Abstract
1. A new technique is presented for determining the volume of extracellular space in bowfin (Amia calva) brain during in vitro incubation. It consists of solving simultaneous equations which are applied to determine the volume of extracellular space as well as intracellular marker concentration. This technique allows for a better insight into the redistribution of marker between incubation medium and extracellular space as well as between extracellular and intracellular space.
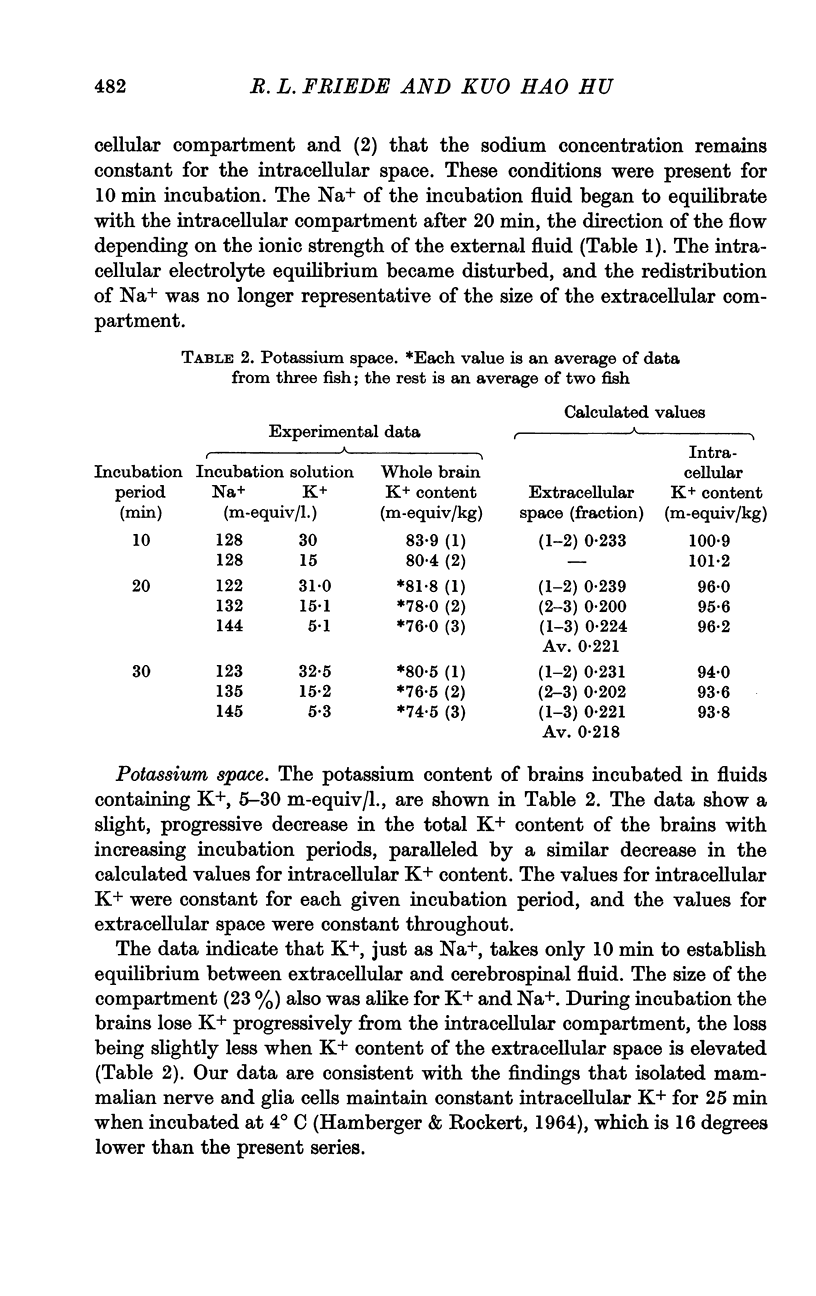
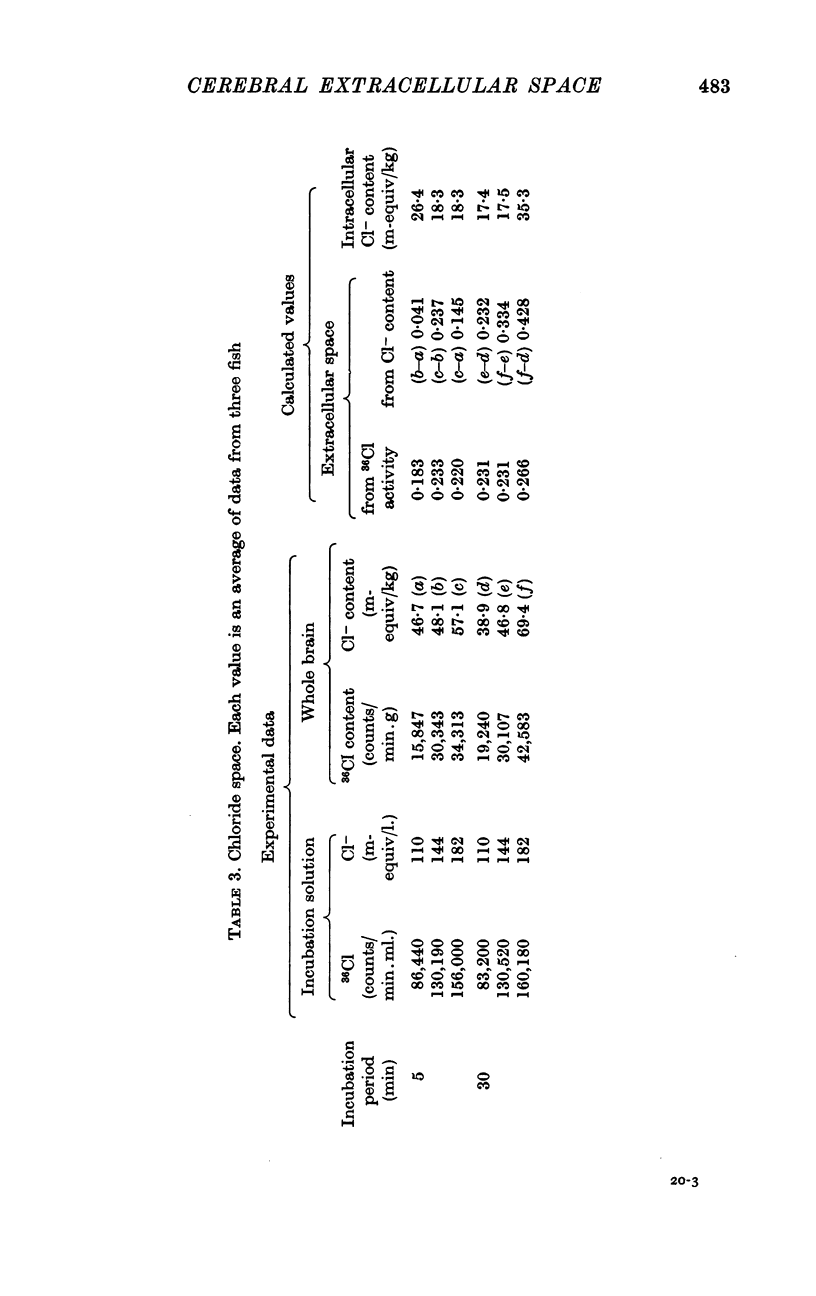
2. Na+, K+ and Cl- equilibrated within 10-15 min between incubation medium and extracellular space. There was no evidence of a homoeostatic mechanism controlling the concentration of these ions in the extracellular fluid, which appeared to be in equilibrium with cerebrospinal fluid. The extracellular spaces of these ions were identical: Na+, 23·4; K+, 23·3 and Cl-, 23·2%.
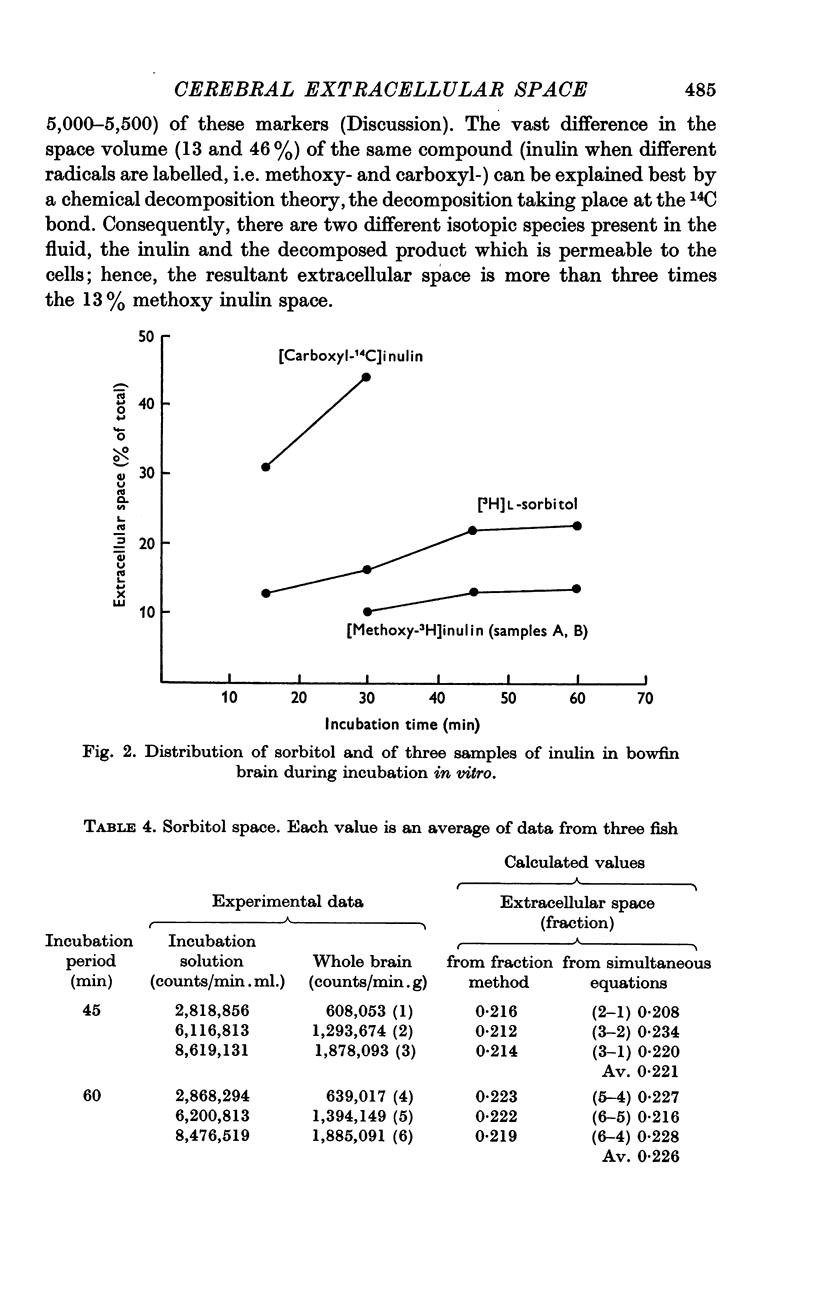
3. Sorbitol equilibrated with the extracellular fluid within 45 min and indicated an extracellular space of 22·6%, nearly identical with that for electrolytes.
4. Vastly different `spaces' were obtained for [3H]methoxy inulin, which equilibrated within 45 min with a 13% space and [14C]carboxyl inulin, which showed a 46% space value for only 30 min. The difference may be explained by marker decomposition. The 9% difference between the [3H]methoxy inulin and sorbitol spaces may be explained by a `packing' factor attributable to molecular size.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW C. F., DOMEK N. S., GOLDBERG M. A., ROTH L. J. Extracellular brain space measured by S35 sulfate. Arch Neurol. 1961 Jul;5:102–110. doi: 10.1001/archneur.1961.00450130104012. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Nicholls J. G. Changes in extracellular potassium concentration produced by neuronal activity in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):555–569. doi: 10.1113/jphysiol.1969.sp008879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito L., Davson H., Levin E., Murray M., Snider N. The concentrations of free amino acids and other electrolytes in cerebrospinal fluid, in vivo dialysate of brain, and blood plasma of the dog. J Neurochem. 1966 Nov;13(11):1057–1067. doi: 10.1111/j.1471-4159.1966.tb04265.x. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Davson H. The transport of potassium between blood, cerebrospinal fluid and brain. J Physiol. 1965 Nov;181(1):151–174. doi: 10.1113/jphysiol.1965.sp007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M. W., Villamil M., Kleeman C. R. Extracellular fluid, ionic distribution and exchange in isolated frog brain. Am J Physiol. 1968 Mar;214(3):643–651. doi: 10.1152/ajplegacy.1968.214.3.643. [DOI] [PubMed] [Google Scholar]
- Brightman M. W. The distribution within the brain of ferritin injected into cerebrospinal fluid compartments. II. Parenchymal distribution. Am J Anat. 1965 Sep;117(2):193–219. doi: 10.1002/aja.1001170204. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Stumpf W. E., Roth L. J. Location of radioactively labelled extracellular fluid indicators in nervous tissue by autoradiography. J Cell Sci. 1969 Jan;4(1):265–288. doi: 10.1242/jcs.4.1.265. [DOI] [PubMed] [Google Scholar]
- COULTER N. A., Jr Filtration coefficient of the capillaries of the brain. Am J Physiol. 1958 Nov;195(2):459–464. doi: 10.1152/ajplegacy.1958.195.2.459. [DOI] [PubMed] [Google Scholar]
- Cohen M. W., Gerschenfeld H. M., Kuffler S. W. Ionic environment of neurones and glial cells in the brain of an amphibian. J Physiol. 1968 Jul;197(2):363–380. doi: 10.1113/jphysiol.1968.sp008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. R., Stampleman P. F., Lajtha A. The temperature-dependent compartmentation of the 'extracellular space' in mouse brain slices as revealed by the markers: inulin, sucrose, d-mannitol, d-sorbitol and sulfate. Brain Res. 1970 Jul 29;21(3):419–434. doi: 10.1016/0006-8993(70)90421-x. [DOI] [PubMed] [Google Scholar]
- Cornog J. L., Gonatas N. K., Feierman J. R. Effects of intracerebral injection of ouabain on the fine structure of rat cerebral cortex. Am J Pathol. 1967 Oct;51(4):573–590. [PMC free article] [PubMed] [Google Scholar]
- Cserr H. Potassium exchange between cerebrospinal fluid, plasma, and brain. Am J Physiol. 1965 Dec;209(6):1219–1226. doi: 10.1152/ajplegacy.1965.209.6.1219. [DOI] [PubMed] [Google Scholar]
- DAVSON H., POLLAY M. The turnover of 24Na in the cerebrospinal fluid and its bearing on the blood-brain barrier. J Physiol. 1963 Jul;167:247–255. doi: 10.1113/jphysiol.1963.sp007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOMER F. R., WHITCOMB M. STUDIES OF K42 MOVEMENT BETWEEN THE BLOOD AND THE CEREBROSPINAL FLUID OF CATS. J Pharmacol Exp Ther. 1964 Jul;145:52–57. [PubMed] [Google Scholar]
- Dryden E. E., Manery J. F. Preparation of tissue and fluid samples for determination of tissue spaces using sorbitol and-or inulin labeled with carbon-14 or tritium. Anal Biochem. 1970 Jun;35(2):384–392. doi: 10.1016/0003-2697(70)90199-5. [DOI] [PubMed] [Google Scholar]
- EDSTROM R., STEINWALL O. The blood-brain barrier phenomenon--the relative importance of permeability and cellular transport mechanisms. Acta Neurol Scand. 1961;37:1–21. doi: 10.1111/j.1600-0404.1961.tb06049.x. [DOI] [PubMed] [Google Scholar]
- ELLIOTT K. A., PAPPIUS H. M. Water distribution in incubated slices of brain and other tissues. Can J Biochem Physiol. 1956 Sep;34(5):1007–1022. [PubMed] [Google Scholar]
- FELDBERG W., FLEISCHHAUER K. Penetration of bromophenol blue from the perfused cerebral ventricles into the brain tissue. J Physiol. 1960 Feb;150:451–462. doi: 10.1113/jphysiol.1960.sp006397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDE R. L. THE ENZYMATIC RESPONSE OF ASTROCYTES TO VARIOUS IONS IN VITRO. J Cell Biol. 1964 Jan;20:5–15. doi: 10.1083/jcb.20.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fencl V., Miller T. B., Pappenheimer J. R. Studies on the respiratory response to disturbances of acid-base balance, with deductions concerning the ionic composition of cerebral interstitial fluid. Am J Physiol. 1966 Mar;210(3):459–472. doi: 10.1152/ajplegacy.1966.210.3.459. [DOI] [PubMed] [Google Scholar]
- Ferguson R. K., Woodbury D. M. Penetration of 14C-inulin and 14C-sucrose into brain, cerebrospinal fluid, and skeletal muscle of developing rats. Exp Brain Res. 1969;7(3):181–194. doi: 10.1007/BF00239028. [DOI] [PubMed] [Google Scholar]
- Friede R. L., Hu K. H., Cechner R. Glial footplates in the bowfin. II. Effects of ouabain and selective damage to footplates on electrolyte composition, glycogen content, fine structure, and electrophysiology of bowfin brain incubated in vitro. J Neuropathol Exp Neurol. 1969 Oct;28(4):540–570. doi: 10.1097/00005072-196910000-00002. [DOI] [PubMed] [Google Scholar]
- Friede R. L., Hu K. H., Johnstone M. Glial footplates in the bowfin. I. Fine structure and chemistry. J Neuropathol Exp Neurol. 1969 Oct;28(4):513–539. doi: 10.1097/00005072-196910000-00001. [DOI] [PubMed] [Google Scholar]
- HAMBERGER A., ROECKERT H. INTRACELLULAR POTASSIUM IN ISOLATED NERVE CELLS AND GLIAL CELLS. J Neurochem. 1964 Oct;11:757–760. doi: 10.1111/j.1471-4159.1964.tb06120.x. [DOI] [PubMed] [Google Scholar]
- Heisey S. R. Brain and choroid plexus blood volumes in vertebrates. Comp Biochem Physiol. 1968 Aug;26(2):489–498. doi: 10.1016/0010-406x(68)90641-5. [DOI] [PubMed] [Google Scholar]
- Hertz L., Schousboe A., Weiss G. B. Estimation of ionic concentrations and intracellular pH in slices from different areas of rat brain. Acta Physiol Scand. 1970 Aug;79(4):506–515. doi: 10.1111/j.1748-1716.1970.tb04751.x. [DOI] [PubMed] [Google Scholar]
- Hu K. H., Friede R. L. Factors affecting glucose and glycogen content of bowfin brain in vitro. Brain Res. 1971 Jan 8;25(1):143–151. doi: 10.1016/0006-8993(71)90573-7. [DOI] [PubMed] [Google Scholar]
- Hu K. H., Friede R. L. Factors affecting glucose transfer into bowfin brain in vitro. Significance of damage to glial footplates. Brain Res. 1971 Jan 8;25(1):153–160. doi: 10.1016/0006-8993(71)90574-9. [DOI] [PubMed] [Google Scholar]
- Hu K. H., Friede R. L. Topographic determination of zinc in human brain by atomic absorption spectrophotometry. J Neurochem. 1968 Jul;15(7):677–685. doi: 10.1111/j.1471-4159.1968.tb08967.x. [DOI] [PubMed] [Google Scholar]
- KATZMAN R. Electrolyte distribution in mammalian central nervous system. Are glia high sodium cells? Neurology. 1961 Jan;11:27–36. doi: 10.1212/wnl.11.1.27. [DOI] [PubMed] [Google Scholar]
- Katzman R., Graziani L., Kaplan R., Escriva A. Exchange of cerebrospinal fluid potassium with blood and brain. Study in normal and Ouabain perfused cats. Arch Neurol. 1965 Nov;13(5):513–524. doi: 10.1001/archneur.1965.00470050061007. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G., Orkand R. K. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- Levi G. Different estimates of tissue extracellular space using [carboxyl-14C]-inulin from different sources. Anal Biochem. 1969 Nov;32(2):348–353. doi: 10.1016/0003-2697(69)90096-7. [DOI] [PubMed] [Google Scholar]
- Levi G., Lattes M. G. Changes with age in water and extracellular space in incubated brain tissue. J Neurochem. 1970 May;17(5):587–596. doi: 10.1111/j.1471-4159.1970.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Malhotra S. K., Van Harreveld A. Distribution of extracellular material in central white matter. J Anat. 1966 Jan;100(Pt 1):99–110. [PMC free article] [PubMed] [Google Scholar]
- Marlow C. G., Sheppard G. Labelled tracers of inulin for physiological measurements. Clin Chim Acta. 1970 Jun;28(3):469–478. doi: 10.1016/0009-8981(70)90075-6. [DOI] [PubMed] [Google Scholar]
- NICHOLLS J. G., KUFFLER S. W. EXTRACELLULAR SPACE AS A PATHWAY FOR EXCHANGE BETWEEN BLOOD AND NEURONS IN THE CENTRAL NERVOUS SYSTEM OF THE LEECH: IONIC COMPOSITION OF GLIAL CELLS AND NEURONS. J Neurophysiol. 1964 Jul;27:645–671. doi: 10.1152/jn.1964.27.4.645. [DOI] [PubMed] [Google Scholar]
- Nicholls J. G., Wolfe D. E. Distribution of 14C-labeled sucrose, inulin, and dextran in extracellular spaces and in cells of the leech central nervous system. J Neurophysiol. 1967 Nov;30(6):1574–1592. doi: 10.1152/jn.1967.30.6.1574. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H., Davson H. Brain extracellular space and the sink action of cerebrospinal fluid. Measurement of rabbit brain extracellular space using sucrose labeled with carbon 14. Arch Neurol. 1967 Aug;17(2):196–205. doi: 10.1001/archneur.1967.00470260086010. [DOI] [PubMed] [Google Scholar]
- Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- PAPPIUS H. M., KLATZO I., ELLIOTT K. A. Further studies on swelling of brain slices. Can J Biochem Physiol. 1962 Jul;40:885–898. [PubMed] [Google Scholar]
- Rambourg A., Leblond C. P. Electron microscope observations on the carbohydrate-rich cell coat present at the surface of cells in the rat. J Cell Biol. 1967 Jan;32(1):27–53. doi: 10.1083/jcb.32.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANHARREVELD A., CROWELL J., MALHOTRA S. K. A STUDY OF EXTRACELLULAR SPACE IN CENTRAL NERVOUS TISSUE BY FREEZE-SUBSTITUTION. J Cell Biol. 1965 Apr;25:117–137. doi: 10.1083/jcb.25.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARON S., McILWAIN H. Fluid content and compartments in isolated cerebral tissues. J Neurochem. 1961 Dec;8:262–275. doi: 10.1111/j.1471-4159.1961.tb13552.x. [DOI] [PubMed] [Google Scholar]
- VERNADAKIS A., WOODBURY D. M. CELLULAR AND EXTRACELLULAR SPACES IN DEVELOPING RAT BRAIN. RADIOACTIVE UPTAKE STUDIES WITH CHLORIDE AND INULIN. Arch Neurol. 1965 Mar;12:284–293. doi: 10.1001/archneur.1965.00460270060008. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A., Collewijn H., Malhotra S. K. Water, electrolytes, and extracellular space in hydrated and dehydrated brains. Am J Physiol. 1966 Feb;210(2):251–256. doi: 10.1152/ajplegacy.1966.210.2.251. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A., Malhotra S. K. Extracellular space in the cerebral cortex of the mouse. J Anat. 1967 Apr;101(Pt 2):197–207. [PMC free article] [PubMed] [Google Scholar]
- Woodward D. L., Reed D. J., Woodbury D. M. Extracellular space of rat cerebral cortex. Am J Physiol. 1967 Feb;212(2):367–370. doi: 10.1152/ajplegacy.1967.212.2.367. [DOI] [PubMed] [Google Scholar]
- Zadunaisky J. A., Curran P. F. Sodium fluxes in isolated frog brain. Am J Physiol. 1963 Nov;205(5):949–956. doi: 10.1152/ajplegacy.1963.205.5.949. [DOI] [PubMed] [Google Scholar]


