Abstract
1. The effect of antidiuretic hormone (ADH) on the movement and distribution of Na was studied. This was done using three different approaches: (a) the measurement of Na and 22Na in slices of epithelium of skins which were exposed to Ringer of varied composition containing 22Na, (b) the measurement of the influx of Na from the outer to the inner bathing solution with 22Na added to the outside, and (c) the use of a recently introduced technique which permits the direct evaluation of the flux from the outer solution → epithelium, (JOT), i.e. the flux across the barrier which is generally regarded as the site of ADH activity.
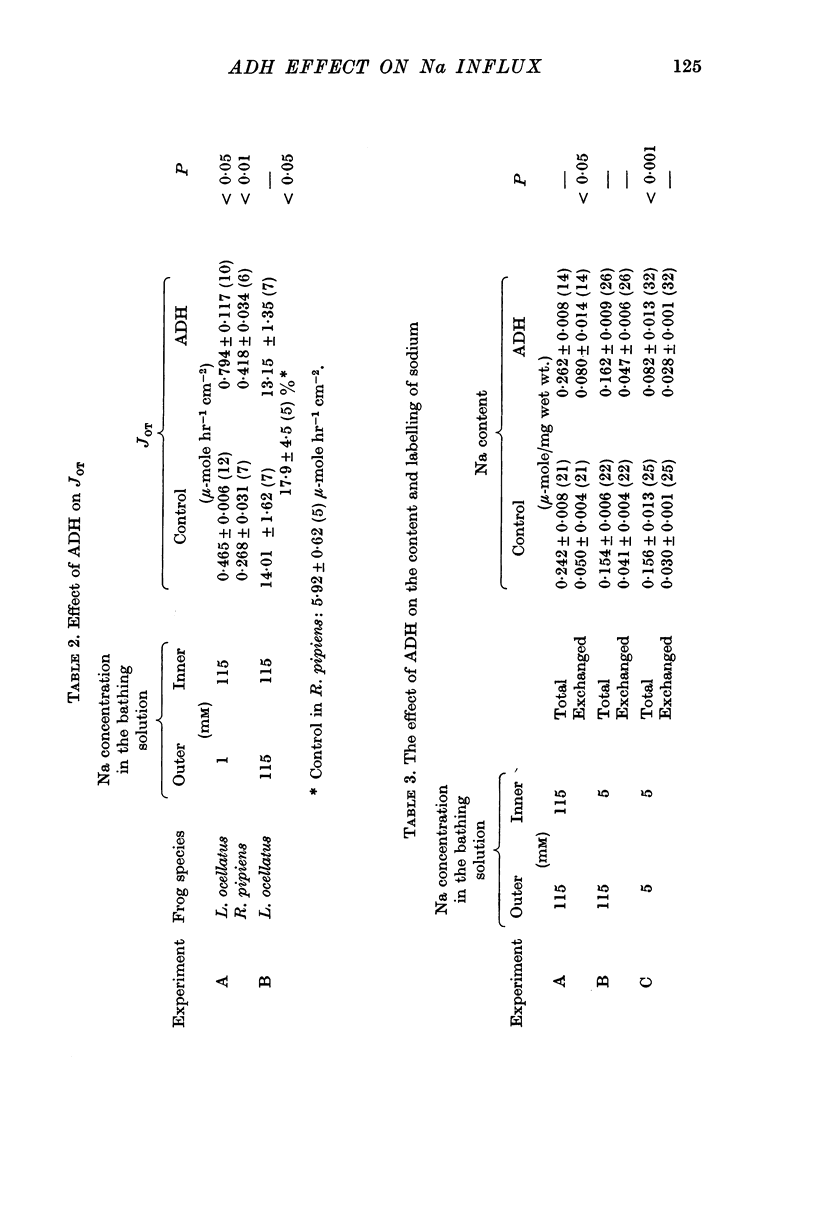
2. ADH increased the influx from the outer to the inner bathing solution of Na (50%) not only when the concentration of Na on the outside was 115 mM (i.e. higher than in the epithelium) but even when the concentration was 1 mM (67%).
3. When the skin was bathed with 1mM-Na Ringer on the outside, ADH increased the unidirectional Na flux JOT by 56% (Rana pipiens) and 71% (Leptodactylus ocellatus). When the concentration was 115 mM a small increase (17%) was observed in paired skins of R. pipiens. Under this condition no change was observed in L. ocellatus.
4. The amount of epithelial sodium which is labelled by 22Na added to the outside was taken to reflect the amount of Na involved in Na transport across the epithelium. Depending on whether the concentration of Na on the outside was high (115 mM) or low (1 mM), ADH produced an increase, or a decrease, of both the total Na content and the amount of 22Na exchanged.
5. When the concentration of Na on the outside was low, ADH increased the total influx and JOT in spite of the fact that it lowers the total Na content and does not affect the exchangeable pool of Na. This observation is inconsistent with the view that the effect of ADH is due to the fact that the increased permeability of the outer barrier allows more Na into the cell, and that the resulting increase of Na concentration in the cytoplasm accelerates the Na pumps at the inner side of the cells.
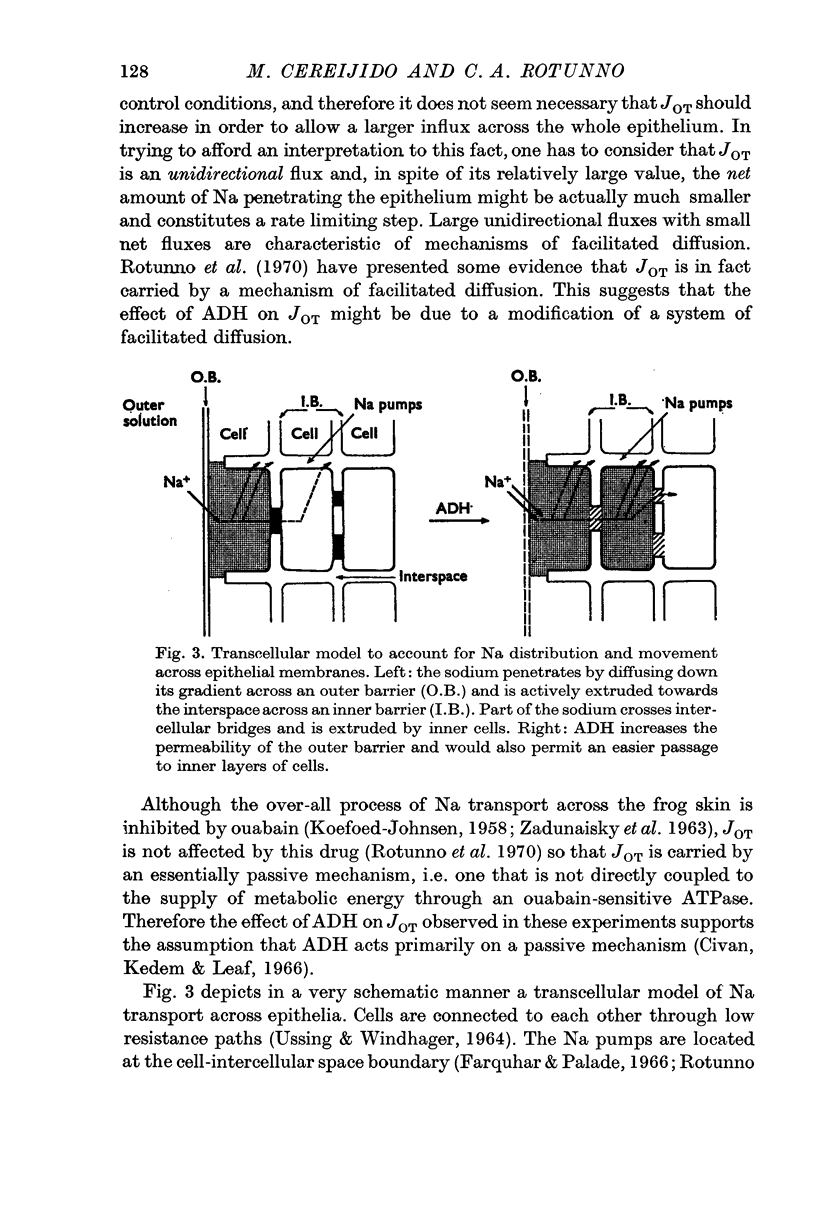
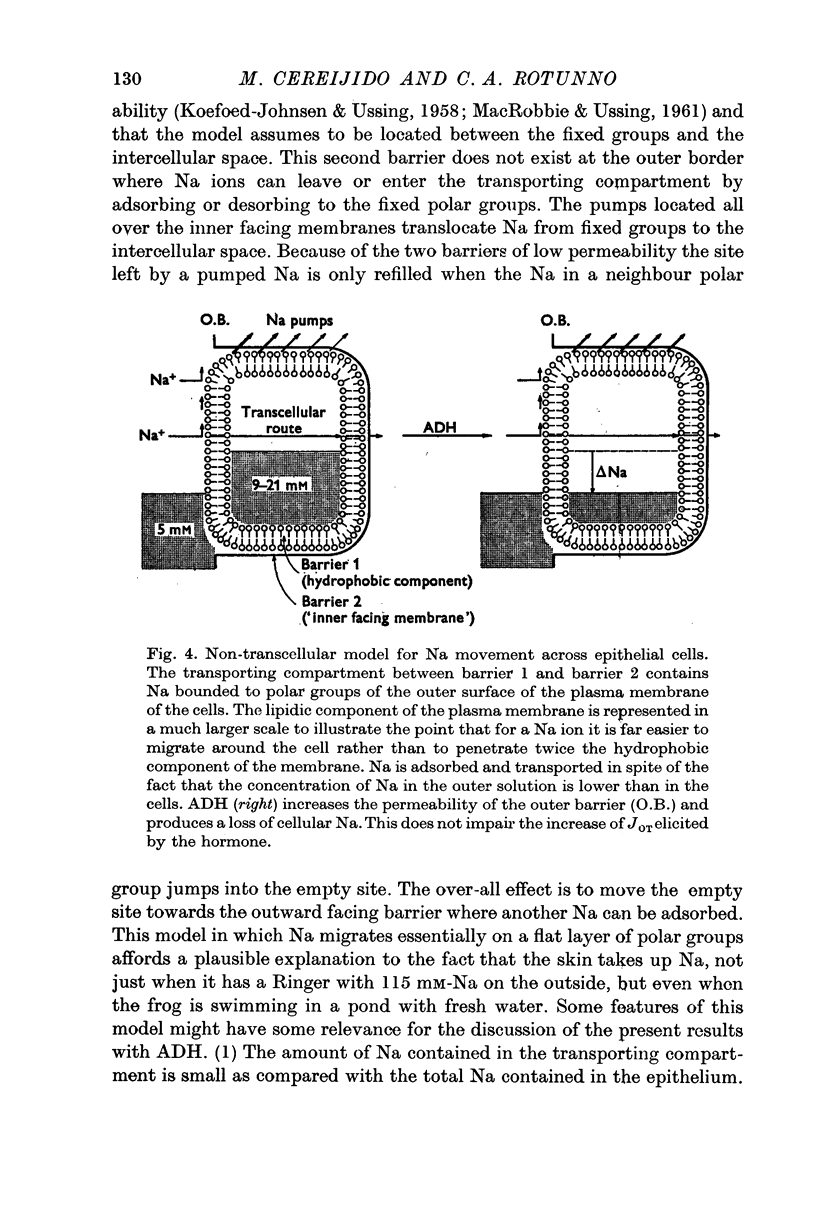
6. It is concluded that ADH speeds up Na movements at the outward facing barrier, and that this exchange which facilitates the penetration of Na into a transporting compartment produces also a gain or a loss of Na in compartments not directly involved in Na transport across the epithelium. One compartment which is not involved in Na transport might be the cytoplasm of the epithelial cells.
Full text
PDF

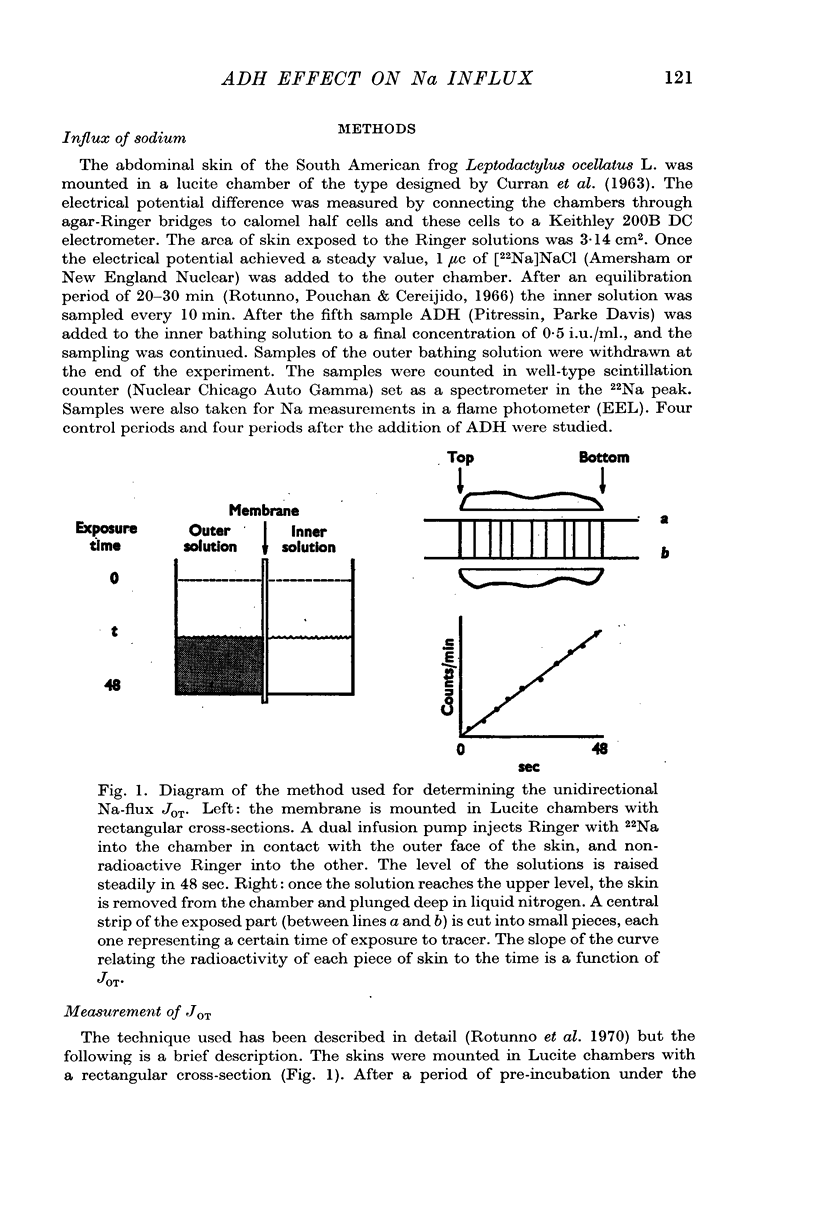









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOURGUET J., MAETZ J. [Arguments in favor of the independence of the mechanisms of action of various neurohypophyseal hormones on the osmotic flux of water and on the active transport of sodium in the same receptor; studies on the bladder and the skin of Rana esculenta L]. Biochim Biophys Acta. 1961 Sep 30;52:552–565. doi: 10.1016/0006-3002(61)90414-0. [DOI] [PubMed] [Google Scholar]
- Bastide F., Jard S. Actions de la noradrénaline et de l'oxytocine sur le transport actif de sodium et la permeabilité à l'eau de la peau de grenouille. Rôle du 3',5'-AMP cyclique. Biochim Biophys Acta. 1968 Jan 3;150(1):113–123. doi: 10.1016/0005-2736(68)90014-x. [DOI] [PubMed] [Google Scholar]
- Biber T. U., Chez R. A., Curran P. F. Na transport across frog skin at low external Na concentrations. J Gen Physiol. 1966 Jul;49(6):1161–1176. doi: 10.1085/jgp.0491161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber T. U., Curran P. F. Direct measurement of uptake of sodium at the outer surface of the frog skin. J Gen Physiol. 1970 Jul;56(1):83–99. doi: 10.1085/jgp.56.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguet J., Morel F. Independance des variations de perméabilité à l'eau et au sodium produites par les hormones neurohypophysaires sur la vessie de grenouille. Biochim Biophys Acta. 1967 Sep 9;135(4):693–700. doi: 10.1016/0005-2736(67)90099-5. [DOI] [PubMed] [Google Scholar]
- CEREIJIDO M., HERRERA F. C., FLANIGAN W. J., CURRAN P. F. THE INFLUENCE OF NA CONCENTRATION ON NA TRANSPORT ACROSS FROG SKIN. J Gen Physiol. 1964 May;47:879–893. doi: 10.1085/jgp.47.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F., GILL J. R., Jr The effect of calcium on sodium transport by frog skin. J Gen Physiol. 1962 Mar;45:625–641. doi: 10.1085/jgp.45.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F., HERRERA F. C., FLANIGAN W. J. The effect of Ca and antidiuretic hormone on Na transport across frog skin. II. Sites and mechanisms of action. J Gen Physiol. 1963 May;46:1011–1027. doi: 10.1085/jgp.46.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M., Reisin I., Rotunno C. A. The effect of sodium concentration on the content and distribution of sodium in the frog skin. J Physiol. 1968 May;196(1):237–253. doi: 10.1113/jphysiol.1968.sp008504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M., Rotunno C. A. Fluxes and distribution of sodium in frog skin. A new model. J Gen Physiol. 1968 May;51(5 Suppl):280S+–280S+. [PubMed] [Google Scholar]
- Cereijido M., Rotunno C. A. Transport and distribution of sodium across frog skin. J Physiol. 1967 Jun;190(3):481–497. doi: 10.1113/jphysiol.1967.sp008223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., Frazier H. S. The site of the stimulatory action of vasopressin on sodium transport in toad bladder. J Gen Physiol. 1968 May;51(5):589–605. doi: 10.1085/jgp.51.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., Kedem O., Leaf A. Effect of vasopressin on toad bladder under conditions of zero net sodium transport. Am J Physiol. 1966 Sep;211(3):569–575. doi: 10.1152/ajplegacy.1966.211.3.569. [DOI] [PubMed] [Google Scholar]
- Crabbé J., De Weer P. Action of aldosterone and vasopressin on the active transport of sodium by the isolated toad bladder. J Physiol. 1965 Oct;180(3):560–568. doi: 10.1113/jphysiol.1965.sp007717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAZIER H. S., DEMPSEY E. F., LEAF A. Movement of sodium across the mucosal surface of the isolated toad bladder and its modification by vasopressin. J Gen Physiol. 1962 Jan;45:529–543. doi: 10.1085/jgp.45.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUHRMAN F. A., USSING H. H. A characteristic response of the isolated frog skin potential to neurohypophysial principles and its relation to the transport of sodium and water. J Cell Physiol. 1951 Aug;38(1):109–130. doi: 10.1002/jcp.1030380109. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. Adenosine triphosphatase localization in amphibian epidermis. J Cell Biol. 1966 Aug;30(2):359–379. doi: 10.1083/jcb.30.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández M. S., Vilallonga F., Rotunno C. A., Cerijdo M. Discrimination of Na + and K + by monolayers of lipids from epithelial cells. Biochim Biophys Acta. 1970 Jun 2;203(3):586–589. doi: 10.1016/0005-2736(70)90198-7. [DOI] [PubMed] [Google Scholar]
- HAYS R. M., LEAF A. Studies on the movement of water through the isolated toad bladder and its modification by vasopressin. J Gen Physiol. 1962 May;45:905–919. doi: 10.1085/jgp.45.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRERA F. C., CURRAN P. F. The effect of Ca and antidiuretic hormone on Na transport across frog skin. I. Examination of interrelationships between Ca and hormone. J Gen Physiol. 1963 May;46:999–1010. doi: 10.1085/jgp.46.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESTRIN-LERNER S., HOKIN L. E. EFFECTS OF HORMONES ON NA AND H2O TRANSPORT AND ON PHOSPHOLIPID METABOLISM IN TOAD BLADDER. Am J Physiol. 1964 Jan;206:136–142. doi: 10.1152/ajplegacy.1964.206.1.136. [DOI] [PubMed] [Google Scholar]
- HOSHIKO T., USSING H. H. The kinetics of Na24 flux across amphibian skin and bladder. Acta Physiol Scand. 1960 May 25;49:74–81. doi: 10.1111/j.1748-1716.1960.tb01931.x. [DOI] [PubMed] [Google Scholar]
- HUF E. G., PARRISH J., WEATHERFORD C. Active salt and water uptake by isolated frog skin. Am J Physiol. 1951 Jan;164(1):137–142. doi: 10.1152/ajplegacy.1950.164.1.137. [DOI] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H. The nature of the frog skin potential. Acta Physiol Scand. 1958 Jun 2;42(3-4):298–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- LEAF A., DEMPSEY E. Some effects of mammalian neurohypophyseal hormones on metabolism and active transport of sodium by the isolated toad bladder. J Biol Chem. 1960 Jul;235:2160–2163. [PubMed] [Google Scholar]
- Leaf A. Transepithelial transport and its hormonal control in toad bladder. Ergeb Physiol. 1965;56:216–263. [PubMed] [Google Scholar]
- MACROBBIE E. A., USSING H. H. Osmotic behaviour of the epithelial cells of frog skin. Acta Physiol Scand. 1961 Nov-Dec;53:348–365. doi: 10.1111/j.1748-1716.1961.tb02293.x. [DOI] [PubMed] [Google Scholar]
- Rotunno C. A., Vilallonga F. A., Fernández M., Cereijido M. The penetration of sodium into the epithelium of the frog skin. J Gen Physiol. 1970 Jun;55(6):716–735. doi: 10.1085/jgp.55.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routunno C. A., Pouchan M. I., Cereijido M. Location of the mechanism of active transport of sodium across the frog skin. Nature. 1966 May 7;210(5036):597–599. doi: 10.1038/210597a0. [DOI] [PubMed] [Google Scholar]
- Snart R. S., Sanyal N. N. Interaction of polypeptide hormones with lipid monolayers. Biochem J. 1968 Jul;108(3):369–373. doi: 10.1042/bj1080369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H., WINDHAGER E. E. NATURE OF SHUNT PATH AND ACTIVE SODIUM TRANSPORT PATH THROUGH FROG SKIN EPITHELIUM. Acta Physiol Scand. 1964 Aug;61:484–504. [PubMed] [Google Scholar]
- Ussing H. H. Transport of electrolytes and water across epithelia. Harvey Lect. 1965;59:1–30. [PubMed] [Google Scholar]
- Vilallonga F., Fernández M., Rotunno C., Cereijido M. The interactions of L-alpha-palmitoyl lecithin monolayers with Na+,K+ or Li+, and its possile role in membrane phenomena. Biochim Biophys Acta. 1969 Jun 3;183(1):98–109. doi: 10.1016/0005-2736(69)90133-3. [DOI] [PubMed] [Google Scholar]
- Watlington C. O., Harlan W. R., Jr Ion transport and lipid content of isolated frog skin. Am J Physiol. 1969 Oct;217(4):1004–1008. doi: 10.1152/ajplegacy.1969.217.4.1004. [DOI] [PubMed] [Google Scholar]
- ZADUNAISKY J. A., CANDIA O. A., CHIARANDINI D. J. THE ORIGIN OF THE SHORT-CIRCUIT CURRENT IN THE ISOLATED SKIN OF THE SOUTH AMERICAN FROG LEPTODACTYLUS OCELLATUS. J Gen Physiol. 1963 Nov;47:393–402. doi: 10.1085/jgp.47.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graeff J., Dempsey E. F., Lameyer L. D., Leaf A. Phospholipids and active sodium transport in toad bladder. Biochim Biophys Acta. 1965 Jul 7;106(1):155–170. doi: 10.1016/0005-2760(65)90104-9. [DOI] [PubMed] [Google Scholar]


