Abstract
1. In anaesthetized male rats, the hypothalamus and pituitary stalk were exposed by a transpharyngeal approach. The compound field potential of the supraoptic nucleus evoked by stimulation of the pituitary stalk, was recorded with glass electrodes inserted near the origin of the anterior cerebral artery.
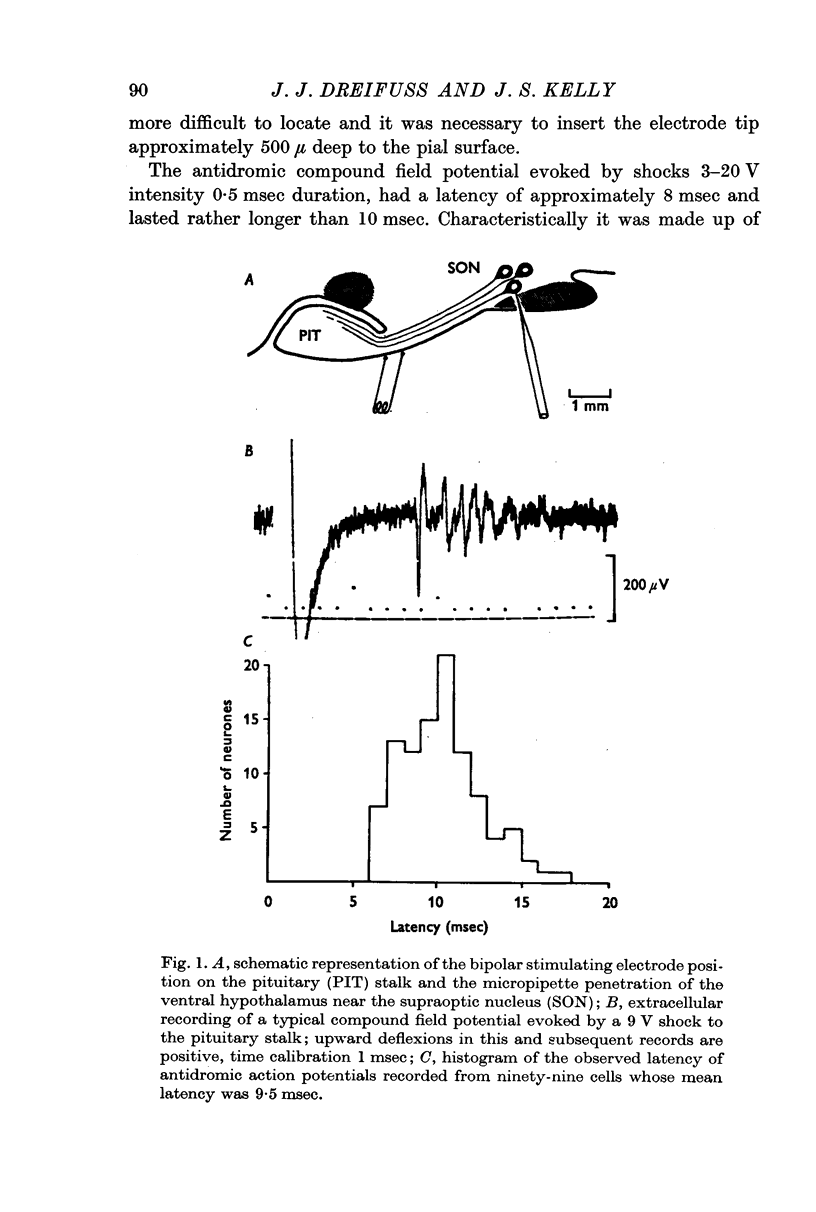
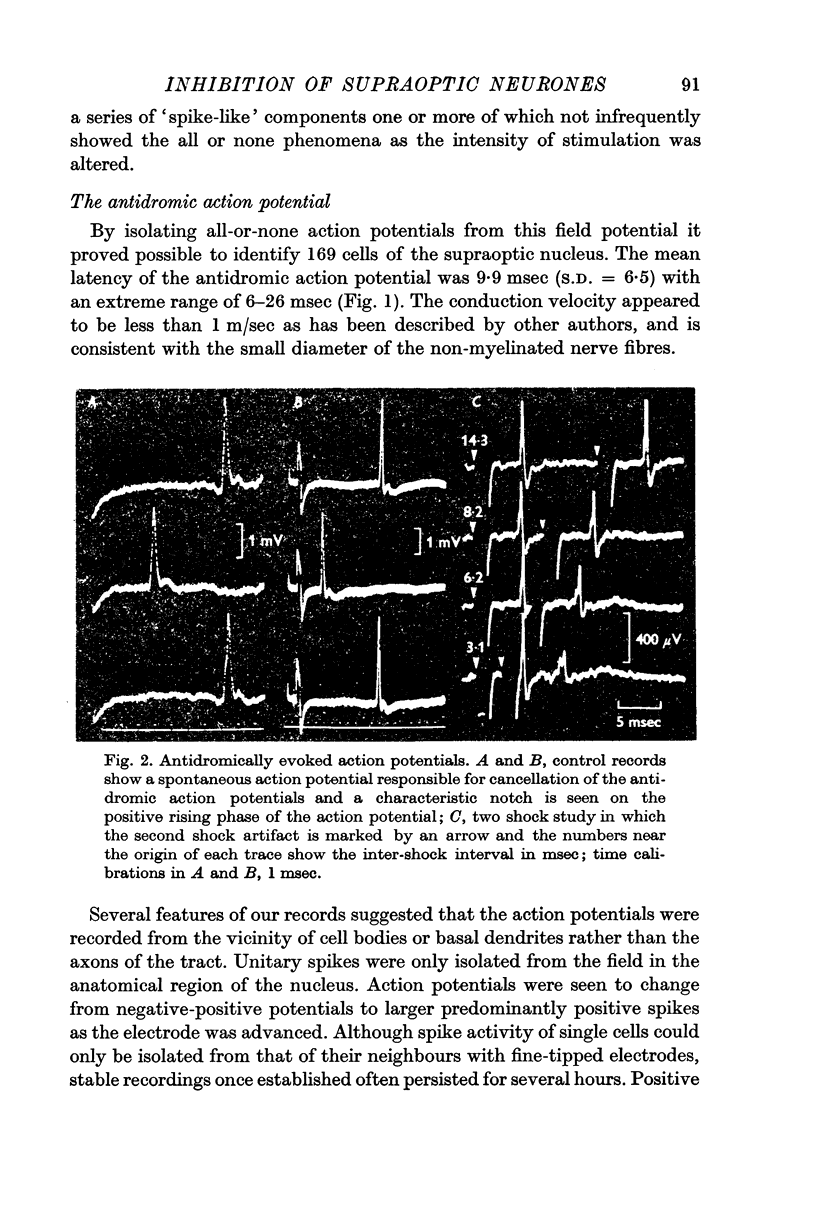
2. The mean latency of 169 antidromically evoked action potentials isolated from the field was 9·9 msec with an extreme range of 6-26 msec. Although the wave form of the antidromic action potential showed a variety of shapes and sizes and the initial wave could be of either polarity, the majority were strikingly similar in form. The initial wave was positive with an inflexion on the rising phase and was followed by a shallow rather longer lasting negative potential.
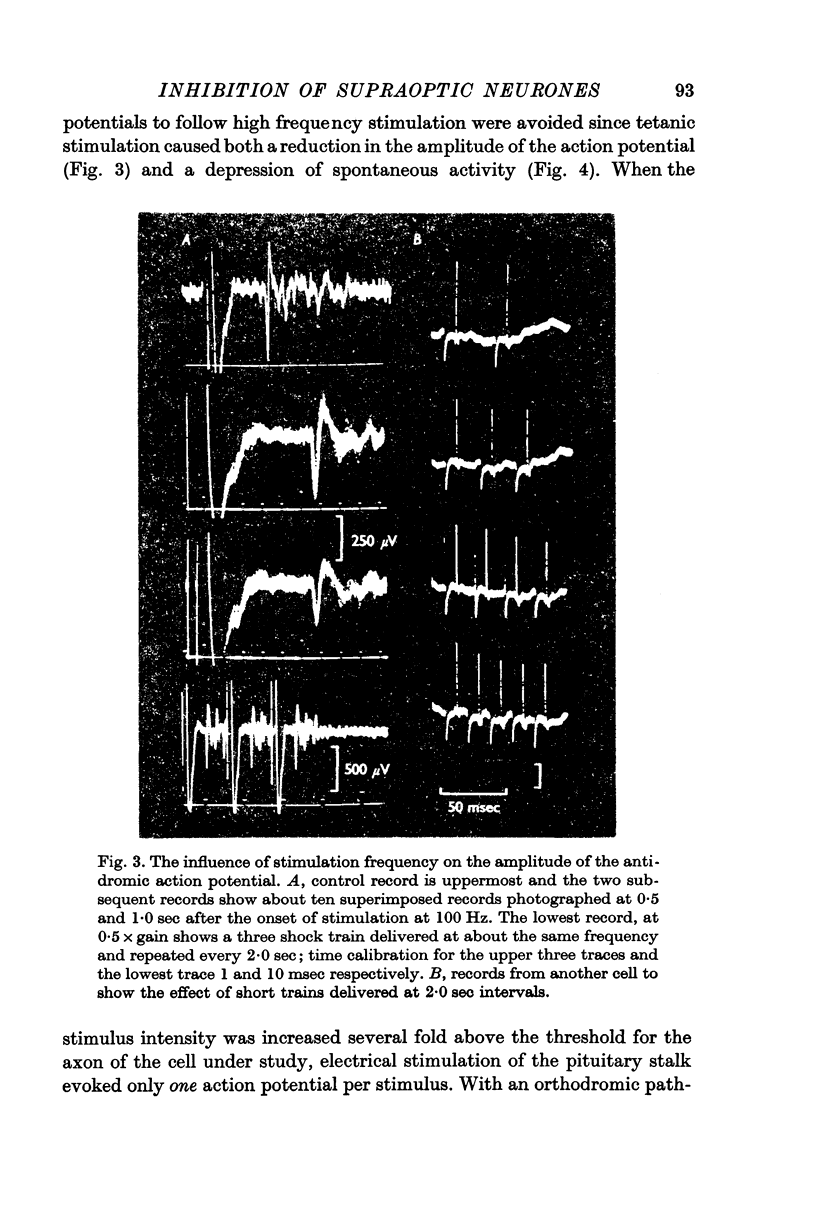
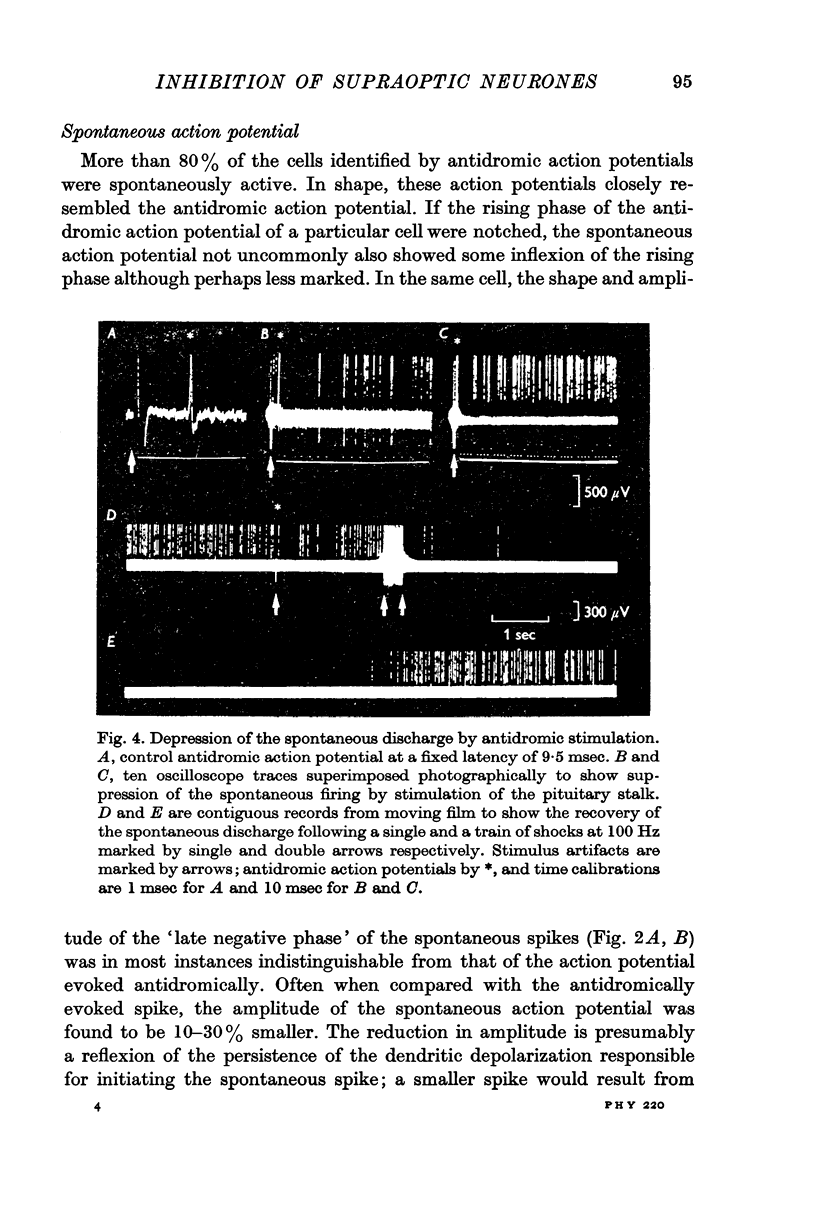
3. The antidromic nature of the action potential was confirmed when the action potential evoked at constant latency after the stimulus was observed to be cancelled by another occurring spontaneously. Although the antidromic action potentials followed stimulation frequencies greater than 100 Hz, the response to high frequency stimulation was seldom tested since the amplitude of the action potential was greatly reduced at frequencies above 30 Hz if the number of shocks exceeded a critical number, as few as 3-6 at 100 Hz.
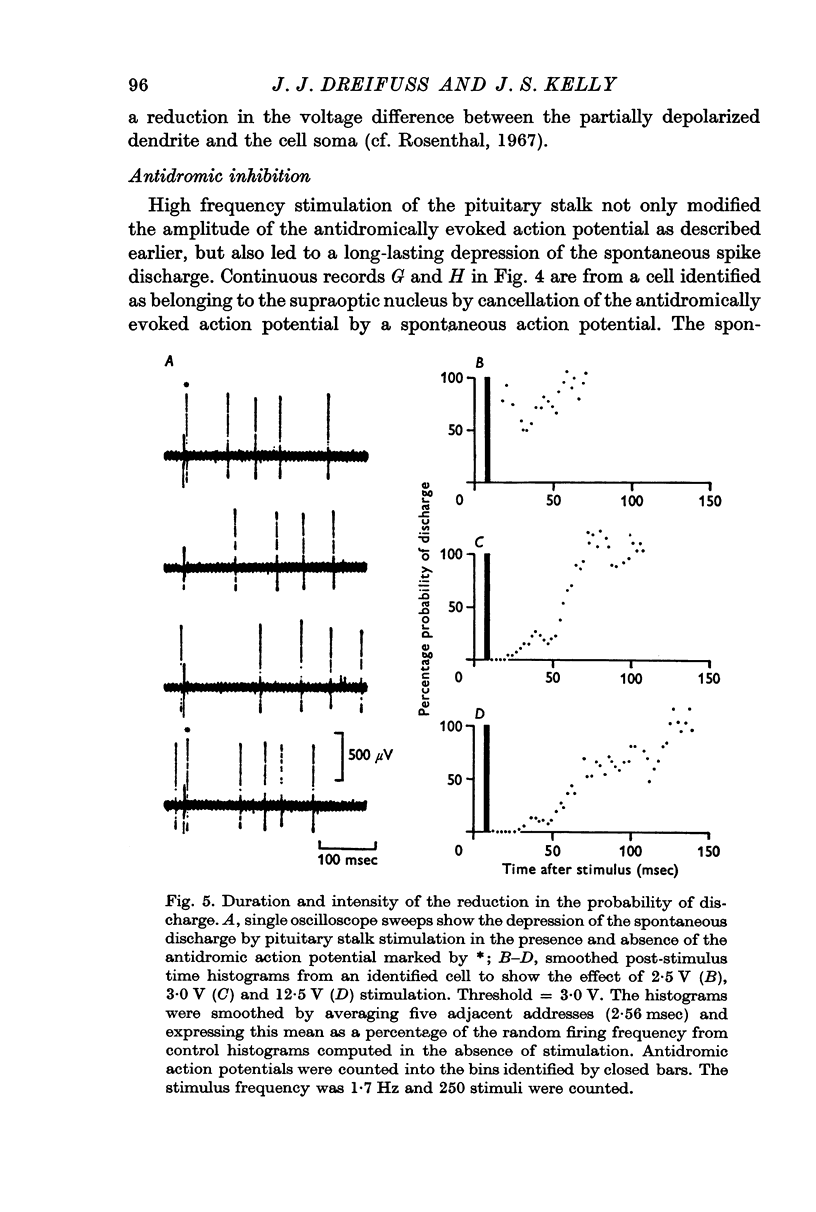
4. Stimulation of the pituitary stalk at intensities below and near threshold for antidromic invasion of the cell under study was shown by means of post-stimulus time histograms to be associated with an inhibitory period lasting on average 80 msec (S.D. = 13, N = 30).
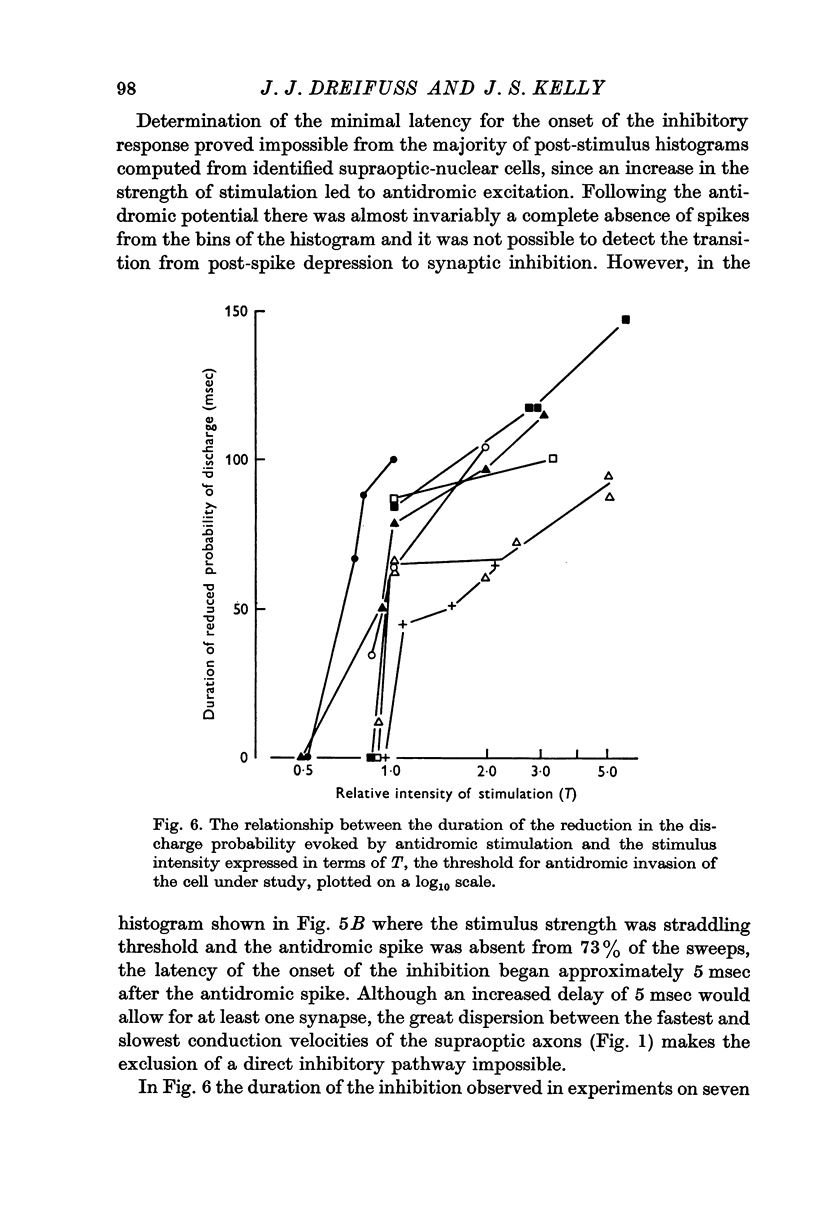
5. An increase in the intensity and duration of the inhibitory period occurred as the intensity of the stimulation was increased as might be expected if the response was mediated synaptically. The inhibitory pathway is believed to involve the recurrent collateral axons already demonstrated anatomically since the stimulation intensities necessary to produce either a marked inhibitory response or antidromic invasion of the cell in question are in most instances nearly the same.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., BROOKS C. M., ECCLES J. C., SEARS T. A. THE VENTRO-BASAL NUCLEUS OF THE THALAMUS: POTENTIAL FIELDS, SYNAPTIC TRANSMISSION AND EXCITABILITY OF BOTH PRESYNAPTIC AND POST-SYNAPTIC COMPONENTS. J Physiol. 1964 Nov;174:348–369. doi: 10.1113/jphysiol.1964.sp007492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. Single-unit recording from antidromically activated optic radiation neurones. J Physiol. 1962 Aug;162:432–450. doi: 10.1113/jphysiol.1962.sp006943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. The identification of single units in central visual pathways. J Physiol. 1962 Aug;162:409–431. doi: 10.1113/jphysiol.1962.sp006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Crayton J. W., Nicoll R. A. Supraopticneurosecretory cells: autonomic modulation. Science. 1971 Jan 15;171(3967):206–207. doi: 10.1126/science.171.3967.206. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The influence of internal sodium on the behaviour of motor nerve endings. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):401–421. doi: 10.1098/rspb.1968.0047. [DOI] [PubMed] [Google Scholar]
- Burke W., Jervie Sefton A. Recovery of responsiveness of cells of lateral geniculate nucleus of rat. J Physiol. 1966 Nov;187(1):213–229. doi: 10.1113/jphysiol.1966.sp008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSS B. A., GREEN J. D. Activity of single neurones in the hypothalamus: effect of osmotic and other stimuli. J Physiol. 1959 Oct;148:554–569. doi: 10.1113/jphysiol.1959.sp006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C., Ryall R. W. Recurrent inhibition in sacral parasympathetic pathways to the bladder. J Physiol. 1968 Jun;196(3):579–591. doi: 10.1113/jphysiol.1968.sp008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Kalnins I., Kelly J. S., Ruf K. B. Action potentials and release of neurohypophysial hormones in vitro. J Physiol. 1971 Jul;215(3):805–817. doi: 10.1113/jphysiol.1971.sp009499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S. The activity of identified supraoptic neurones and their response to acetylcholine applied by iontophoresis. J Physiol. 1972 Jan;220(1):105–118. doi: 10.1113/jphysiol.1972.sp009697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball R. E., Koizumi K. Electrical activity in the supraoptic and paraventricular nuclei associated with neurohypophysial hormone release. J Physiol. 1969 May;201(3):711–722. doi: 10.1113/jphysiol.1969.sp008783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball R. E. Oxytocin and ADH secretion in relation to electrical activity in antidromically identified supraoptic and paraventricular units. J Physiol. 1971 Apr;214(2):245–256. doi: 10.1113/jphysiol.1971.sp009430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURUKAWA T., FURSHPAN E. J. Two inhibitory mechanisms in the Mauthner neurons of goldfish. J Neurophysiol. 1963 Jan;26:140–176. doi: 10.1152/jn.1963.26.1.140. [DOI] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DESCENDING INFLUENCES ON THE EXTEROCEPTIVE ORGANIZATIONS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:291–319. doi: 10.1113/jphysiol.1964.sp007457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G. W., Manabe Y., Ruf K. B. A study of the parameters of electrical stimulation of unmyelinated fibres in the pituitary stalk. J Physiol. 1969 Jul;203(1):67–81. doi: 10.1113/jphysiol.1969.sp008850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A. The oxytocin release and the compound action potential evoked by electrical stimulation on the isolated neurohypophysis of the rat. Jpn J Physiol. 1970 Feb 15;20(1):84–96. doi: 10.2170/jjphysiol.20.84. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R. ELECTRICAL PROPERTIES OF HYPOTHALAMIC NEUROENDOCRINE CELLS. J Gen Physiol. 1964 Mar;47:691–717. doi: 10.1085/jgp.47.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A., BRINLEY F. J., Jr Electrophysiology of hippocampal neurons. I. Sequential invasion and synaptic organization. J Neurophysiol. 1961 May;24:225–242. doi: 10.1152/jn.1961.24.3.225. [DOI] [PubMed] [Google Scholar]
- Kelly J. S., Dreifuss J. J. Antidromic inhibition of identified rat supraoptic neurones. Brain Res. 1970 Sep 16;22(3):406–409. doi: 10.1016/0006-8993(70)90483-x. [DOI] [PubMed] [Google Scholar]
- Novin D., Sundsten J. W., Cross B. A. Some properties of antidromically activated units in the paraventricular nucleus of the hypothalamus. Exp Neurol. 1970 Feb;26(2):330–341. doi: 10.1016/0014-4886(70)90130-5. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G. Actions of antidromic pyramidal volleys on single Betz cells in the cat. Q J Exp Physiol Cogn Med Sci. 1959 Jan;44(1):1–25. doi: 10.1113/expphysiol.1959.sp001364. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G., POWELL T. P., SHEPHERD G. M. RESPONSES OF MITRAL CELLS TO STIMULATION OF THE LATERAL OLFACTORY TRACT IN THE RABBIT. J Physiol. 1963 Aug;168:65–88. doi: 10.1113/jphysiol.1963.sp007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The after-effects of repetitive stimulation on mammalian non-medullated fibres. J Physiol. 1956 Dec 28;134(3):698–711. doi: 10.1113/jphysiol.1956.sp005676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal F. A dendritic component in extracellular records from single cortical pyramidal tract neurons. J Neurophysiol. 1967 Jul;30(4):753–768. doi: 10.1152/jn.1967.30.4.753. [DOI] [PubMed] [Google Scholar]
- STEFANIS C., JASPER H. RECURRENT COLLATERAL INHIBITION IN PYRAMIDAL TRACT NEURONS. J Neurophysiol. 1964 Sep;27:855–877. doi: 10.1152/jn.1964.27.5.855. [DOI] [PubMed] [Google Scholar]
- Yagi K., Azuma T., Matsuda K. Neurosecretory cell: capable of conducting impulse in rats. Science. 1966 Nov 11;154(3750):778–779. doi: 10.1126/science.154.3750.778. [DOI] [PubMed] [Google Scholar]
- Yamashita H., Koizumi K., Brooks C. M. Electrophysiological studies of neurosecretory cells in the cat hypothalamus. Brain Res. 1970 Jun 15;20(3):462–466. doi: 10.1016/0006-8993(70)90176-9. [DOI] [PubMed] [Google Scholar]


