Abstract
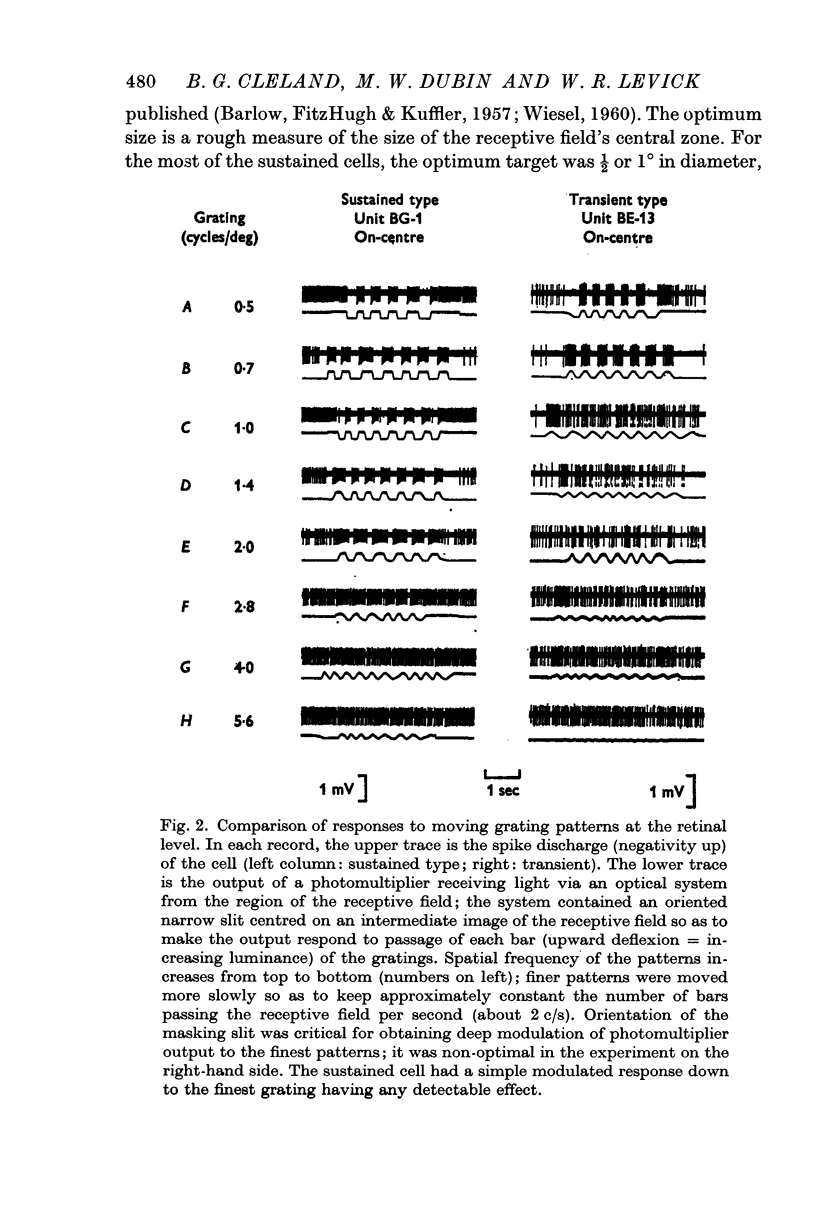
1. Cat retinal ganglion cells may be subdivided into sustained and transient response-types by the application of a battery of simple tests based on responses to standing contrast, fine grating patterns, size and speed of contrasting targets, and on the presence or absence of the periphery effect. The classification is equivalent to the `X'/`Y' (linear/nonlinear) subdivision of Enroth-Cugell & Robson which is thus confirmed and extended.
2. The sustained/transient classification applied to both on-centre and off-centre cells.
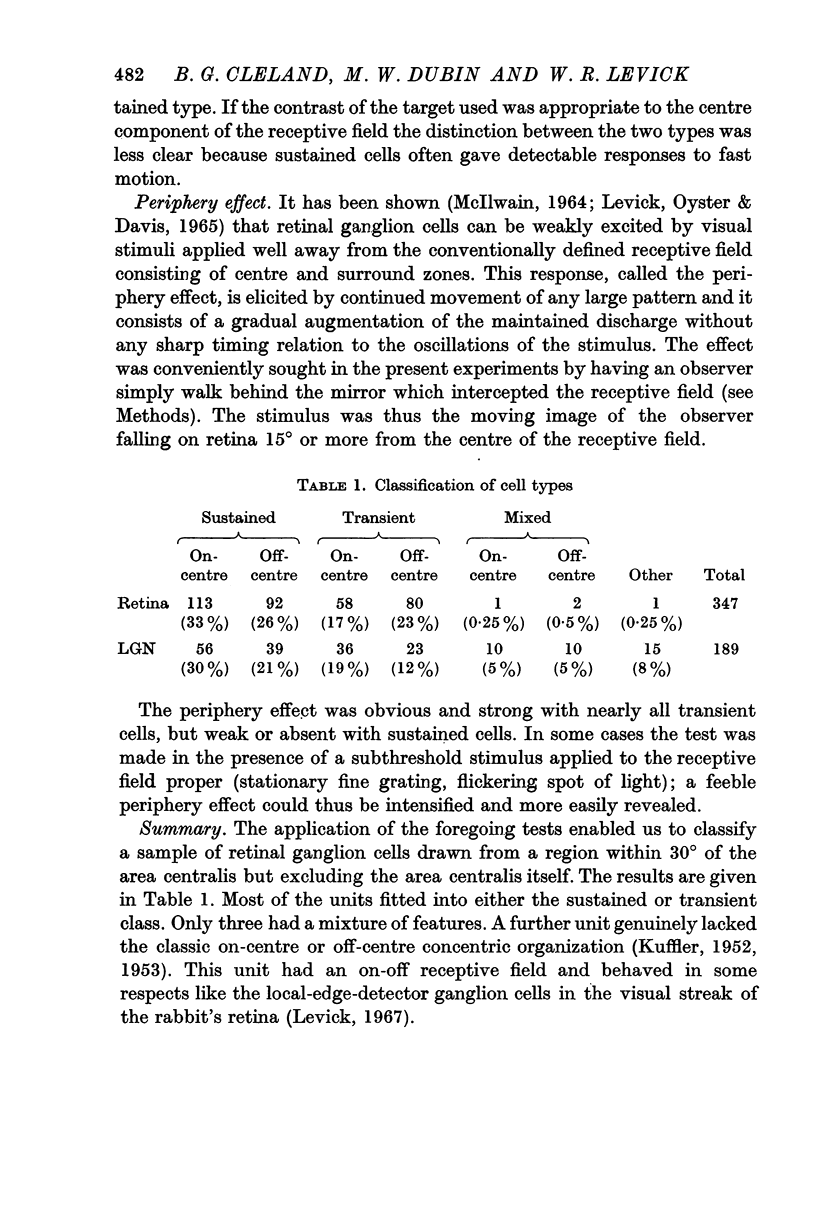
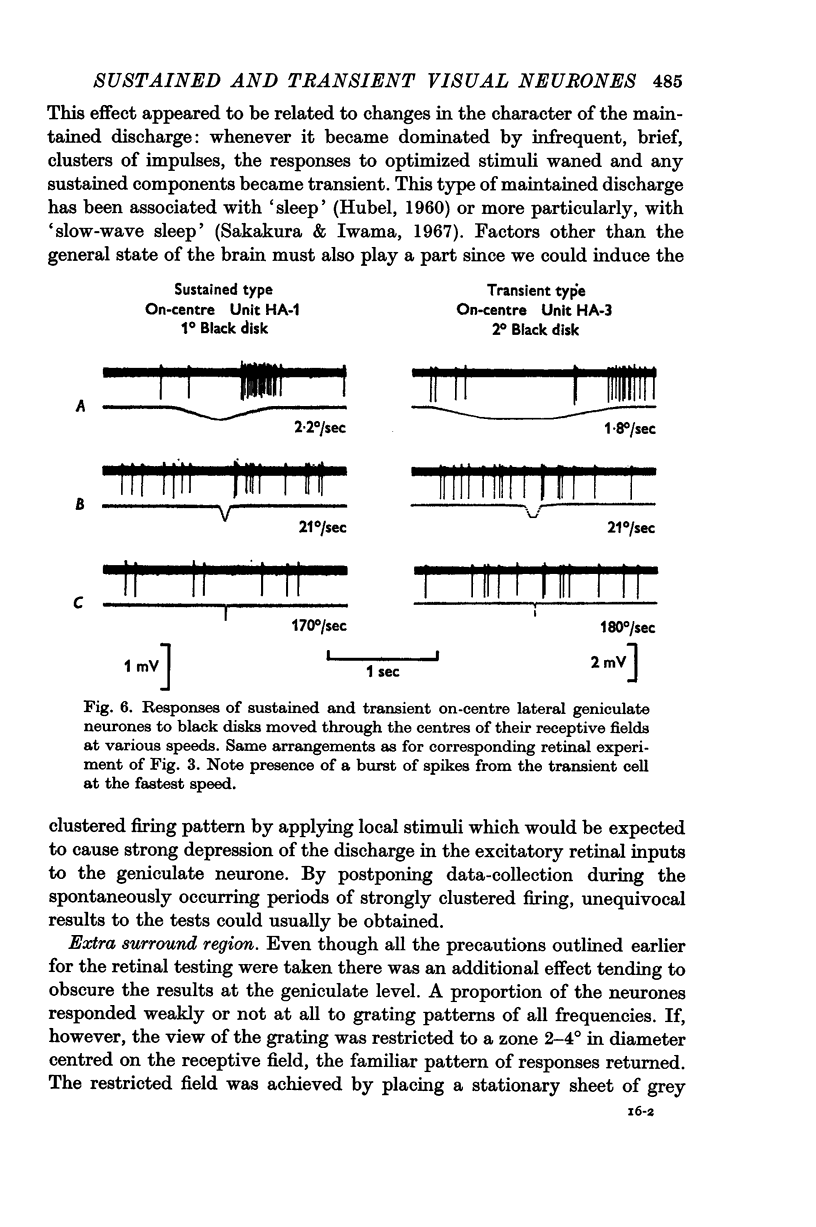
3. Lateral geniculate neurones may be similarly classified by the same tests. Occasional concentrically organized cells had a mixture of sustained and transient properties.
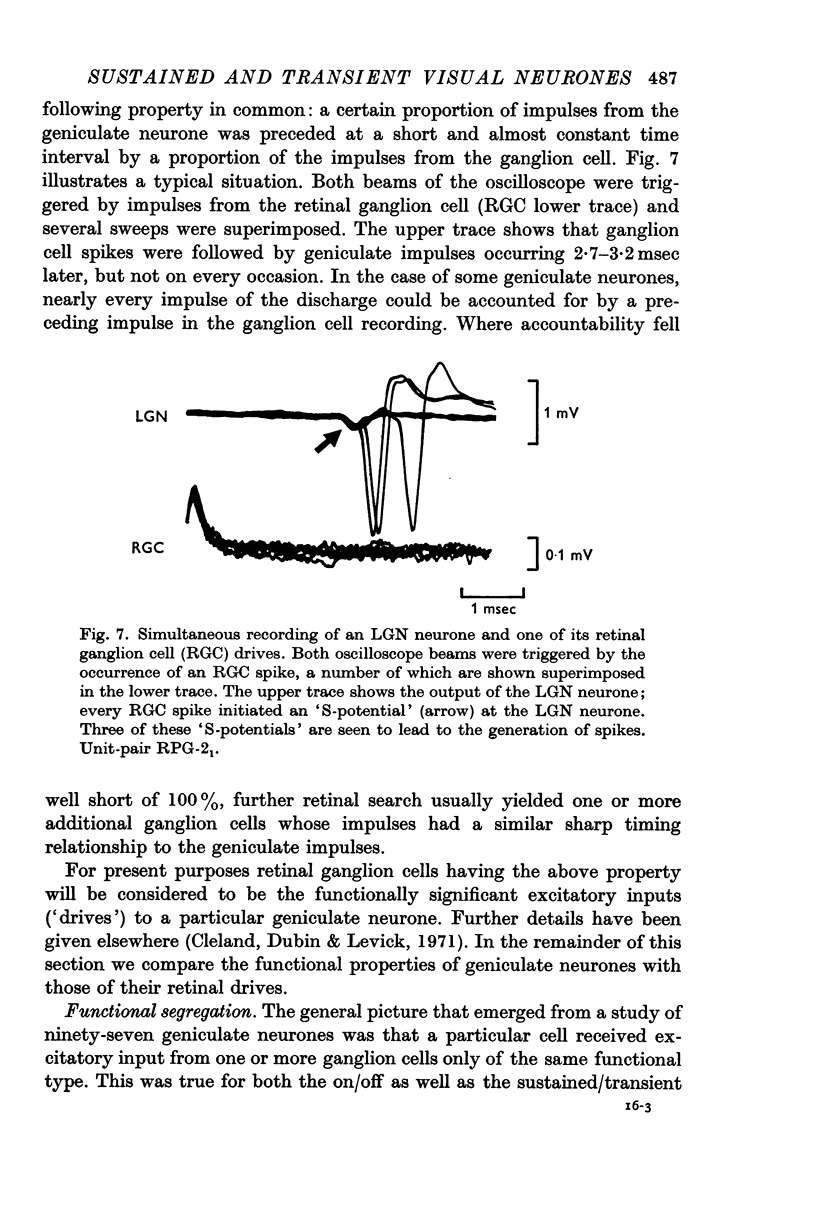
4. A technique for simultaneous recording from a geniculate neurone and one or more retinal ganglion cells providing its excitatory input showed that the connexions were specific with respect to the sustained/transient classification as well as the on-centre/off-centre classification. Most geniculate neurones are excitatorily driven only by retinal ganglion cells of the same functional type. In a few cases the inputs were mixed but only with respect to the sustained/transient classification.
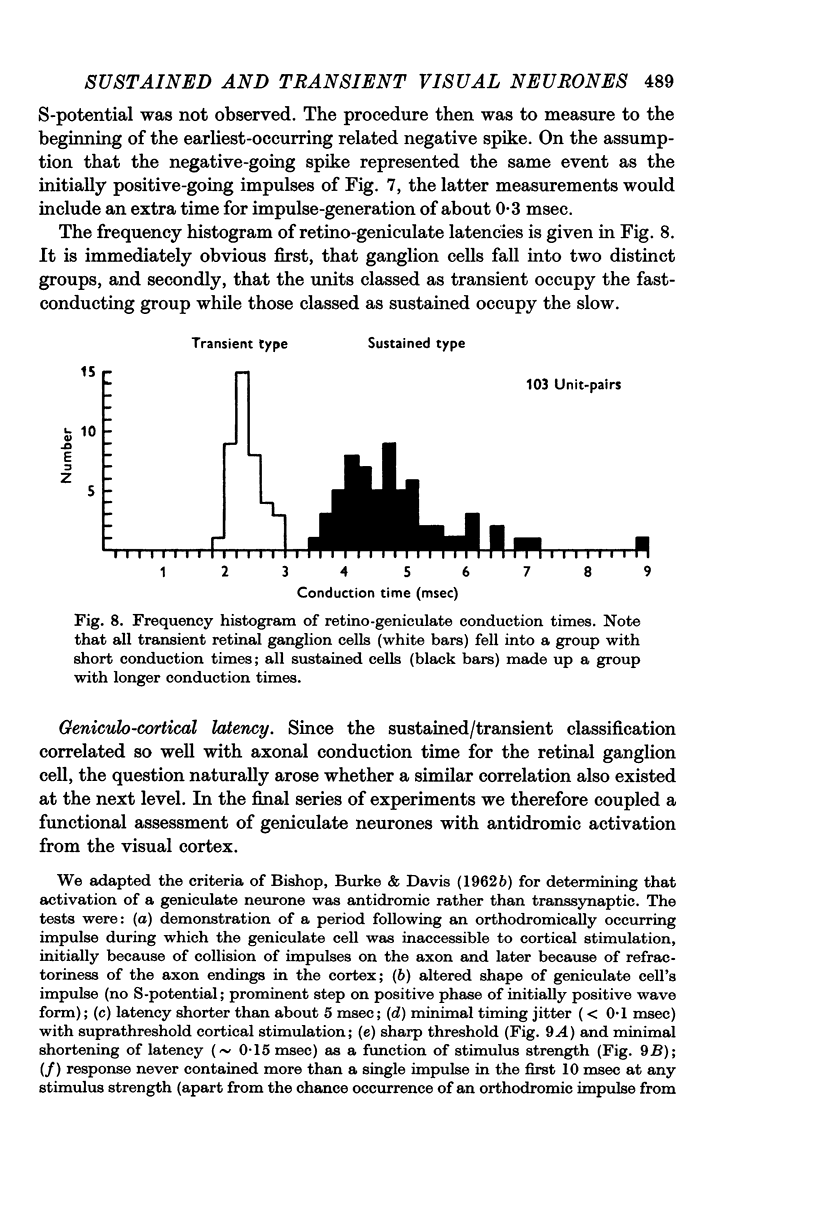
5. Sustained retinal ganglion cells had slower-conducting axons than the transient type. The same was true for lateral geniculate neurones but in this case the distributions showed considerable overlap.
6. The sustained/transient classification is the functional correlate for the well-known segregation of optic nerve fibres into two conduction groups.
7. The pathways carrying sustained and transient information remain essentially separate from retina through the lateral geniculate nucleus to the striate cortex.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP G. H., CLARE M. H. Organization and distribution of fibers in the optic tract of the cat. J Comp Neurol. 1955 Oct;103(2):269–304. doi: 10.1002/cne.901030204. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. Single-unit recording from antidromically activated optic radiation neurones. J Physiol. 1962 Aug;162:432–450. doi: 10.1113/jphysiol.1962.sp006943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. The identification of single units in central visual pathways. J Physiol. 1962 Aug;162:409–431. doi: 10.1113/jphysiol.1962.sp006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. The interpretation of the extracellular response of single lateral geniculate cells. J Physiol. 1962 Aug;162:451–472. doi: 10.1113/jphysiol.1962.sp006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., JEREMY D., LANCE J. W. The optic nerve; properties of a central tract. J Physiol. 1953 Aug;121(2):415–432. doi: 10.1113/jphysiol.1953.sp004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O. PROPERTIES OF AFFERENT SYNAPSES AND SENSORY NEURONS IN THE LATERAL GENICULATE NUCLEUS. Int Rev Neurobiol. 1964;6:191–255. doi: 10.1016/s0074-7742(08)60770-9. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O. Synaptic transmission; an analysis of the electrical activity of the lateral geniculate nucleus in the cat after optic nerve stimulation. Proc R Soc Lond B Biol Sci. 1953 Jul 15;141(904):362–392. doi: 10.1098/rspb.1953.0048. [DOI] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965 Jun;178(3):477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969 Jan;200(1):1–24. doi: 10.1113/jphysiol.1969.sp008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke W., Jervie Sefton A. Discharge patterns of principal cells and interneurones in lateral geniculate nucleus of rat. J Physiol. 1966 Nov;187(1):201–212. doi: 10.1113/jphysiol.1966.sp008083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG H. T. Functional organization of central visual pathways. Res Publ Assoc Res Nerv Ment Dis. 1952;30:430–453. [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Simultaneous recording of input and output of lateral geniculate neurones. Nat New Biol. 1971 Jun 9;231(23):191–192. doi: 10.1038/newbio231191a0. [DOI] [PubMed] [Google Scholar]
- DODT E. Geschwindigkeit der Nervenleitung innerhalb der Netzhaut. Experientia. 1956 Jan 15;12(1):34–35. doi: 10.1007/BF02156996. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr An analysis of extracellular potentials from single neurons in the lateral geniculate nucleus of the cat. J Gen Physiol. 1958 Jan 20;41(3):543–564. doi: 10.1085/jgp.41.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, FRANK K. Extracellular potentials from single spinal motoneurons. J Gen Physiol. 1959 Mar 20;42(4):749–760. doi: 10.1085/jgp.42.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y., Motokawa K., Norton A. C., Tasaki K. Functional significance of conduction velocity in the transfer of flicker information in the optic nerve of the cat. J Neurophysiol. 1966 Jul;29(4):698–714. doi: 10.1152/jn.1966.29.4.698. [DOI] [PubMed] [Google Scholar]
- GUYTON A. C., REEDER R. C. Quantitative studies on the autonomic actions of curare. J Pharmacol Exp Ther. 1950 Feb;98(2):188–193. [PubMed] [Google Scholar]
- Gouras P. Antidromic responses of orthodromically identified ganglion cells in monkey retina. J Physiol. 1969 Oct;204(2):407–419. doi: 10.1113/jphysiol.1969.sp008920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J Physiol. 1960 Jan;150:91–104. doi: 10.1113/jphysiol.1960.sp006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Sumitomo I., Iwama K. Activation of lateral geniculate neurons by electrical stimulation of superior colliculus in cats. Jpn J Physiol. 1967 Dec 15;17(6):638–651. doi: 10.2170/jjphysiol.17.638. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Neurons in the retina; organization, inhibition and excitation problems. Cold Spring Harb Symp Quant Biol. 1952;17:281–292. doi: 10.1101/sqb.1952.017.01.026. [DOI] [PubMed] [Google Scholar]
- LENNOX M. A. The on responses to colored flash in single optic tract fibers of cat: correlation with conduction velocity. J Neurophysiol. 1958 Jan;21(1):70–84. doi: 10.1152/jn.1958.21.1.70. [DOI] [PubMed] [Google Scholar]
- LEVICK W. R., OYSTER C. W., DAVIS D. L. EVIDENCE THAT MCILWAIN'S PERIPHERY EFFECT IS NOT A STRAY LIGHT ARTIFACT. J Neurophysiol. 1965 May;28:555–559. doi: 10.1152/jn.1965.28.3.555. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Receptive fields and trigger features of ganglion cells in the visual streak of the rabbits retina. J Physiol. 1967 Feb;188(3):285–307. doi: 10.1113/jphysiol.1967.sp008140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick W. R., Zacks J. L. Responses of cat retinal ganglion cells to brief flashes of light. J Physiol. 1970 Mar;206(3):677–700. doi: 10.1113/jphysiol.1970.sp009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCILWAIN J. T. RECEPTIVE FIELDS OF OPTIC TRACT AXONS AND LATERAL GENICULATE CELLS: PERIPHERAL EXTENT AND BARBITURATE SENSITIVITY. J Neurophysiol. 1964 Nov;27:1154–1173. doi: 10.1152/jn.1964.27.6.1154. [DOI] [PubMed] [Google Scholar]
- MOTOKAWA K., OIKAWA T., TASAKI K. Studies on neuronal processes in the retina by antidromic' stimulation. Jpn J Physiol. 1957 Jun 15;7(2):119–131. doi: 10.2170/jjphysiol.7.119. [DOI] [PubMed] [Google Scholar]
- McIlwain J. T., Buser P. Receptive fields of single cells in the cat's superior colliculus. Exp Brain Res. 1968;5(4):314–325. doi: 10.1007/BF00235906. [DOI] [PubMed] [Google Scholar]
- Noda H., Iwama K. Unitary analysis of retino-geniculate response time in rats. Vision Res. 1967 Mar;7(3):205–213. doi: 10.1016/0042-6989(67)90085-5. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Pettigrew J. D., Bishop P. O., Nikara T. Residual eye movements in receptive-field studies of paralyzed cats. Vision Res. 1967 Jan;7(1):107–110. doi: 10.1016/0042-6989(67)90031-4. [DOI] [PubMed] [Google Scholar]
- Sakakura H., Iwama K. Effects of bilateral eye enucleation upon single unit activity of the lateral geniculate body in free behaving cats. Brain Res. 1967 Dec;6(4):667–678. doi: 10.1016/0006-8993(67)90124-2. [DOI] [PubMed] [Google Scholar]
- Sanderson K. J., Darian-Smith I., Bishop P. O. Binocular corresponding receptive fields of single units in the cat dorsal lateral geniculate nucleus. Vision Res. 1969 Oct;9(10):1297–1303. doi: 10.1016/0042-6989(69)90117-5. [DOI] [PubMed] [Google Scholar]
- Sumitomo I., Iwama K. Discharge frequency of the lateral geniculate neurons as a function of response latency to optic tract stimulation. Brain Res. 1967 Oct;6(2):395–397. doi: 10.1016/0006-8993(67)90210-7. [DOI] [PubMed] [Google Scholar]
- Sumitomo I., Iwama K. Responsiveness to flicker stimulation and maintained discharges in rat lateral geniculate neurons. Vision Res. 1968 Aug;8(8):1123–1126. doi: 10.1016/0042-6989(68)90083-7. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]


