Abstract
1. The pharmacological properties of the nerve—muscle junctions of vagus-innervated frog sartorius muscles were investigated. Vagus-evoked junctional potentials were ten times more sensitive to hexamethonium than were control end-plate potentials from muscles re-innervated with sartorius nerve. Hexamethonium did not affect passive electrical muscle membrane properties or quantal content. The sensitivity of vagus junctions to D-tubocurarine (dTC) did not differ from the controls.
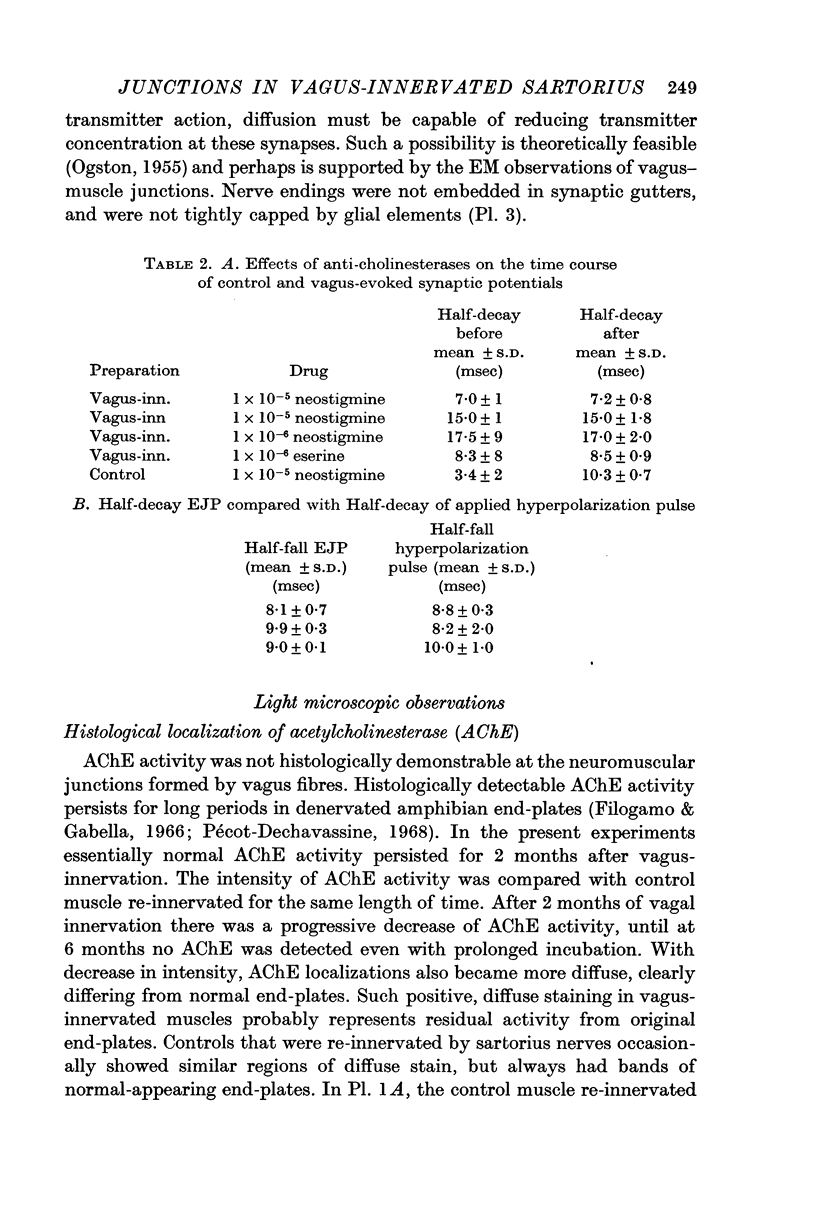
2. The amplitude and time course of vagus-evoked junctional potentials were not affected by eserine or neostigmine. Furthermore, histologically detectable acetylcholinesterase activity was not demonstrable at vagus-muscle junctions.
3. The ultrastructure of vagus-innervated muscle fibres was normal in appearance indicating that the vagus was capable of structurally maintaining the muscle fibres.
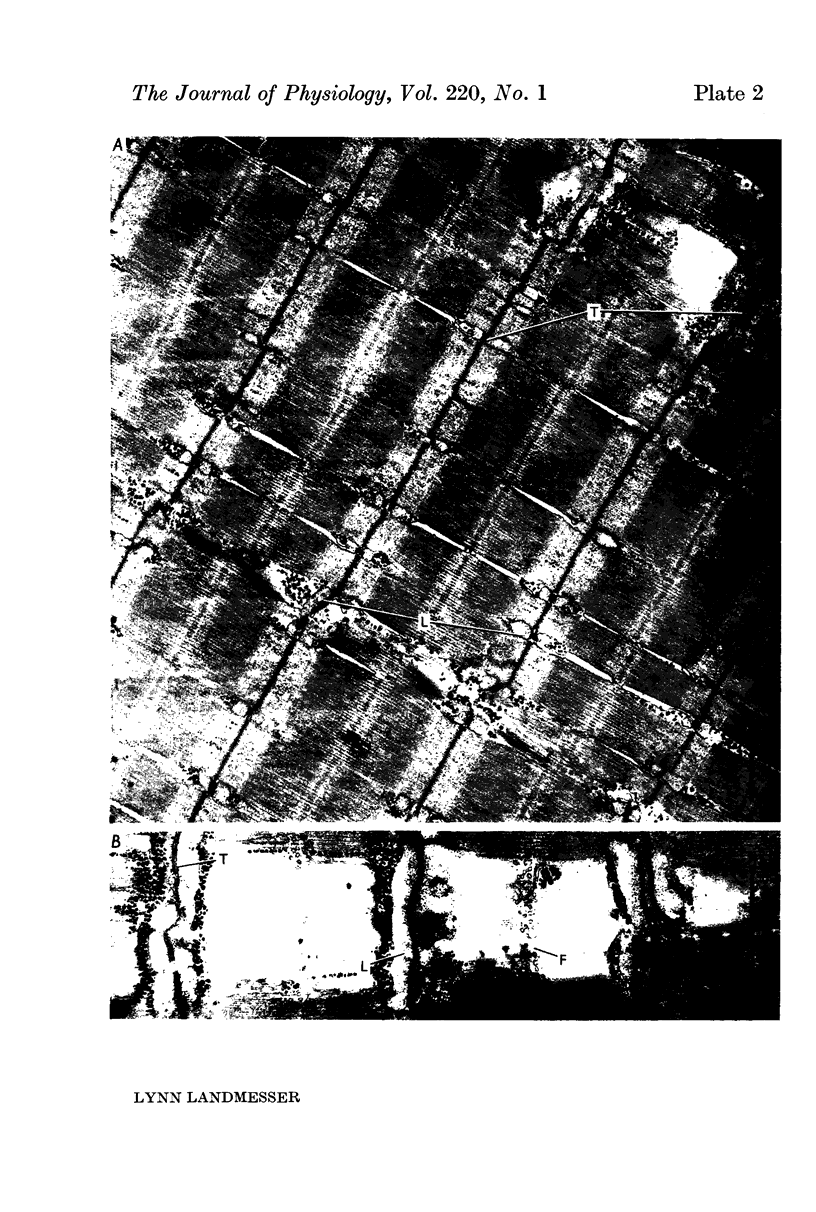
4. Junctional contacts were made by varicosities of unmyelinated vagal fibres. Although such varicose vagal fibres ran along the muscle fibres for long distances, they seemed to make synaptic contacts primarily at old end-plate regions, as deduced from persisting junctional folds.
5. It is concluded that at least several `trophic' factors are probably involved in the different effects of the vagus nerve on the sartorius muscle.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRKS R., HUXLEY H. E., KATZ B. The fine structure of the neuromuscular junction of the frog. J Physiol. 1960 Jan;150:134–144. doi: 10.1113/jphysiol.1960.sp006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRKS R., KATZ B., MILEDI R. Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol. 1960 Jan;150:145–168. doi: 10.1113/jphysiol.1960.sp006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beránek R., Vyskocil F. The action of tubocurarine and atropine on the normal and denervated rat diaphragm. J Physiol. 1967 Jan;188(1):53–66. doi: 10.1113/jphysiol.1967.sp008123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beránek R., Vyskocil F. The effect of atropine on the frog sartorius neuromuscular junction. J Physiol. 1968 Mar;195(2):493–503. doi: 10.1113/jphysiol.1968.sp008470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Crowcroft P. J., Devine C. E., Holman M. E., Yonemura K. Transmission from pregnanglionic fibres in the hypogastric nerve to peripheral ganglia of male guinea-pigs. J Physiol. 1969 May;201(3):723–743. doi: 10.1113/jphysiol.1969.sp008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE W. V. Motor endings in the striated muscle of vertebrates. J Comp Neurol. 1955 Jun;102(3):671–715. doi: 10.1002/cne.901020306. [DOI] [PubMed] [Google Scholar]
- DE CASTRO F. Aspects anatomiques de la transmission synaptique ganglionnaire chez les mammifères. Arch Int Physiol. 1951 Dec;59(4):479–525. doi: 10.3109/13813455109150845. [DOI] [PubMed] [Google Scholar]
- DIAMOND J., MILEDI R. A study of foetal and new-born rat muscle fibres. J Physiol. 1962 Aug;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Mallart A. Differences in the action of acetylcholine on the denervated muscle fiber and on the normal end-plate. Brain Res. 1970 Aug 27;22(2):264–267. doi: 10.1016/0006-8993(70)90014-4. [DOI] [PubMed] [Google Scholar]
- Fex S., Sonesson B., Thesleff S., Zelená J. Nerve implants in botulinum poisoned mammalian muscle. J Physiol. 1966 Jun;184(4):872–882. doi: 10.1113/jphysiol.1966.sp007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filogamo G., Gabella G. Cholinesterase behaviour in the denervated and reinnervated muscles. Acta Anat (Basel) 1966;63(2):199–214. doi: 10.1159/000142789. [DOI] [PubMed] [Google Scholar]
- GUTH L., ZALEWSKI A. A. Disposition of cholinesterase following implantation of nerve into innervated and denervated muscle. Exp Neurol. 1963 Apr;7:316–326. doi: 10.1016/0014-4886(63)90078-5. [DOI] [PubMed] [Google Scholar]
- Hník P., Jirmanová I., Vyklický L., Zelená J. Fast and slow muscles of the chick after nerve cross-union. J Physiol. 1967 Nov;193(2):309–325. doi: 10.1113/jphysiol.1967.sp008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOELLE G. B. The histochemical differentiation of types of cholinesterases and their localizations in tissues of the cat. J Pharmacol Exp Ther. 1950 Oct;100(2):158–179. [PubMed] [Google Scholar]
- Koenig J. Contribution a l'étude de la morphologie des plaques motrices des grands dorsaux antérieur et postérieur du poulet après innervation croisée. Arch Anat Microsc Morphol Exp. 1970 Oct-Dec;59(4):403–425. [PubMed] [Google Scholar]
- Kosterlitz H. W., Wallis D. I. The effects of hexamethonium and morphine on transmission in the superior cervical ganglion of the rabbit. Br J Pharmacol Chemother. 1966 Feb;26(2):334–344. doi: 10.1111/j.1476-5381.1966.tb01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L. Contractile and electrical responses of vagus-innervated frog sartorius muscles. J Physiol. 1971 Mar;213(3):707–725. doi: 10.1113/jphysiol.1971.sp009410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon A. M., Vera C. L., Rex A. L., Luco J. V. Cholinesterase activity of the nictitating membrane reinnervated by cholinergic fibers. J Neurophysiol. 1967 Nov;30(6):1523–1530. doi: 10.1152/jn.1967.30.6.1523. [DOI] [PubMed] [Google Scholar]
- MATSUMURA M., KOELLE G. B. The nature of synaptic transmission in the superior cervical ganglion following reinnervation by the afferent vagus. J Pharmacol Exp Ther. 1961 Oct;134:28–46. [PubMed] [Google Scholar]
- MILEDI R. Induced innervation of end-plate free muscle segments. Nature. 1962 Jan 20;193:281–282. doi: 10.1038/193281a0. [DOI] [PubMed] [Google Scholar]
- MILEDI R. Junctional and extra-junctional acetylcholine receptors in skeletal muscle fibres. J Physiol. 1960 Apr;151:24–30. [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclagan J., Vrbová G. The importance of peripheral changes in determining the sensitivity of striated muscle to depolarizing drugs. J Physiol. 1966 Jun;184(3):618–630. doi: 10.1113/jphysiol.1966.sp007935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Kuffler S. W. Visual identification of synaptic boutons on living ganglion cells and of varicosities in postganglionic axons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):485–508. doi: 10.1098/rspb.1971.0044. [DOI] [PubMed] [Google Scholar]
- OGSTON A. G. Removal of acetylcholine from a limited volume by diffusion. J Physiol. 1955 Apr 28;128(1):222–223. doi: 10.1113/jphysiol.1955.sp005300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payton B. W. Use of the frog neuromuscular junction for assessing the action of drugs affecting synaptic transmission. Br J Pharmacol Chemother. 1966 Oct;28(1):35–43. doi: 10.1111/j.1476-5381.1966.tb01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Pécot-Dechavassine M. Evolution de l'activité des cholinestérases et de leur capacité fonctionnelle au niveau des jonctions neuromusculaires et musculotendineuses de la grenoluille après section du nerf moteur. Arch Int Pharmacodyn Ther. 1968 Nov;176(1):118–133. [PubMed] [Google Scholar]
- Saito A., Zacks S. I. Fine structure of neuromuscular junctions after nerve section and implantation of nerve in denervated muscle. Exp Mol Pathol. 1969 Jun;10(3):256–273. doi: 10.1016/0014-4800(69)90056-2. [DOI] [PubMed] [Google Scholar]
- THESLEFF S., QUASTEL D. M. NEUROMUSCULAR PHARMACOLOGY. Annu Rev Pharmacol. 1965;5:263–284. doi: 10.1146/annurev.pa.05.040165.001403. [DOI] [PubMed] [Google Scholar]
- Vera C. L., Luco J. V. Reinnervation of smooth and striated muscle by sensory nerve fibers. J Neurophysiol. 1967 May;30(3):620–627. doi: 10.1152/jn.1967.30.3.620. [DOI] [PubMed] [Google Scholar]











