Abstract
1. The accumulation of [14C]glycine by slices of mammalian spinal cord has been measured.
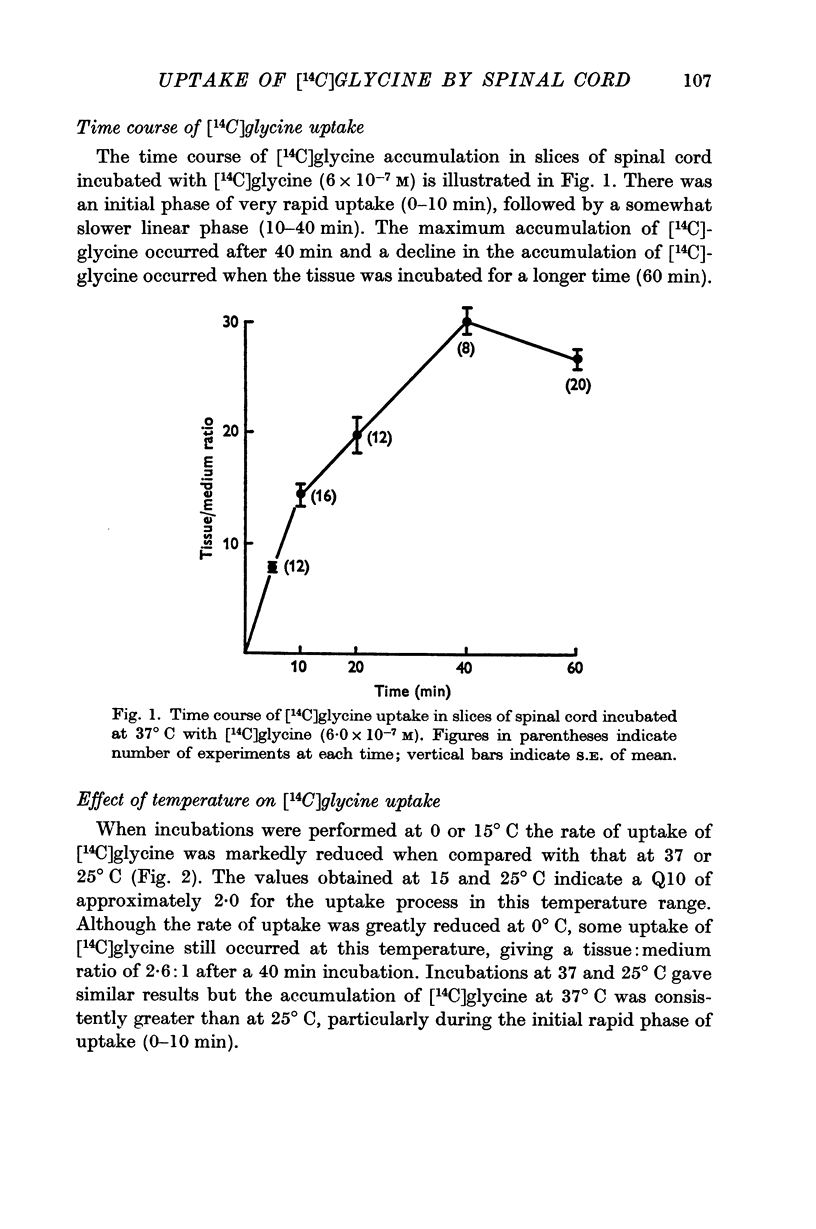
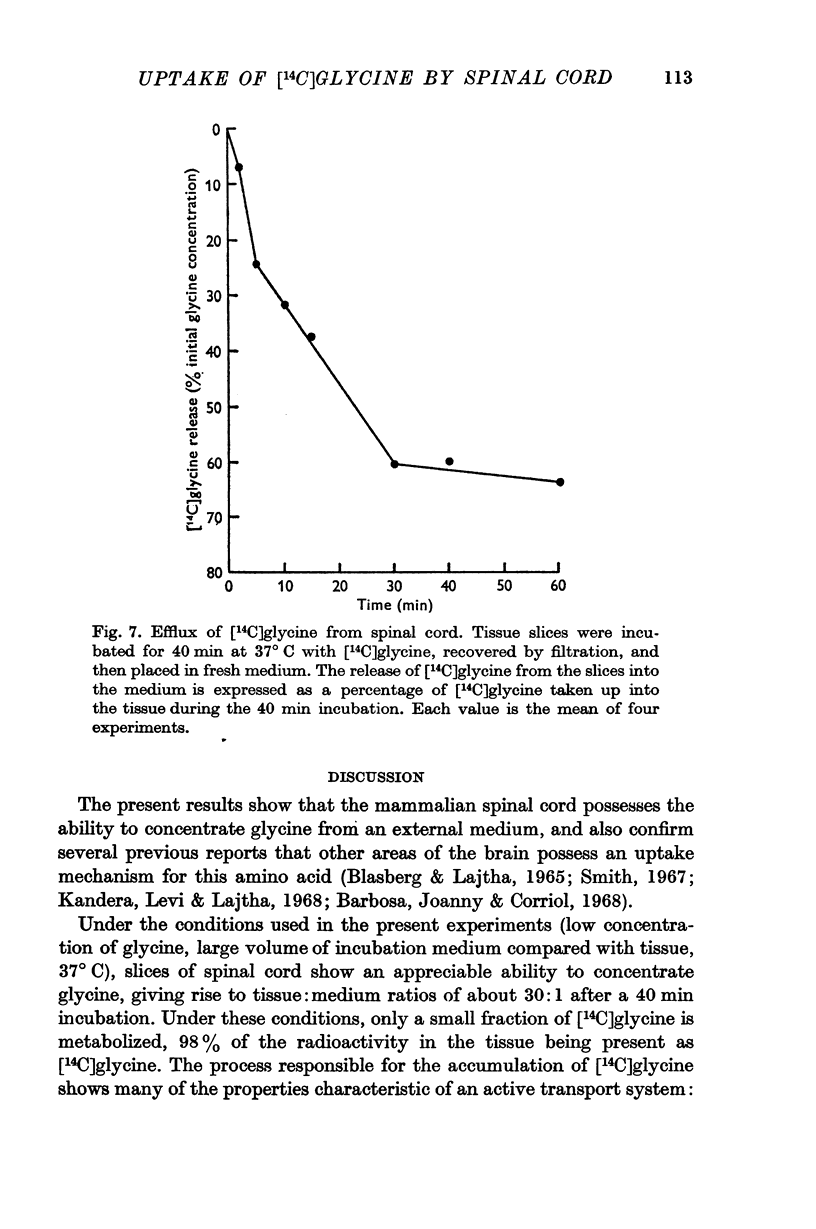
2. When slices of rat cord were incubated at 37° C in a medium containing [14C]glycine, tissue:medium ratios of about 30:1 were attained after a 40 min incubation.
3. After incubations at 37° C for 40 min, almost all (98%) the radioactivity in the tissue was present as unchanged [14C]glycine.
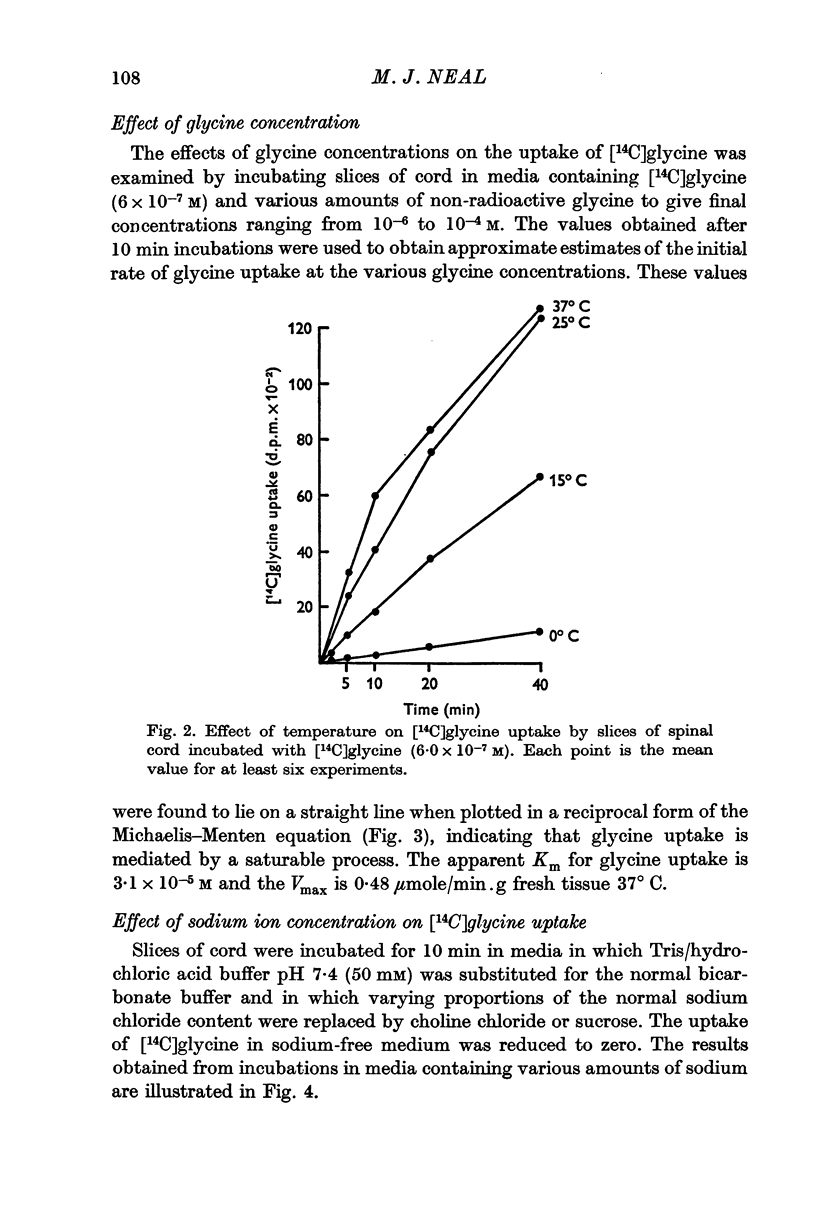
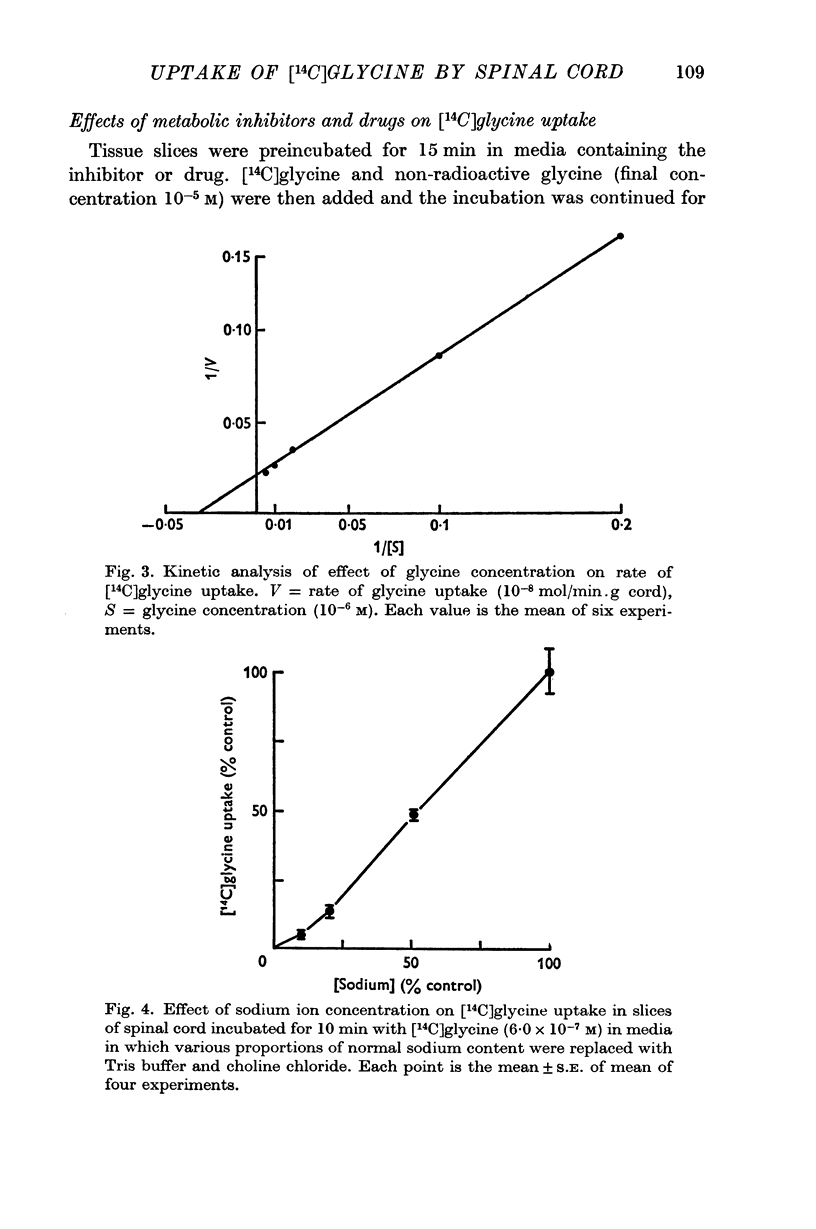
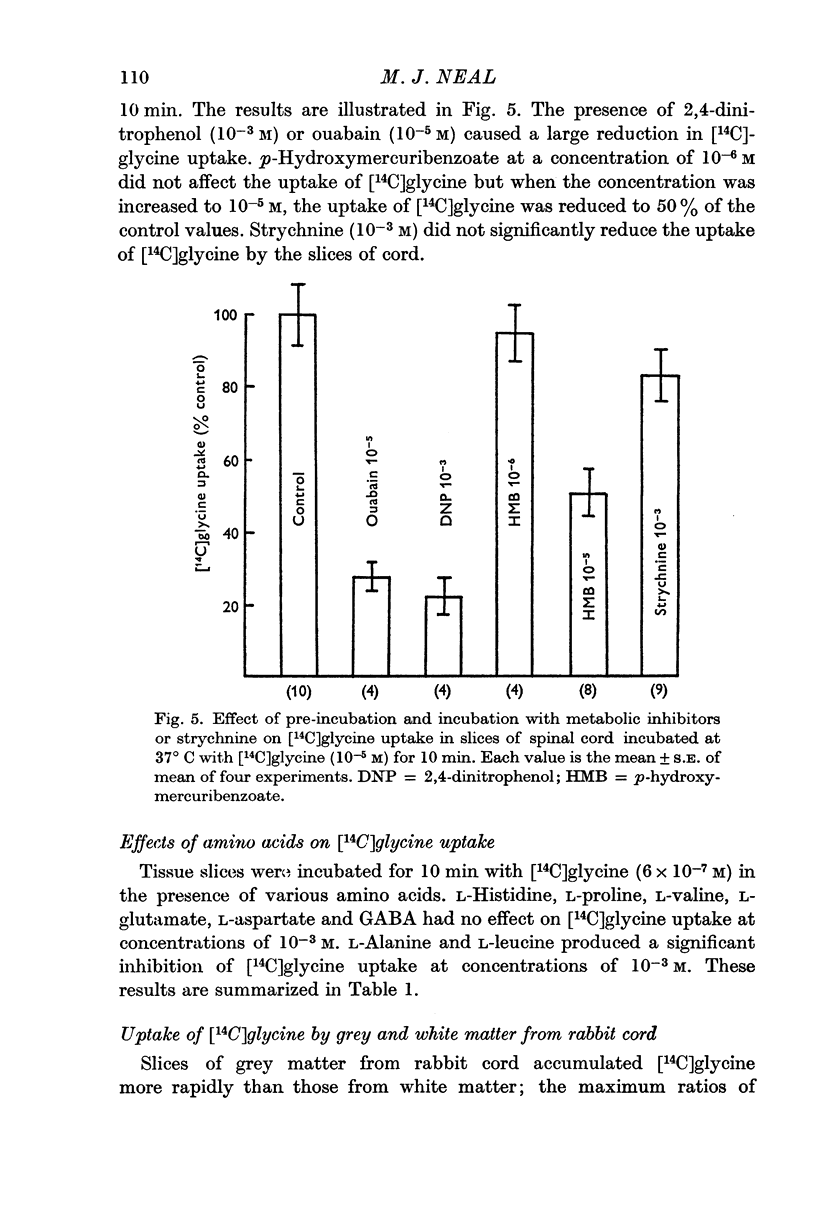
4. The process responsible for [14C]glycine uptake showed many of the properties of an active transport system: it was temperature sensitive, required the presence of sodium ions in the external medium, was inhibited by dinitrophenol and ouabain and showed saturation kinetics.
5. The estimated Km value of glycine was 3·1 × 10-5 M, and Vmax was 0·48 μ-mole/min.g cord.
6. The uptake of [14C]glycine was not affected by the presence of large molar excesses of L-histidine, L-proline, L-aspartate, L-glutamate, L-valine or GABA, but was inhibited in the presence of L-alanine and L-leucine.
7. The uptake of [14C]glycine was not reduced by strychnine, but a significant reduction in uptake was produced by p-hydroxymercuribenzoate.
8. The uptake of [14C]glycine by the grey matter of rabbit spinal cord was 2 to 6 times greater than the uptake by slices of white matter incubated under the same conditions.
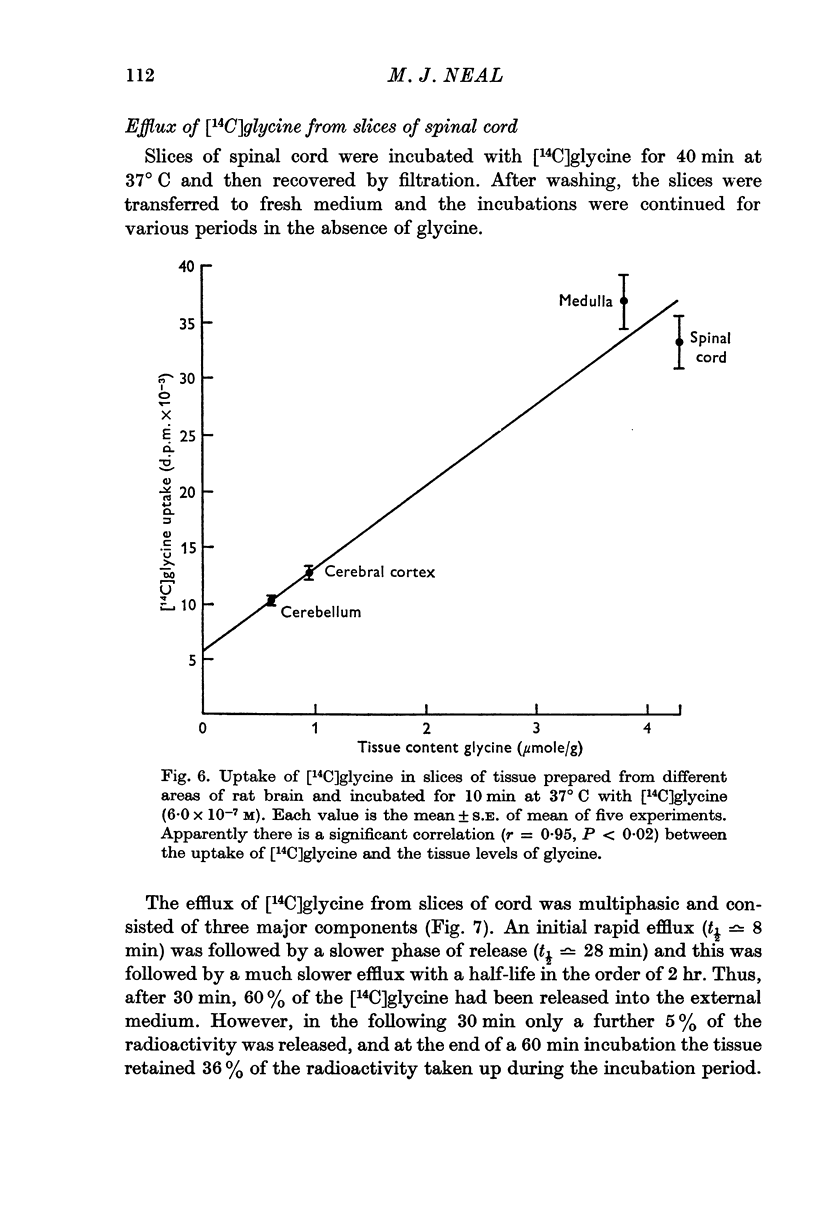
9. Rat cerebral cortex, cerebellar cortex and medulla also accumulated [14C]glycine, and the uptake by the tissue slices in vitro appeared to parallel the concentration of glycine in these areas in vivo.
10. It is suggested that the glycine uptake system may represent a possible mechanism for the inactivation of glycine at inhibitory synapses in the spinal cord.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aprison M. H., Werman R. The distribution of glycine in cat spinal cord and roots. Life Sci. 1965 Nov;4(21):2075–2083. doi: 10.1016/0024-3205(65)90325-5. [DOI] [PubMed] [Google Scholar]
- Barbosa D., Joanny P., Corriol J. Uptake of some amino acids by rat brain slices: effect of various substrates. Experientia. 1968 Dec 15;24(12):1196–1197. doi: 10.1007/BF02146616. [DOI] [PubMed] [Google Scholar]
- Battistin L., Grynbaum A., Lajtha A. Distribution and uptake of amino acids in various regions of the cat brain in vitro. J Neurochem. 1969 Oct;16(10):1459–1468. doi: 10.1111/j.1471-4159.1969.tb09898.x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Johnston G. A. The inactivation of extracellularly administered amino acids in the feline spinal cord. Exp Brain Res. 1970 Jun 25;10(5):447–462. doi: 10.1007/BF00234262. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A. A pharmacological study of the depression of spinal neurones by glycine and related amino acids. Exp Brain Res. 1968;6(1):1–18. doi: 10.1007/BF00235443. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A. Inhibition of spinal neurons by glycine. Nature. 1967 Sep 30;215(5109):1502–1503. doi: 10.1038/2151502a0. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A., Johnston I. H. The hyperpolarization of spinal motoneurones by glycine and related amino acids. Exp Brain Res. 1968;5(3):235–258. doi: 10.1007/BF00238666. [DOI] [PubMed] [Google Scholar]
- Dale H. H., Feldberg W. The chemical transmitter of vagus effects to the stomach. J Physiol. 1934 Jun 9;81(3):320–334. doi: 10.1113/jphysiol.1934.sp003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale H. H., Feldberg W., Vogt M. Release of acetylcholine at voluntary motor nerve endings. J Physiol. 1936 May 4;86(4):353–380. doi: 10.1113/jphysiol.1936.sp003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff R. A., Aprison M. H., Werman R. The effects of strychnine on the inhibition of interneurons by glycine and gamma-aminobutyric acid. Int J Neuropharmacol. 1969 Mar;8(2):191–194. doi: 10.1016/0028-3908(69)90013-6. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A., Shank R. P., Graham L. T., Jr, Aprison M. H., Werman R. Association of glycine with spinal interneurones. Nature. 1967 May 13;214(5089):680–681. doi: 10.1038/214680a0. [DOI] [PubMed] [Google Scholar]
- Graham L. T., Jr, Shank R. P., Werman R., Aprison M. H. Distribution of some synaptic transmitter suspects in cat spinal cord: glutamic acid, aspartic acid, gamma-aminobutyric acid, glycine and glutamine. J Neurochem. 1967 Apr;14(4):465–472. doi: 10.1111/j.1471-4159.1967.tb09545.x. [DOI] [PubMed] [Google Scholar]
- Heinz E. Transport through biological membranes. Annu Rev Physiol. 1967;29:21–58. doi: 10.1146/annurev.ph.29.030167.000321. [DOI] [PubMed] [Google Scholar]
- Hopkin J. M., Neal M. J. Thr release of 14C-glycine from electrically stimulated rat spinal cord slices. Br J Pharmacol. 1970 Sep;40(1):136P–138P. [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Neal M. J. The uptake of [3H]GABA by slices of rat cerebral cortex. J Neurochem. 1968 Oct;15(10):1141–1149. doi: 10.1111/j.1471-4159.1968.tb06831.x. [DOI] [PubMed] [Google Scholar]
- Kandera J., Levi G., Lajtha A. Control of cerebral metabolite levels. II. Amino acid uptake and levels in various areas of the rat brain. Arch Biochem Biophys. 1968 Jul;126(1):249–260. doi: 10.1016/0003-9861(68)90581-x. [DOI] [PubMed] [Google Scholar]
- MCILWAIN H., BUDDLE H. L. Techniques in tissue metabolism. I. A mechanical chopper. Biochem J. 1953 Feb;53(3):412–420. doi: 10.1042/bj0530412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. F., Neal M. J., Srinivasan V. The release of amino-acids from electrically stimulated rat cerebral cortex slices. Br J Pharmacol. 1969 May;36(1):201P–202P. [PMC free article] [PubMed] [Google Scholar]
- Neal M. J., Pickles H. G. Uptake of 14C glycine by spinal cord. Nature. 1969 May 17;222(5194):679–680. doi: 10.1038/222679a0. [DOI] [PubMed] [Google Scholar]
- Neal M. J. Uptake of [14C]-glycine by rat spinal cord. Br J Pharmacol. 1969 May;36(1):205P–206P. [PMC free article] [PubMed] [Google Scholar]
- Shank R. P., Aprison M. H. The metabolism in vivo of glycine and serine in eight areas of the rat central nervous system. J Neurochem. 1970 Oct;17(10):1461–1475. doi: 10.1111/j.1471-4159.1970.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Smith S. E. Kinetics of neutral amino acid transport in rat brain in vitro. J Neurochem. 1967 Mar;14(3):291–300. doi: 10.1111/j.1471-4159.1967.tb09526.x. [DOI] [PubMed] [Google Scholar]
- Srinivasan V., Neal M. J., Mitchell J. F. The effect of electrical stimulation and high potassium concentrations on the efflux of (3H) gamma-aminobutyric acid from brain slices. J Neurochem. 1969 Aug;16(8):1235–1244. doi: 10.1111/j.1471-4159.1969.tb05971.x. [DOI] [PubMed] [Google Scholar]
- Ten Bruggencate G., Engberg I. Analysis of glycine actions on spinal interneurones by intracellular recording. Brain Res. 1968 Nov;11(2):446–450. doi: 10.1016/0006-8993(68)90037-1. [DOI] [PubMed] [Google Scholar]
- Werman R., Davidoff R. A., Aprison M. H. Inhibition of motoneurones by iontophoresis of glycine. Nature. 1967 May 13;214(5089):681–683. doi: 10.1038/214681a0. [DOI] [PubMed] [Google Scholar]
- Werman R., Davidoff R. A., Aprison M. H. Inhibitory of glycine on spinal neurons in the cat. J Neurophysiol. 1968 Jan;31(1):81–95. doi: 10.1152/jn.1968.31.1.81. [DOI] [PubMed] [Google Scholar]


