Abstract
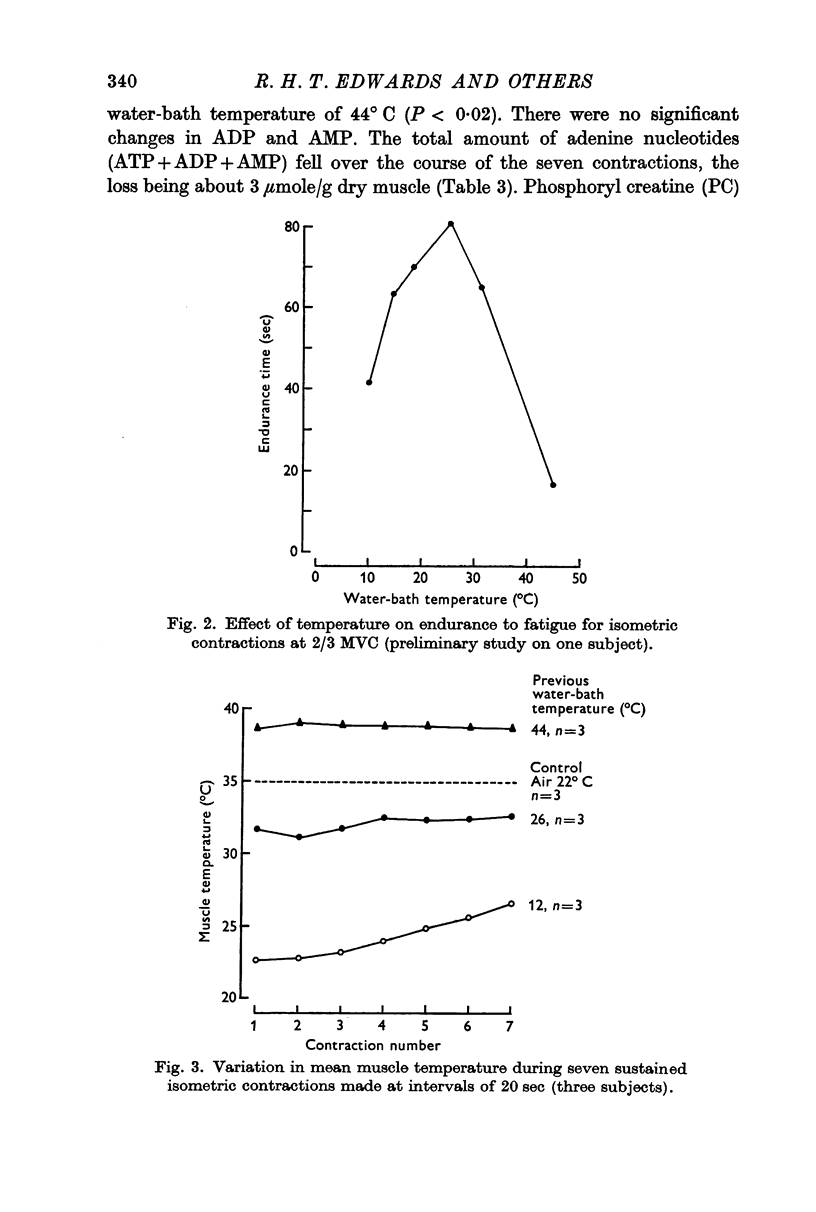
1. The effects of altered tissue temperature on muscle metabolism during successive isometric contractions, sustained to fatigue, have been studied in the quadriceps muscle of man by combining biochemical analyses of metabolites in needle biopsy samples with measurements of endurance time with a force of 2/3 maximum voluntary contraction. Fatigue and recovery were observed repeatedly in a series of seven contractions at intervals of 20 sec, following immersion of the test leg in water at 12, 26 or 44° C for 45 min. Muscle temperatures corresponding to these water temperatures were 22·5, 32·6 and 38·6° C respectively.
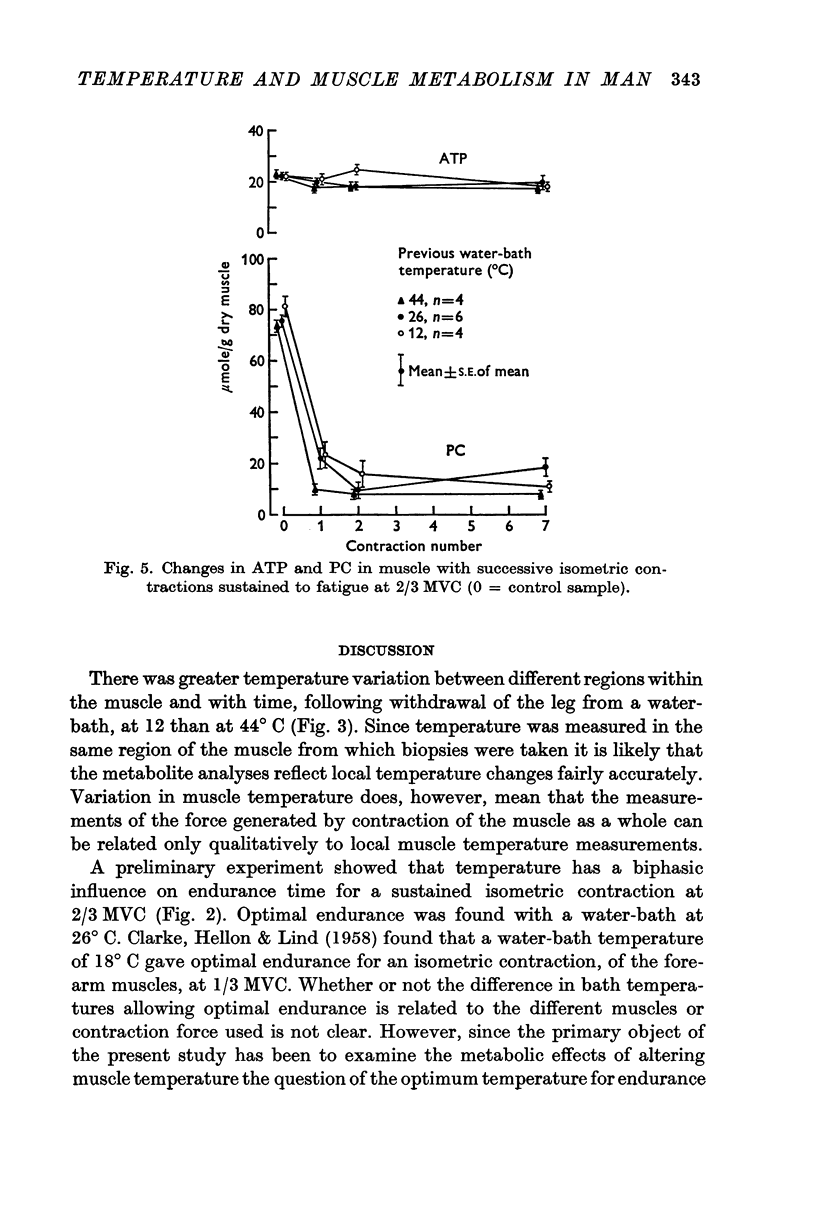
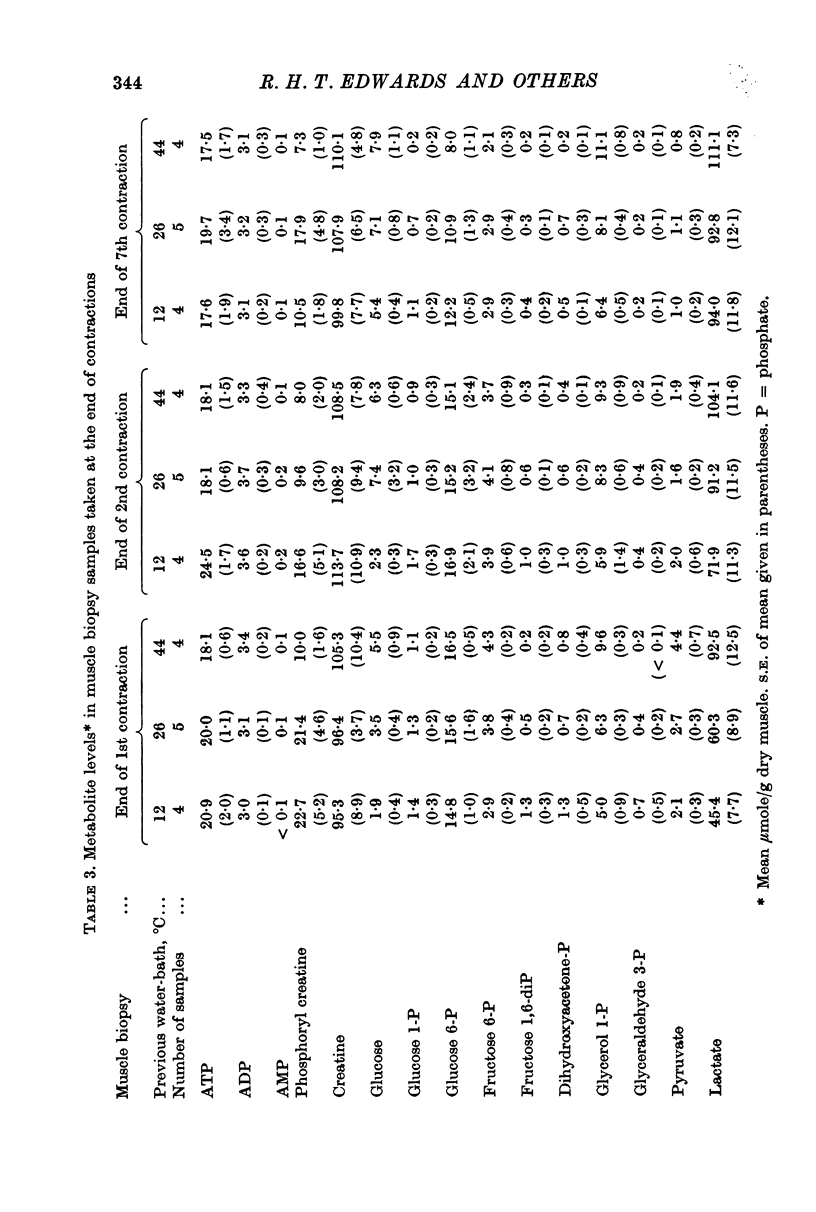
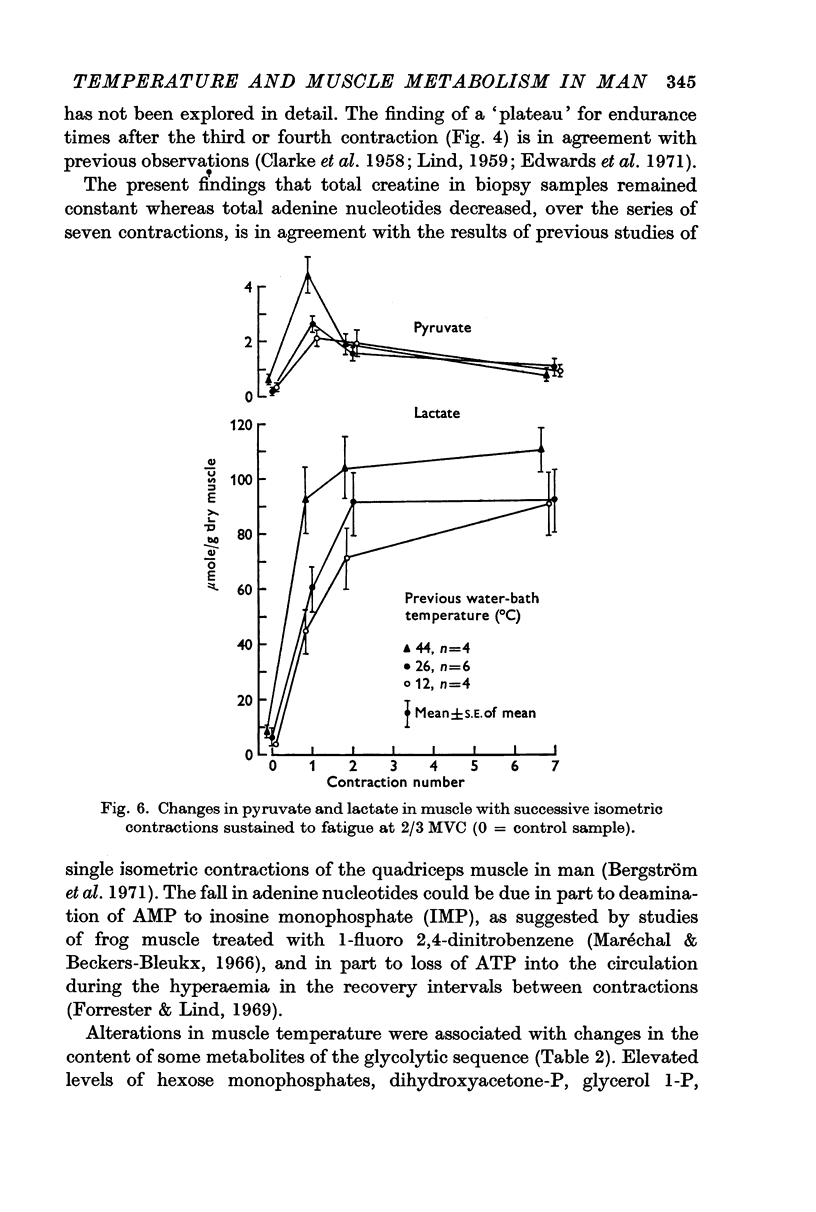
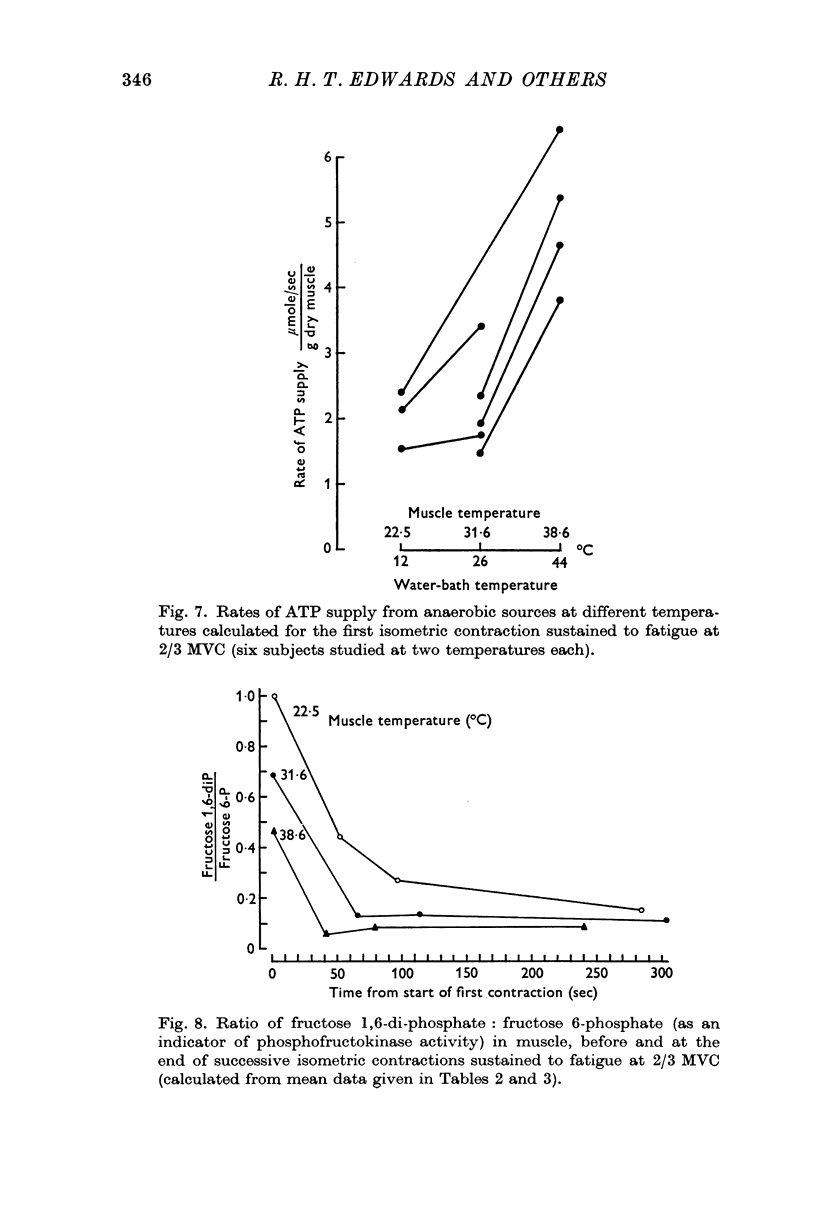
2. Increased levels of several glycolytic intermediates at rest in the heated muscle suggested an increased rate of glycolysis. ATP and phosphoryl creatine were lower at the end of the first contraction and the calculated rate of ATP utilization (including the contribution from anaerobic glycolysis) was highest in the heated nuscle.
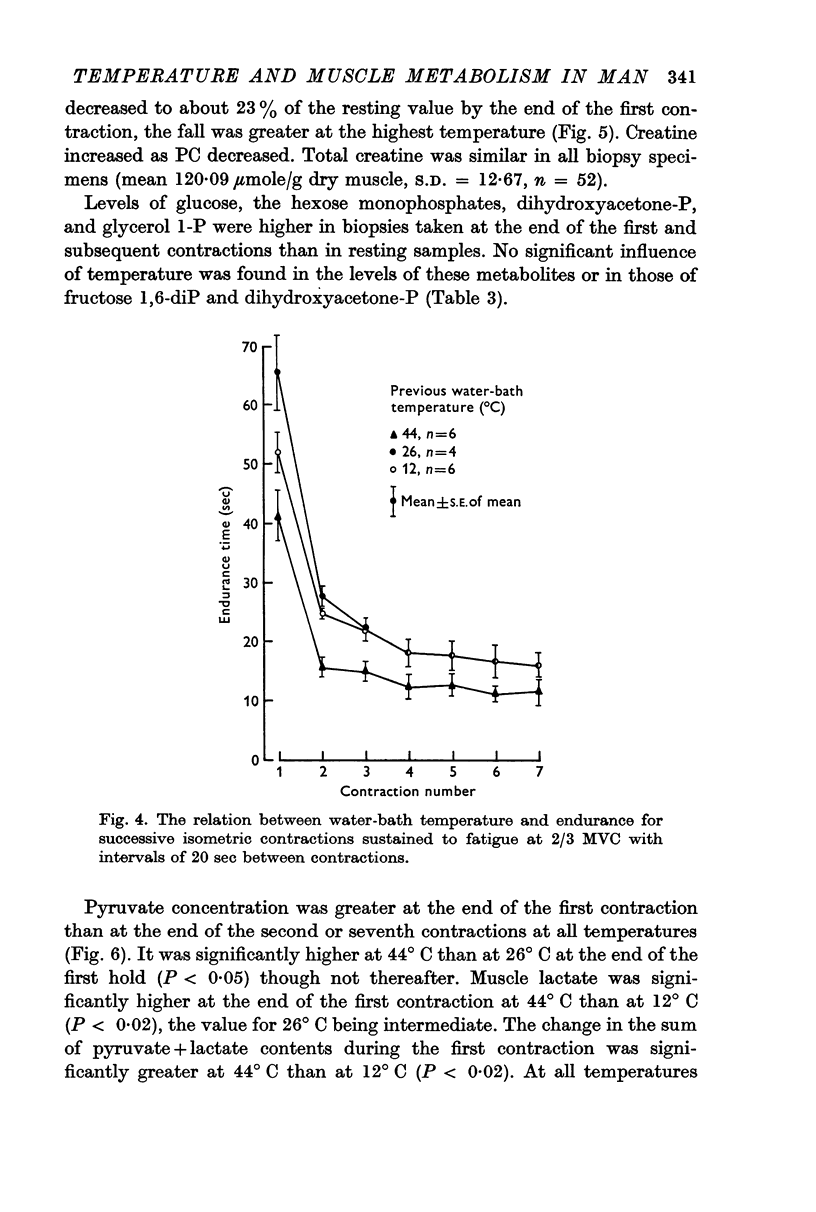
3. Significantly shorter endurance times were found for the heated muscle. These could not be attributed to depletion of local energy resources in muscle. Fatigue may be due to a reduction in the rate of regeneration of ATP from anaerobic glycolysis below that needed to maintain the contraction force. Lower values for the ratio of fructose 1,6-diphosphate: fructose 6-phosphate at the end of contractions, particularly at the highest temperature, are compatible with the hypothesis that there is partial inhibition of the rate controlling enzyme phosphofructokinase, possibly due to the accumulation of hydrogen ions in muscle.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMSON D. I., KAHN A., TUCK S., Jr, TURMAN G. A., REJAL H., FLEISCHER C. J. Relationship between a range of tissue temperature and local oxygen uptake in the human forearm. I. Changes observed under resting conditions. J Clin Invest. 1958 Jul;37(7):1031–1038. doi: 10.1172/JCI103684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDRES R., CADER G., ZIERLER K. L. The quantitatively minor role of carbohydrate in oxidative metabolism by skeletal muscle in intact man in the basal state; measurements of oxygen and glucose uptake and carbon dioxide and lactate production in the forearm. J Clin Invest. 1956 Jun;35(6):671–682. doi: 10.1172/JCI103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K., KREBS H. A. The free-energy changes associated with the individual steps of the tricarboxylic acid cycle, glycolysis and alcoholic fermentation and with the hydrolysis of the pyrophosphate groups of adenosinetriphosphate. Biochem J. 1953 Apr;54(1):94–107. doi: 10.1042/bj0540094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcroft H., Millen J. L. The blood flow through muscle during sustained contraction. J Physiol. 1939 Nov 14;97(1):17–31. doi: 10.1113/jphysiol.1939.sp003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIN D. F., DAVIES R. E. Breakdown of adenosine triphosphate during a single contraction of working muscle. Biochem Biophys Res Commun. 1962 Aug 7;8:361–366. doi: 10.1016/0006-291x(62)90008-6. [DOI] [PubMed] [Google Scholar]
- CLARKE R. S., HELLON R. F. Hyperaemia following sustained and rhythmic exercise in the human forearm at various temperatures. J Physiol. 1959 Mar 12;145(3):447–458. doi: 10.1113/jphysiol.1959.sp006153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE R. S., HELLON R. F., LIND A. R. The duration of sustained contractions of the human forearm at different muscle temperatures. J Physiol. 1958 Oct 31;143(3):454–473. doi: 10.1113/jphysiol.1958.sp006071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE R. K., SOLS A. The association of hexokinase with particulate fractions of brain and other tissue homogenates. J Biol Chem. 1953 Jul;203(1):273–292. [PubMed] [Google Scholar]
- Close R., Hoh J. F. Influence of temperature on isometric contractions of rat skeletal muscles. Nature. 1968 Mar 23;217(5134):1179–1180. doi: 10.1038/2171179a0. [DOI] [PubMed] [Google Scholar]
- Corsi A., Midrio M., Granata A. L. In situ utilization of glycogen and blood glucose by skeletal muscle during tetanus. Am J Physiol. 1969 Jun;216(6):1534–1541. doi: 10.1152/ajplegacy.1969.216.6.1534. [DOI] [PubMed] [Google Scholar]
- Craig F. N., Froehlich H. L. Endurance of preheated men in exhausting work. J Appl Physiol. 1968 May;24(5):636–639. doi: 10.1152/jappl.1968.24.5.636. [DOI] [PubMed] [Google Scholar]
- Davies R. E., Goldspink G., Larson R. E. ATP utilization by fast and slow muscles during the development and maintenance of isometric tension. J Physiol. 1970 Feb;206(2):28P–29P. [PubMed] [Google Scholar]
- Forrester T., Lind A. R. Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J Physiol. 1969 Oct;204(2):347–364. doi: 10.1113/jphysiol.1969.sp008917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink G., Larson R. E., Davies R. E. Fluctuations in sarcomere length in the chick anterior and posterior latissimus dorsi muscles during isometric contraction. Experientia. 1970 Jan 15;26(1):16–18. doi: 10.1007/BF01900361. [DOI] [PubMed] [Google Scholar]
- Hartree W., Hill A. V. The regulation of the supply of energy in muscular contraction. J Physiol. 1921 May 24;55(1-2):133–158. doi: 10.1113/jphysiol.1921.sp001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIND A. R. Muscle fatigue and recovery from fatigue induced by sustained contractions. J Physiol. 1959 Jun 23;147(1):162–171. doi: 10.1113/jphysiol.1959.sp006231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- MORGAN H. E., PARMEGGIANI A. REGULATION OF GLYCOGENOLYSIS IN MUSCLE. 3. CONTROL OF MUSCLE GLYCOGEN PHOSPHORYLASE ACTIVITY. J Biol Chem. 1964 Aug;239:2440–2445. [PubMed] [Google Scholar]
- START K. B., HOLMES R. Local muscle endurance with open and occluded intramuscular circulation. J Appl Physiol. 1963 Jul;18:804–807. doi: 10.1152/jappl.1963.18.4.804. [DOI] [PubMed] [Google Scholar]
- SYLVEST O., HVID N. Pressure measurements in human striated muscles during contraction. Acta Rheumatol Scand. 1959;5:216–222. doi: 10.3109/rhe1.1959.5.issue-1-4.26. [DOI] [PubMed] [Google Scholar]
- Saltin B., Gagge A. P., Stolwijk J. A. Muscle temperature during submaximal exercise in man. J Appl Physiol. 1968 Dec;25(6):679–688. doi: 10.1152/jappl.1968.25.6.679. [DOI] [PubMed] [Google Scholar]


