolarization and electrogenic Na pump in stretch receptor neurone of crayfish. J Physiol. 1966 Nov;187(1):105–127. doi: 10.1113/jphysiol.1966.sp008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE E., STORN S. R. CAT HEART MUSCLE IN VITRO. 8. ACTIVE TRANSPORT OF SODIUM IN PAPILLARY MUSCLES. J Gen Physiol. 1965 May;48:957–972. doi: 10.1085/jgp.48.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai T., Kagiyama S. Studies of cat heart muscle during recovery after prolonged hypothermia. Hyperpolarization of cell membranes and its dependence on the sodium pump with electrogenic characteristics. Circ Res. 1968 Mar;22(3):423–433. doi: 10.1161/01.res.22.3.423. [DOI] [PubMed] [Google Scholar]
- Taylor G. S., Paton D. M., Daniel E. E. Characteristics of electrogenic sodium pumping in rat myometrium. J Gen Physiol. 1970 Sep;56(3):360–375. doi: 10.1085/jgp.56.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. S., Paton D. M., Daniel E. E. Evidence for an electrogenic sodium pump in smooth muscle. Life Sci. 1969 Jul 1;8(13):769–773. doi: 10.1016/0024-3205(69)90268-9. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]


