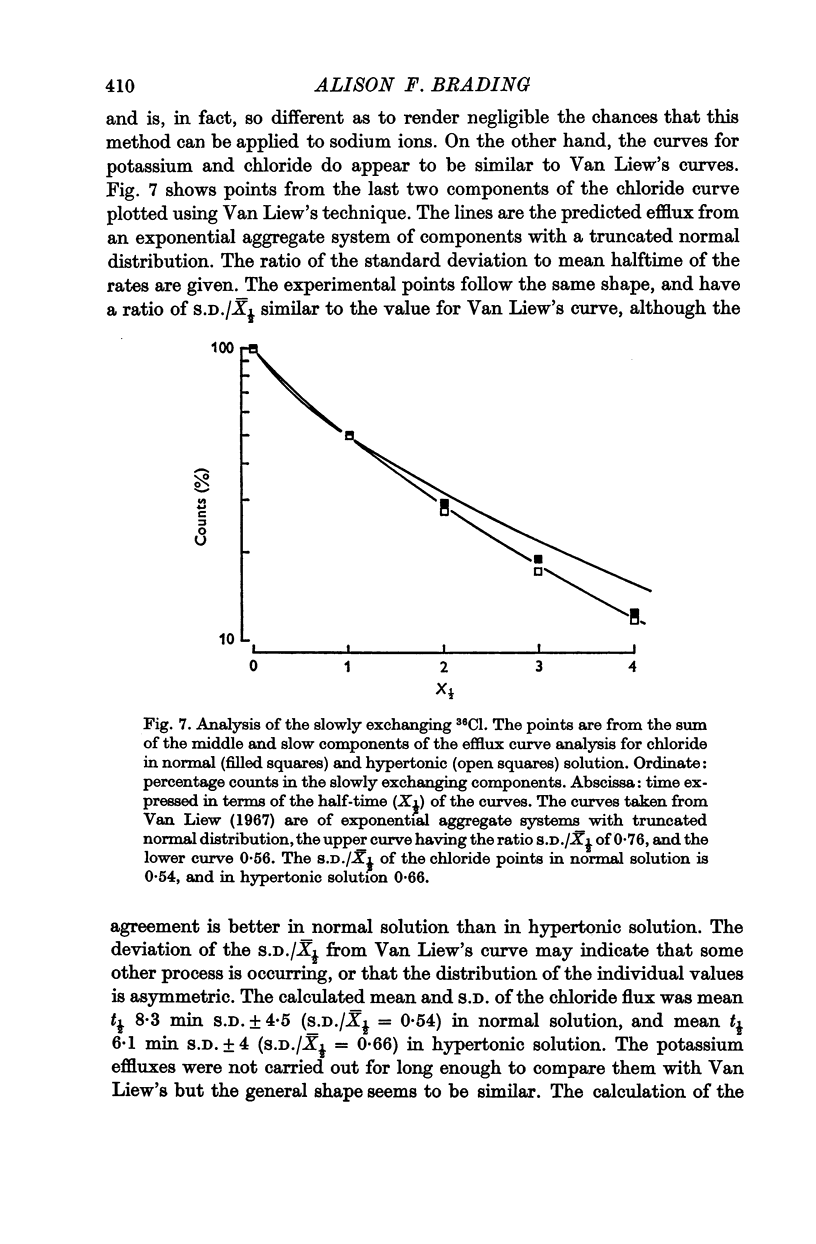
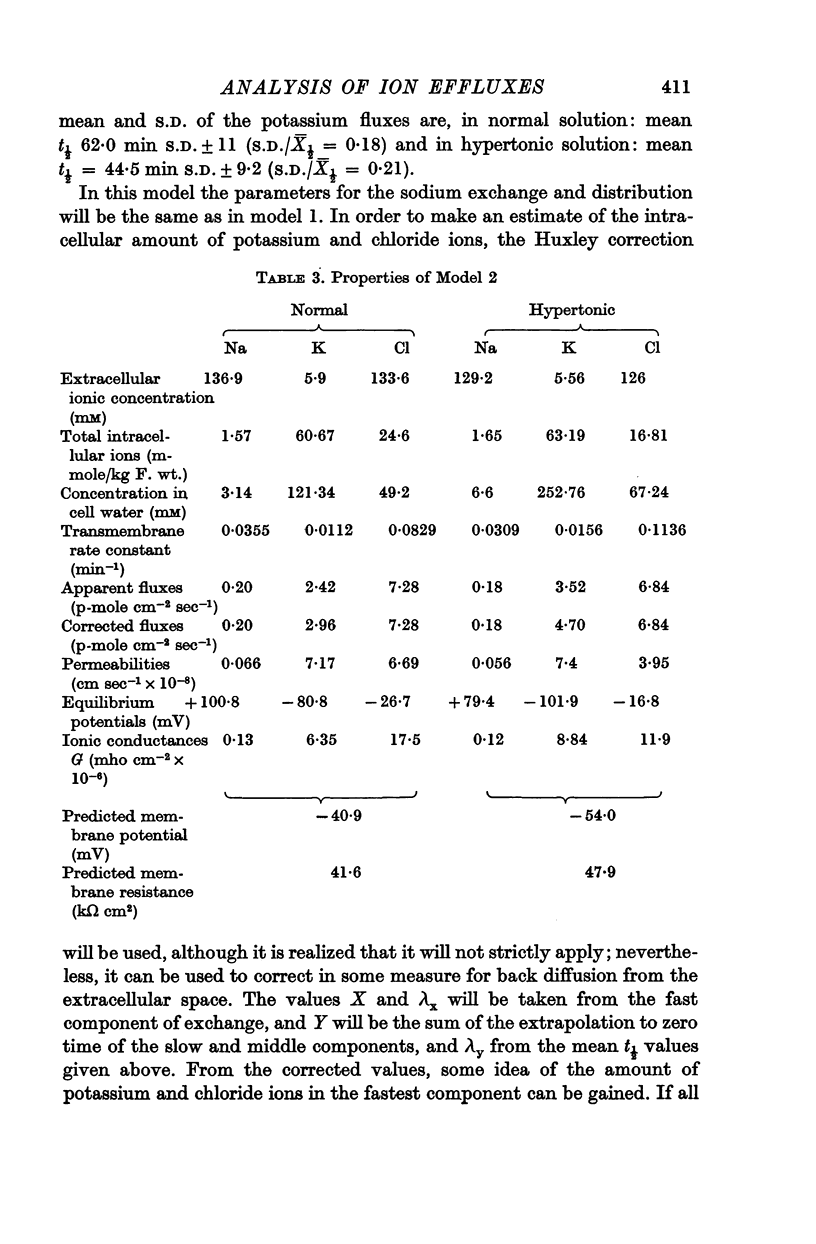
Abstract
1. Efflux curves of 24Na, 42K and 36Cl from the guinea-pig taenia coli were obtained in normal Krebs solution, and in hypertonic Krebs solution in which the osmolarity had been doubled by the addition of sucrose.
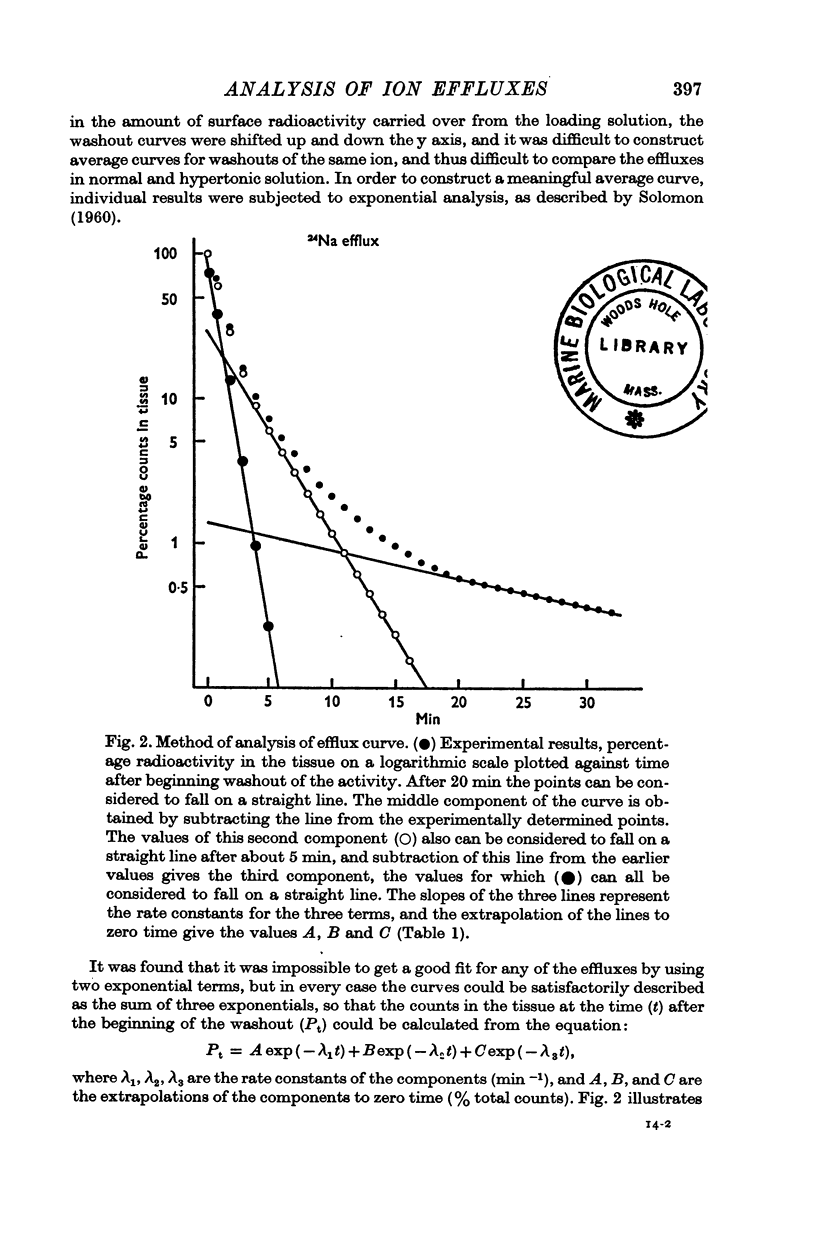
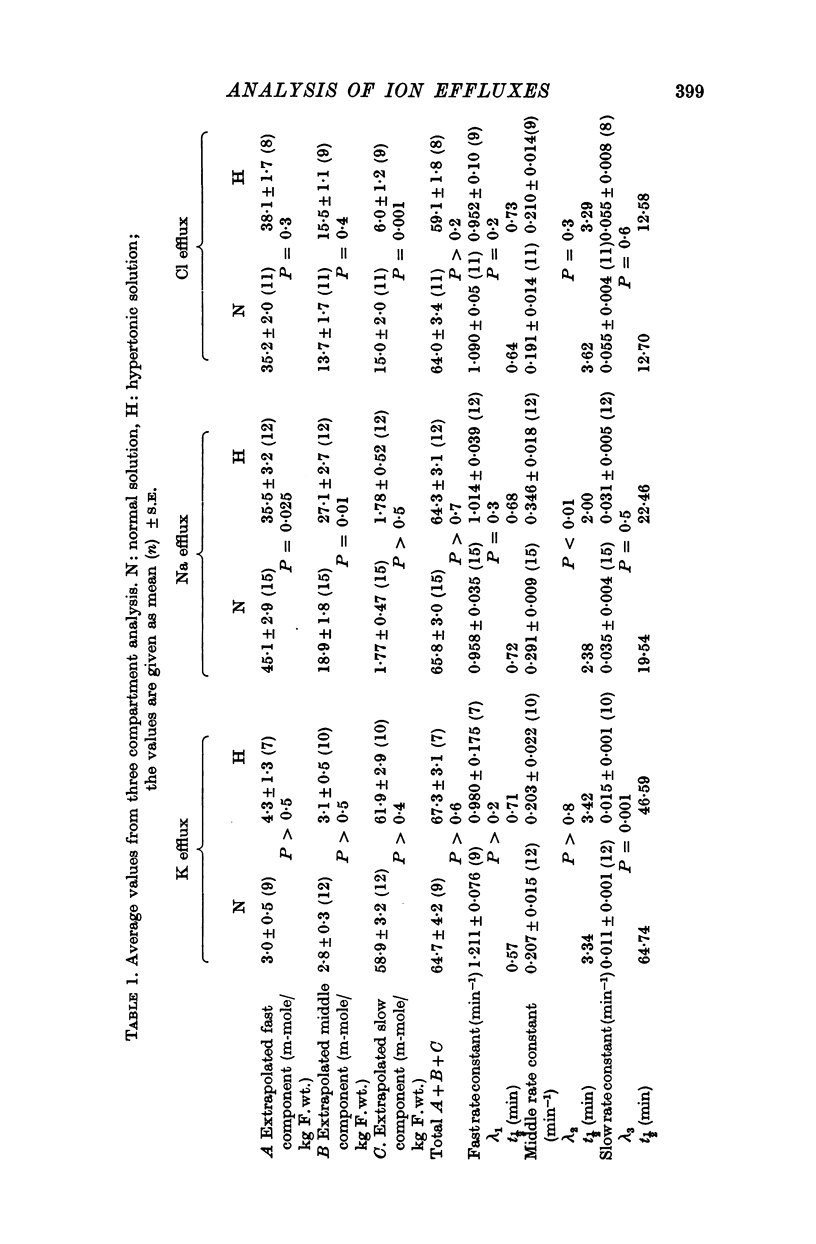
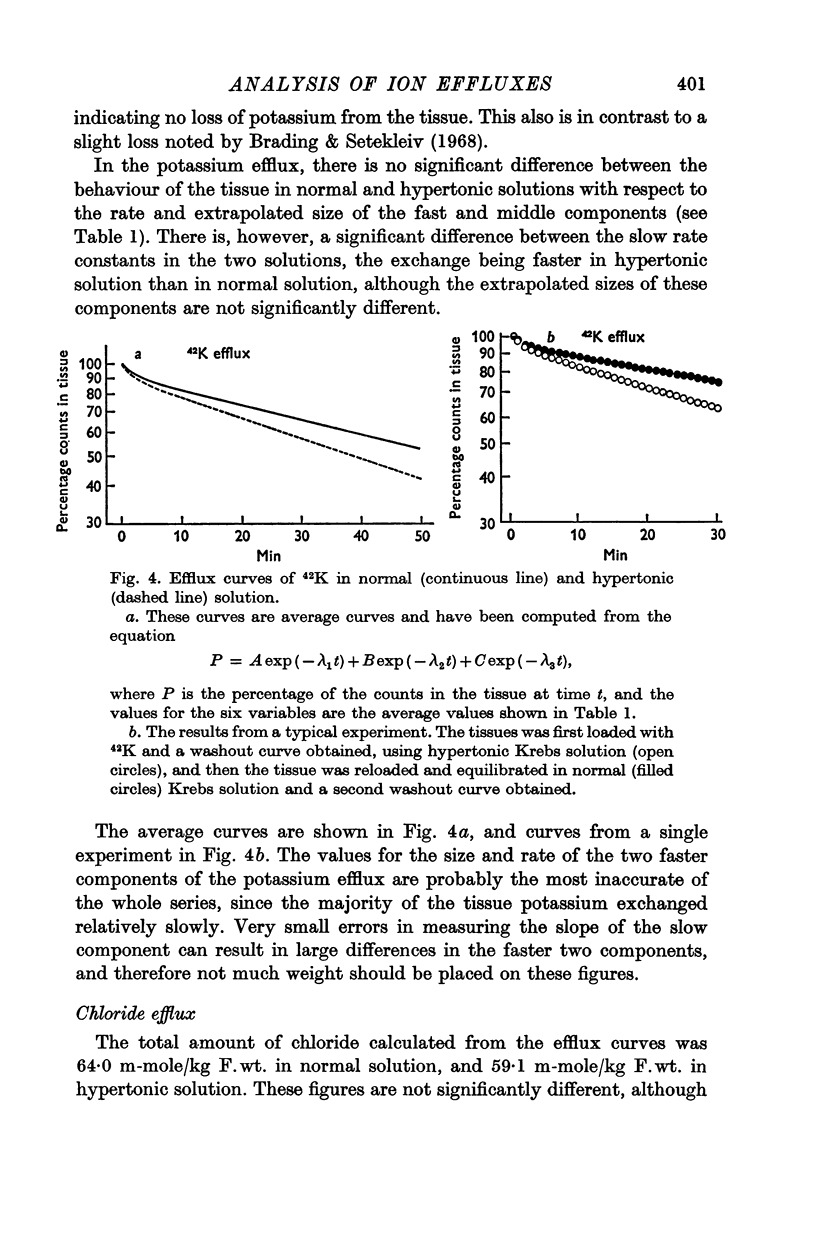
2. The efflux curves were complex, and in order to get average curves each was analysed as the sum of three exponential terms, and average curves were constructed from the means of the constants found.
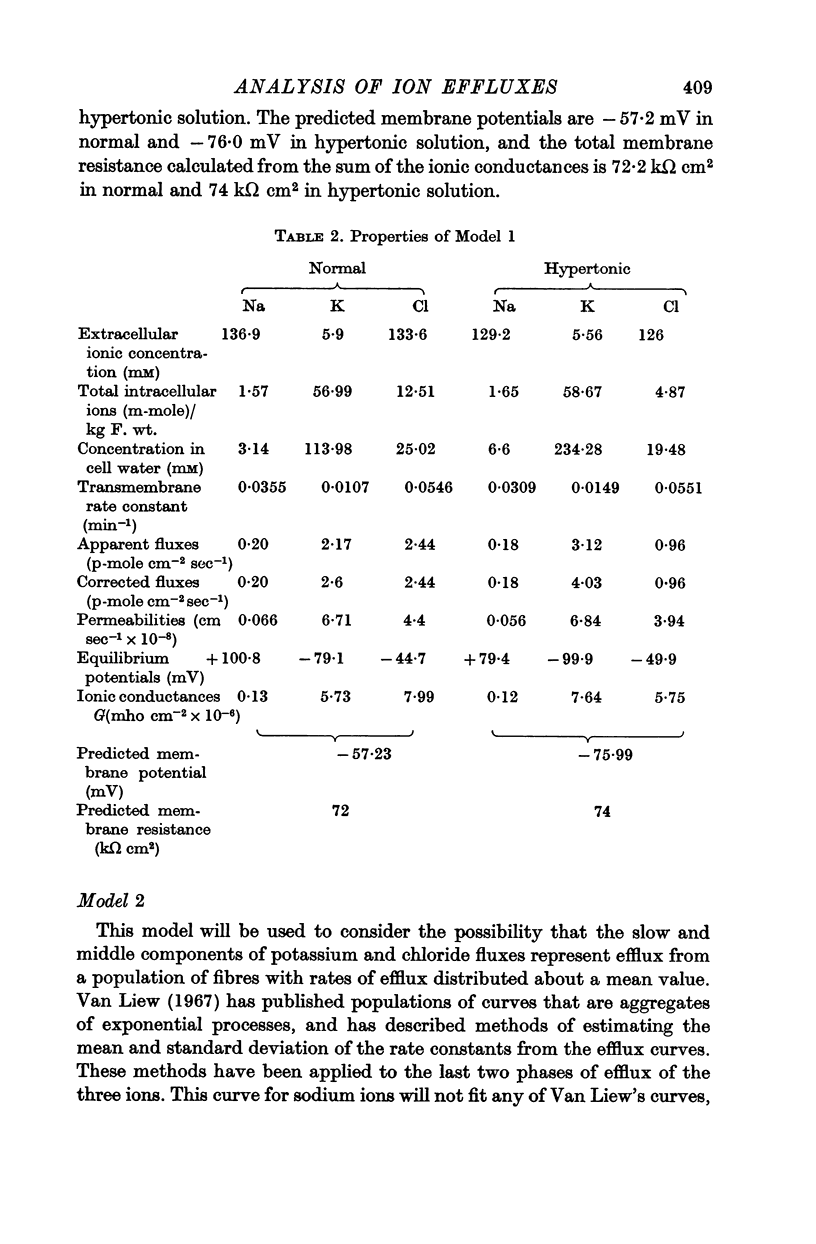
3. In order to estimate the membrane permeability to the ions, it was necessary to make assumptions as to the distribution of ions in the tissue. Several models have been examined and the predictions of the models with respect to the membrane properties were compared with the data obtained by using electrophysiological methods by other workers.
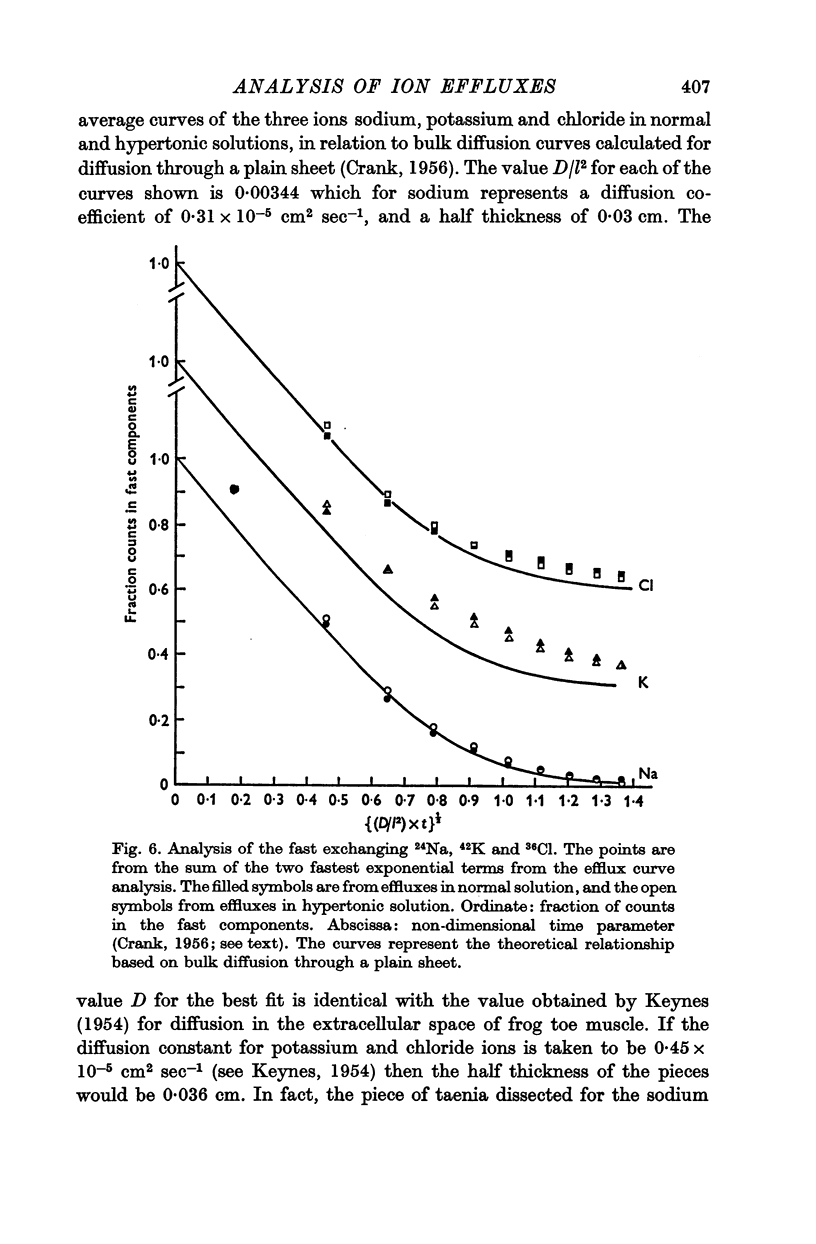
4. It was found that reasonable predictions of membrane properties could only be made using models in which the majority of the rapidly exchanging sodium is considered to be extracellular. This amount of sodium is more than can be accounted for as freely dissolved in the extracellular water.
5. A possible interpretation of the ion exchange and distribution would be to suppose that some proportion of the three ions is contained in an extracellular compartment not available to the normal extracellular markers, and limited in its exchange by the rate of diffusion in the extracellular phase, and that the truly intracellular fractions of the tissue ions do not exchange with the external solution in a simple exponential manner, but in a manner described by an aggregate of exponential terms due to inherent variation in the permeabilities of the individual cell membranes.
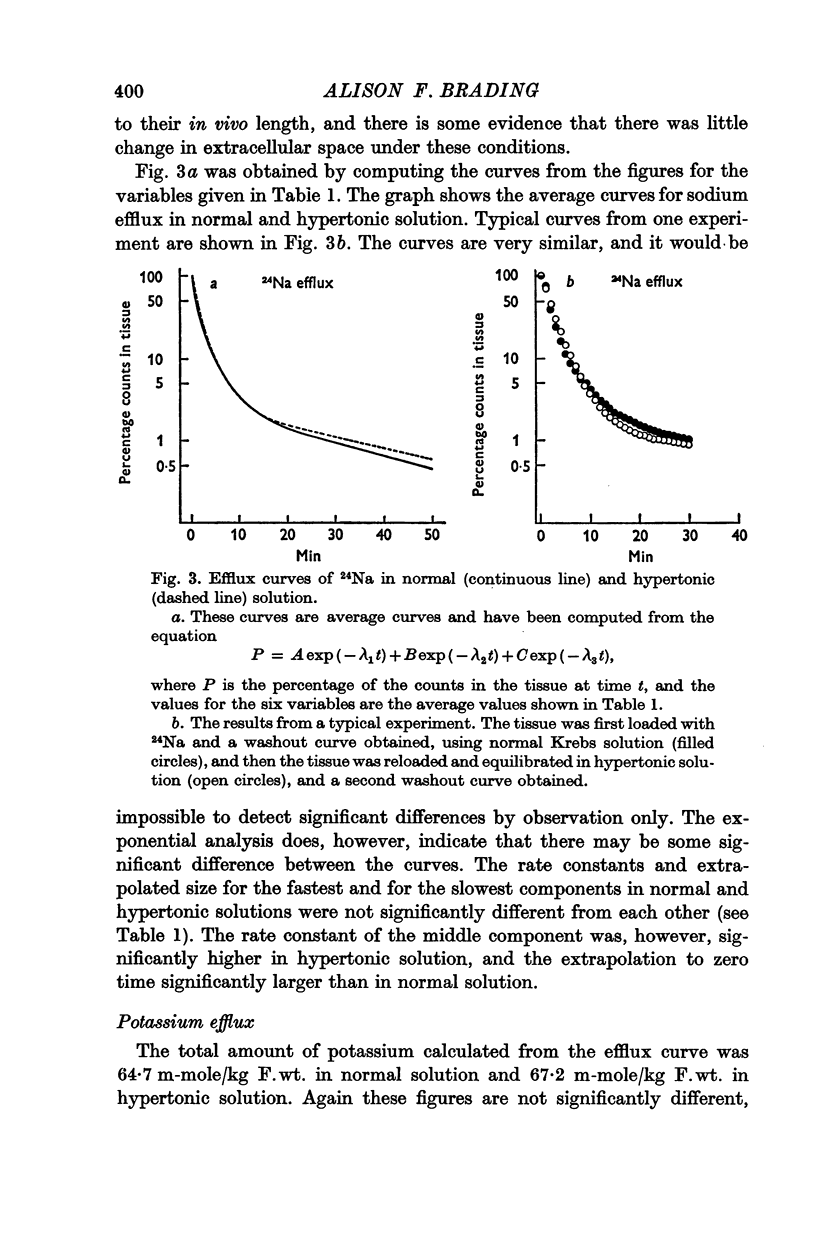
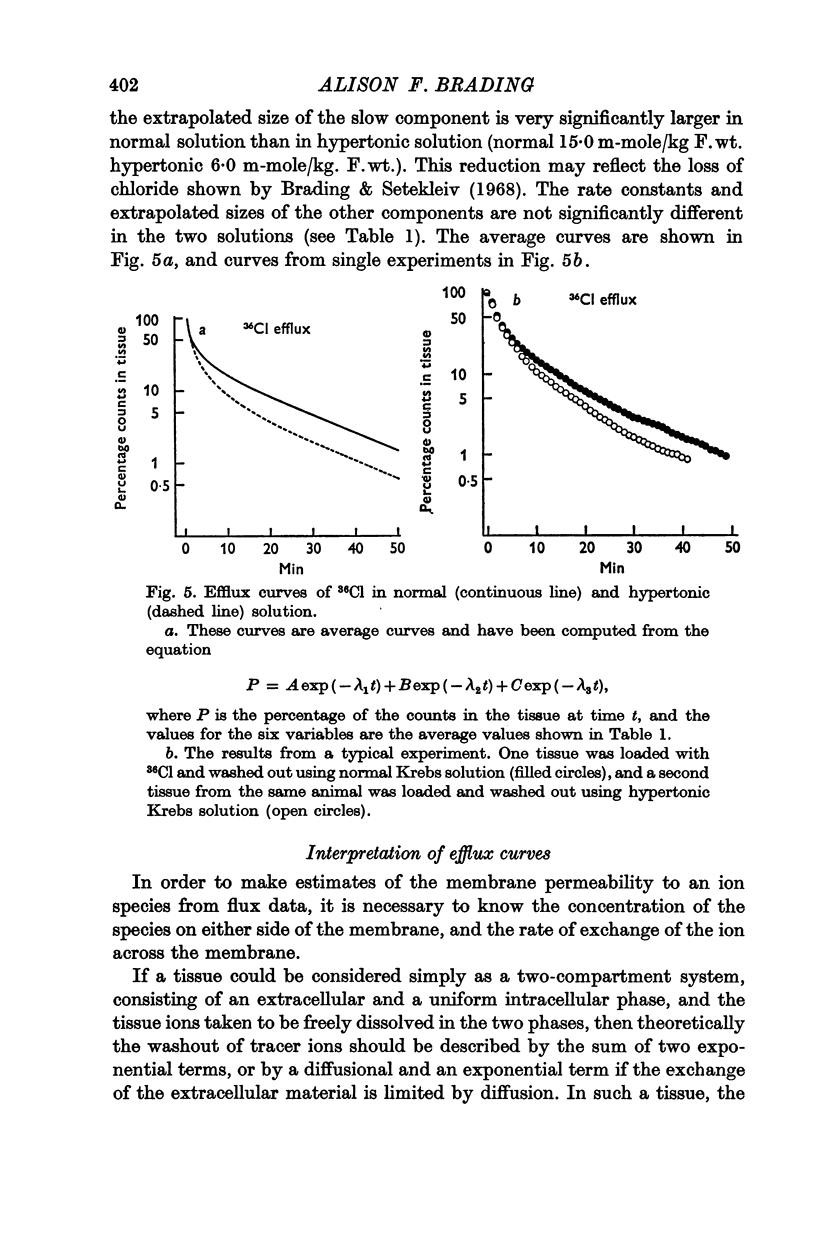
6. There is no evidence for any change in the membrane permeabilities to sodium and potassium in hypertonic solution, but there is evidence for a decrease in chloride permeability in this solution.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F. A constant flow apparatus for measuring radioactive ion effluxes from guinea-pig taenia coli. J Physiol. 1967 Sep;192(2):15P–16P. [PubMed] [Google Scholar]
- Brading A. F., Jones A. W. Distribution and kinetics of CoEDTA in smooth muscle, and its use as an extracellular marker. J Physiol. 1969 Feb;200(2):387–401. doi: 10.1113/jphysiol.1969.sp008700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Setekleiv J. The effect of hypo- and hypertonic solutions on volume and ion distribution of smooth muscle of guinea-pig taenia coli. J Physiol. 1968 Mar;195(1):107–118. doi: 10.1113/jphysiol.1968.sp008449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTEELS R., KURIYAMA H. MEMBRANE POTENTIAL AND IONIC CONTENT IN PREGNANT AND NON-PREGNANT RAT MYOMETRIUM. J Physiol. 1965 Mar;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. Calculation of the membrane potential in smooth muscle cells of the guinea-pig's taenia coli by the Goldman equation. J Physiol. 1969 Nov;205(1):193–208. doi: 10.1113/jphysiol.1969.sp008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN-NARROD M., GOODFORD P. J. Sodium and potassium content of the smooth muscle of the guinea-pig taenia coli at different temperatures and tensions. J Physiol. 1962 Oct;163:399–410. doi: 10.1113/jphysiol.1962.sp006985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFORD P. J., HERMANSEN K. Sodium and potassium movements in the unstriated muscle of the guinea-pig taenia coli. J Physiol. 1961 Oct;158:426–448. doi: 10.1113/jphysiol.1961.sp006778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodford P. J. An interaction between potassium and sodium in the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Sep;186(1):11–26. doi: 10.1113/jphysiol.1966.sp008017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The resting exchange of radioactive potassium in crab nerve. J Physiol. 1951 Mar;113(1):73–98. doi: 10.1113/jphysiol.1951.sp004557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic fluxes in frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):359–382. doi: 10.1098/rspb.1954.0030. [DOI] [PubMed] [Google Scholar]
- KLEINZELLER A., JANACEK K., KNOTKOVA A. A simple method for measuring steady-state ion compartments and rate constants of ion fluxes in tissue slices. Biochim Biophys Acta. 1962 May 7;59:239–241. doi: 10.1016/0006-3002(62)90724-2. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H. The influence of potassium, sodium and chloride on the membrane potential of the smooth muscle of taenia coli. J Physiol. 1963 Apr;166:15–28. doi: 10.1113/jphysiol.1963.sp007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Steinhardt R. A. The components of the sodium efflux in frog muscle. J Physiol. 1968 Oct;198(3):581–599. doi: 10.1113/jphysiol.1968.sp008627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipicky R. J., Bryant S. H. Sodium, potassium, and chloride fluxes in intercostal muscle from normal goats and goats with hereditary myotonia. J Gen Physiol. 1966 Sep;50(1):89–111. doi: 10.1085/jgp.50.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrical responses of smooth muscle to external stimulation in hypertonic solution. J Physiol. 1966 Mar;183(2):450–468. doi: 10.1113/jphysiol.1966.sp007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Membrane capacity and resistance of mammalian smooth muscle. J Theor Biol. 1966 Nov;12(2):216–227. doi: 10.1016/0022-5193(66)90114-7. [DOI] [PubMed] [Google Scholar]
- Van Liew H. D. Graphic analysis of aggregates of linear and exponential processes. J Theor Biol. 1967 Jul;16(1):43–53. doi: 10.1016/0022-5193(67)90052-5. [DOI] [PubMed] [Google Scholar]
- Zierler K. L. Interpretation of tracer washout curves from a population of muscle fibers. J Gen Physiol. 1966 Jan;49(3):423–431. doi: 10.1085/jgp.49.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]


