Abstract
1. Cortical slices from kidneys of adult rats were equilibrated for 24 and 48 hr in the refrigerator in solutions containing 5 mM-K, 2·5 mM-Ca and Mg, M/15 phosphate buffer (pH 7·4), 5 mM cyanide and iodoacetate with 5, 6, 7 and 8% polyethylene glycol (PEG 6000) and 77, 154, 308, 462 and 770 mM-NaCl.
2. There were no consistent differences in water content between slices after 24 and after 48 hr.
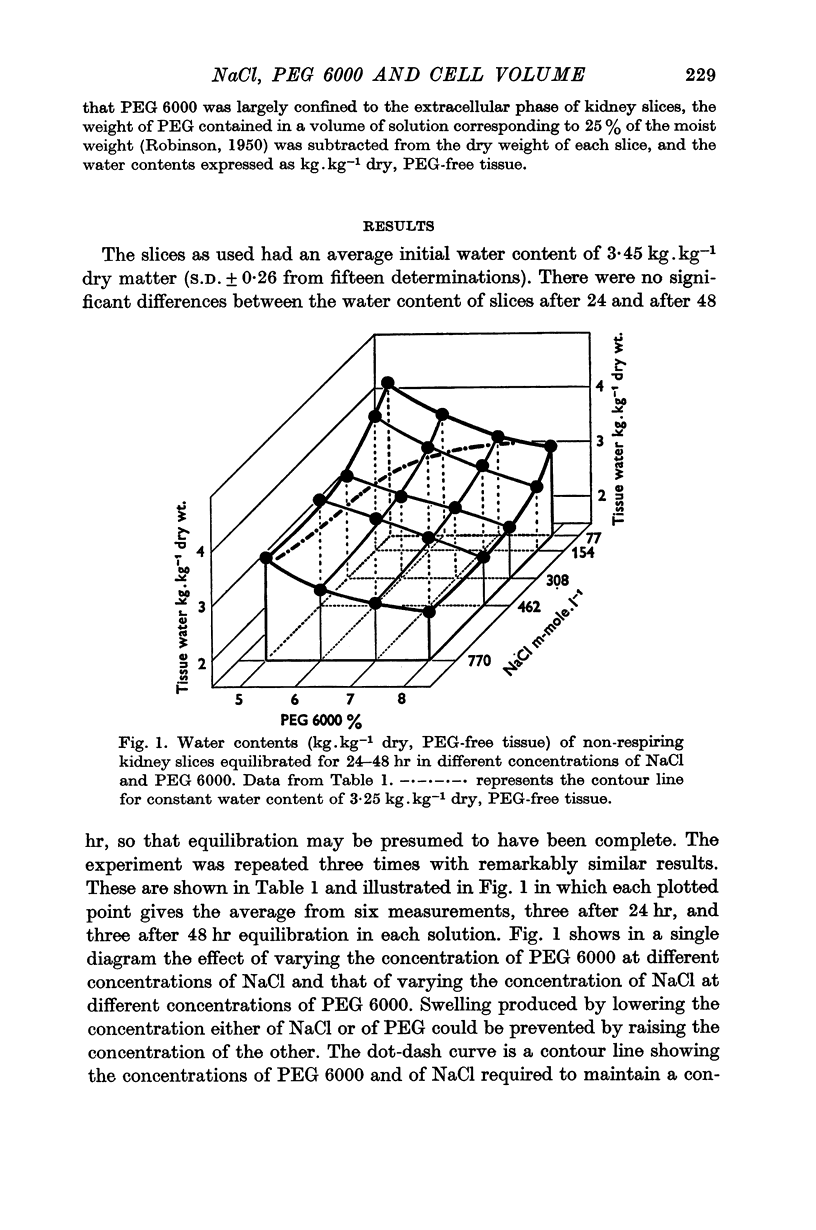
3. Slices contained more water when either the concentration of NaCl or that of PEG was reduced. The increase in water content induced by lowering the concentration of either was prevented by a higher concentration of the other.
4. Water content was held constant at 3·25 kg.kg-1 dry PEG-free tissue despite a fall in [NaCl] from 770 to 77 mM (more than 1200 m-osmole.kg-1) by increasing PEG from 5·1 to 7·7% corresponding to an increase of the order of 15 m-osmole.kg-1.
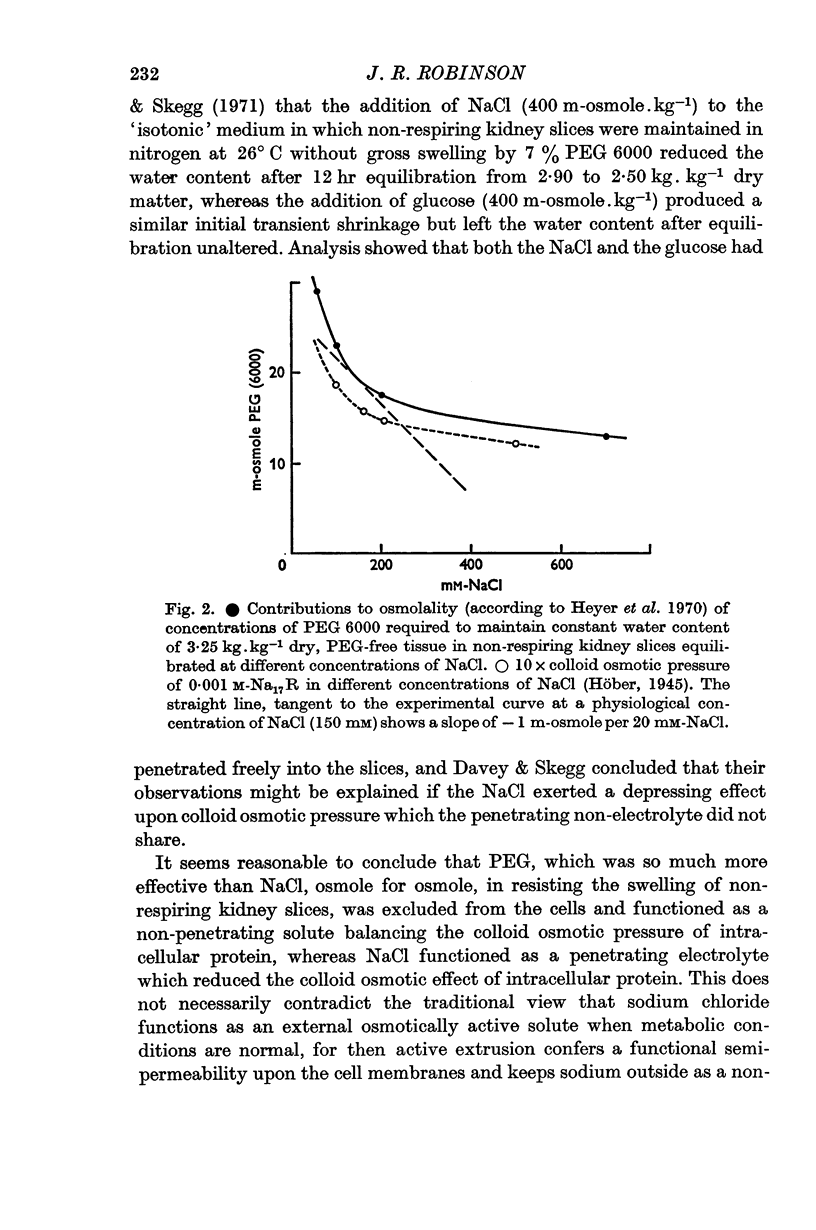
5. It is suggested that PEG 6000 was so much more effective than NaCl, osmole for osmole, because PEG 6000 acted as a non-penetrating solute, whereas NaCl penetrated the slices and reduced the `Donnan excess' component of the intracellular colloid osmotic pressure so that this could be balanced by a smaller concentration of PEG.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davey K. J., Skegg D. C. The effects of high concentrations of an electrolyte on the swelling of non-metabolizing tissue slices. J Physiol. 1971 Feb;212(3):641–653. doi: 10.1113/jphysiol.1971.sp009347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaister D., Kerly M. The oxygen consumption and carbohydrate metabolism of the retractor muscle of the foot of Mytilus edulis. J Physiol. 1936 Jun 10;87(1):56–66. doi: 10.1113/jphysiol.1936.sp003388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer E., Cass A., Mauro A. A demonstration of the effect of permeant and impermeant solutes, and unstirred boundary layers on osmoti flow. Yale J Biol Med. 1969 Dec;42(3-4):139–153. [PMC free article] [PubMed] [Google Scholar]
- LEAF A. Maintenance of concentration gradients and regulation of cell volume. Ann N Y Acad Sci. 1959 Feb 6;72(12):396–404. doi: 10.1111/j.1749-6632.1959.tb44168.x. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN L. M. The effect of albumin on osmotic hemolysis. Exp Cell Res. 1960 Jun;20:56–65. doi: 10.1016/0014-4827(60)90221-4. [DOI] [PubMed] [Google Scholar]
- PETERS J. P. Sodium, water and edema. J Mt Sinai Hosp N Y. 1950 Sep-Oct;17(3):159–175. [PubMed] [Google Scholar]
- ROBINSON J. R. Osmoregulation in surviving slices from the kidneys of adult rats. Proc R Soc Lond B Biol Sci. 1950 Oct 13;137(888):378–402. doi: 10.1098/rspb.1950.0048. [DOI] [PubMed] [Google Scholar]
- ROBINSON J. R. The effect of sodium and chloride ions upon swelling of rat kidney slices treated with a mercurial diuretic. J Physiol. 1956 Oct 29;134(1):216–228. doi: 10.1113/jphysiol.1956.sp005638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. R. Water regulation in mammalian cells. Symp Soc Exp Biol. 1965;19:237–258. [PubMed] [Google Scholar]


