Abstract
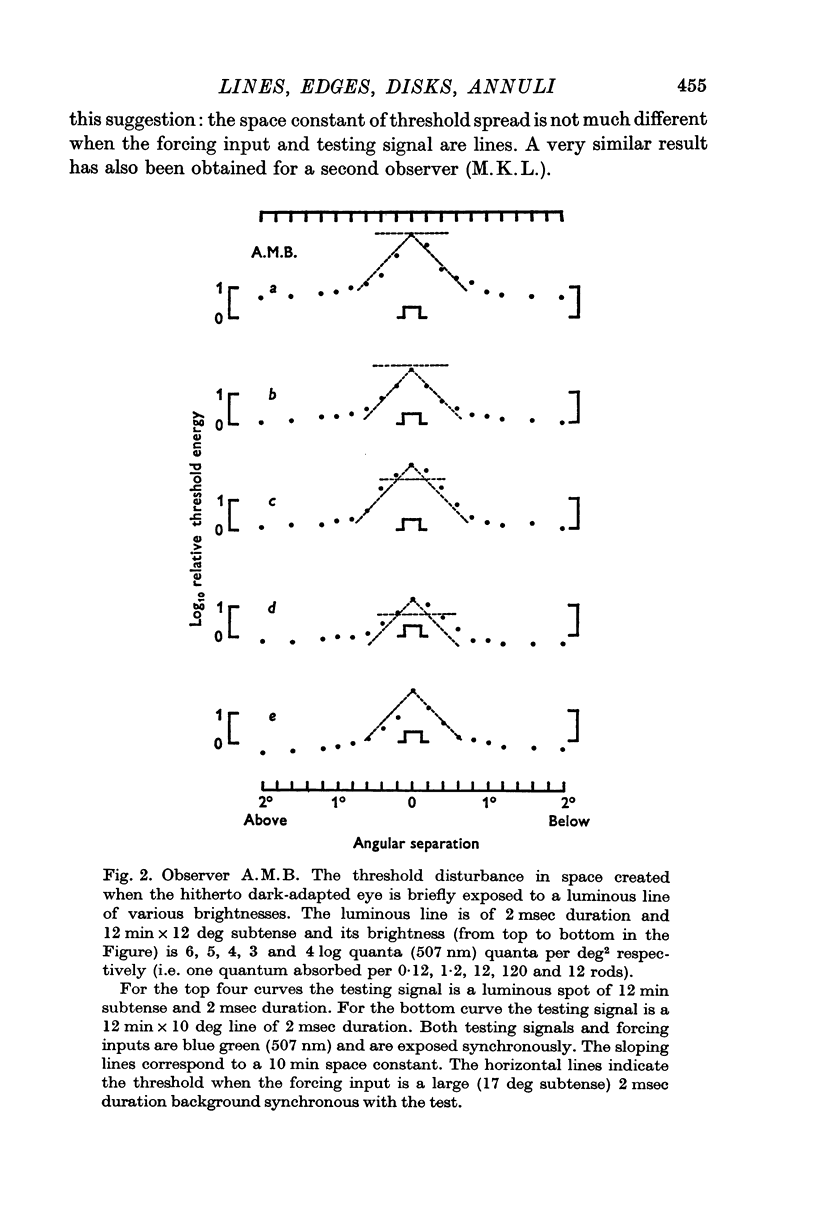
1. When the dark-adapted eye is exposed to a brief duration (2 msec) luminous line the resulting threshold disturbance is much sharper (decay constant of ca. 10 min arc) than would be expected in a system which is known to integrate the effects of light quanta over a distance of 1 deg or so.
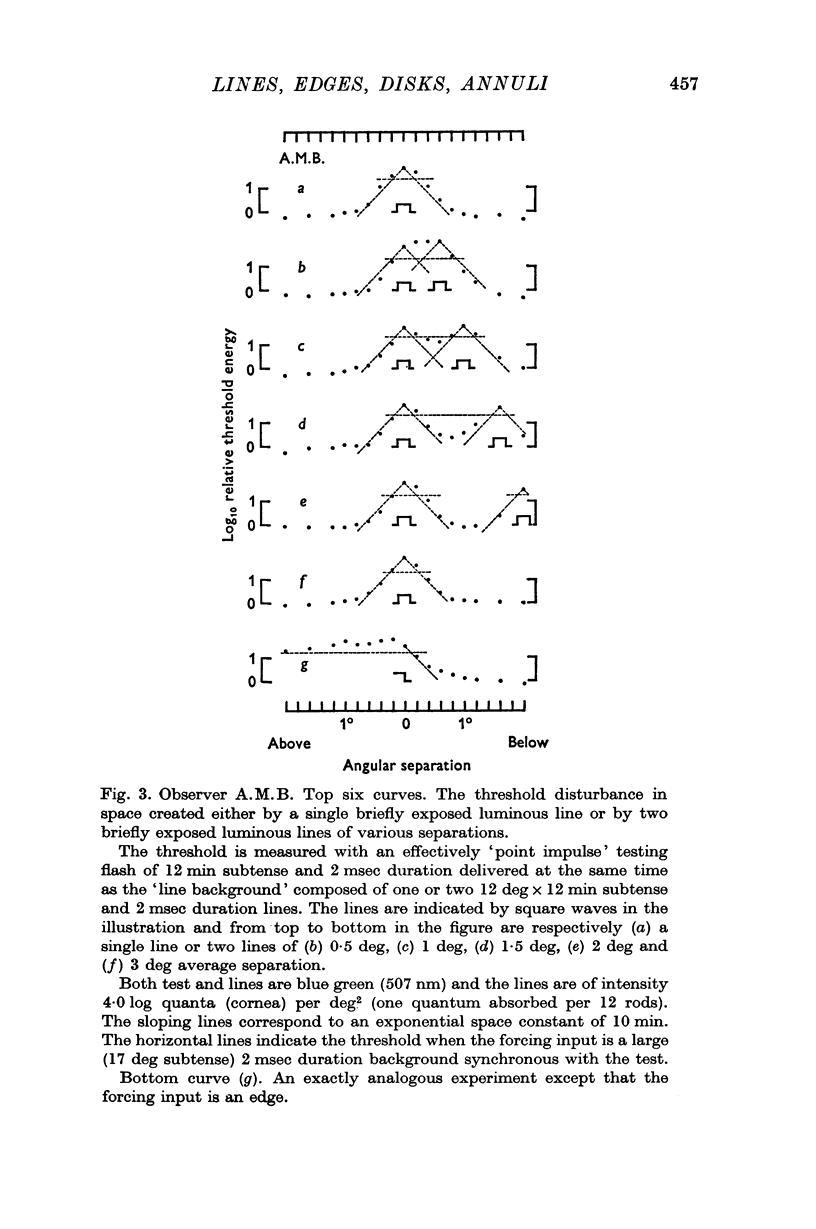
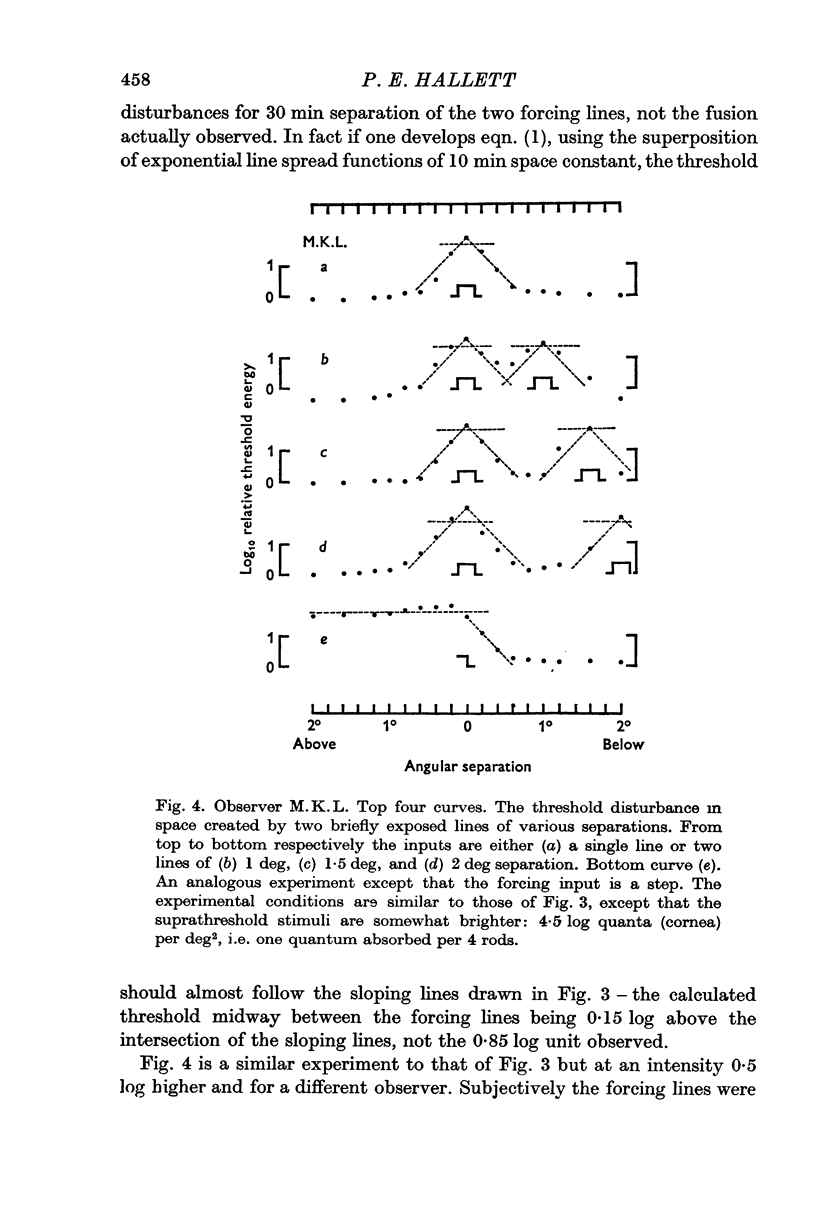
2. When the forcing input is a pair of brief duration parallel luminous lines the threshold disturbance falls off sharply at the outsides of the pattern but on the inside a considerable spread of threshold-raising effects may occur unless the lines are sufficiently far apart.
3. The threshold disturbance due to a briefly exposed edge shows an overshoot reminiscent of `lateral inhibition'.
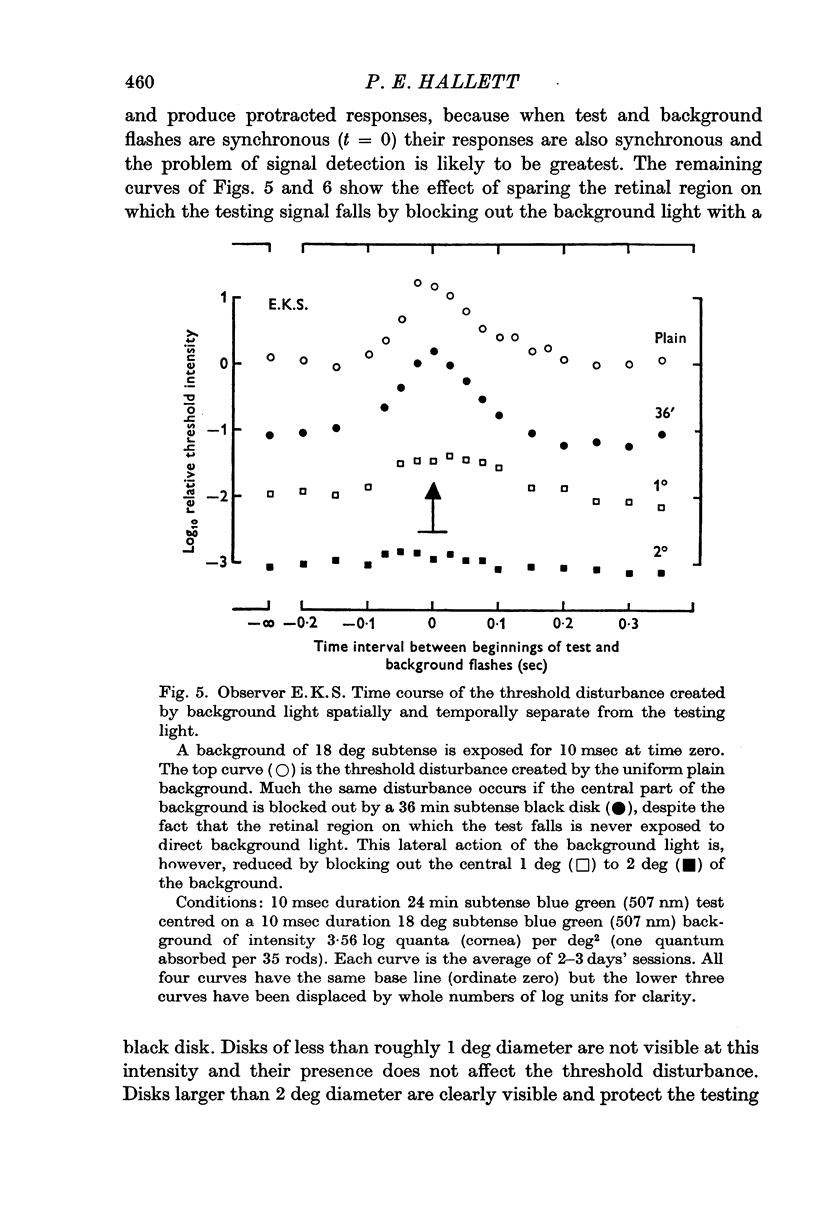
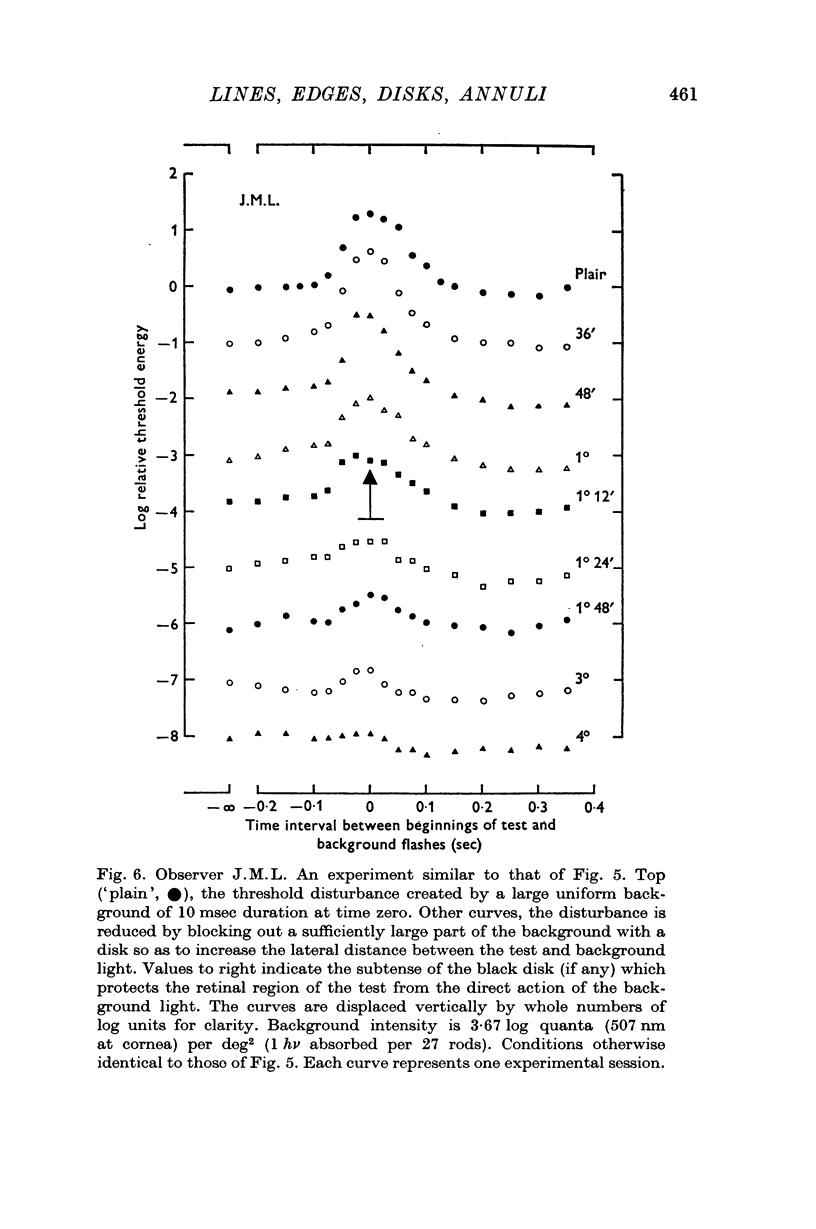
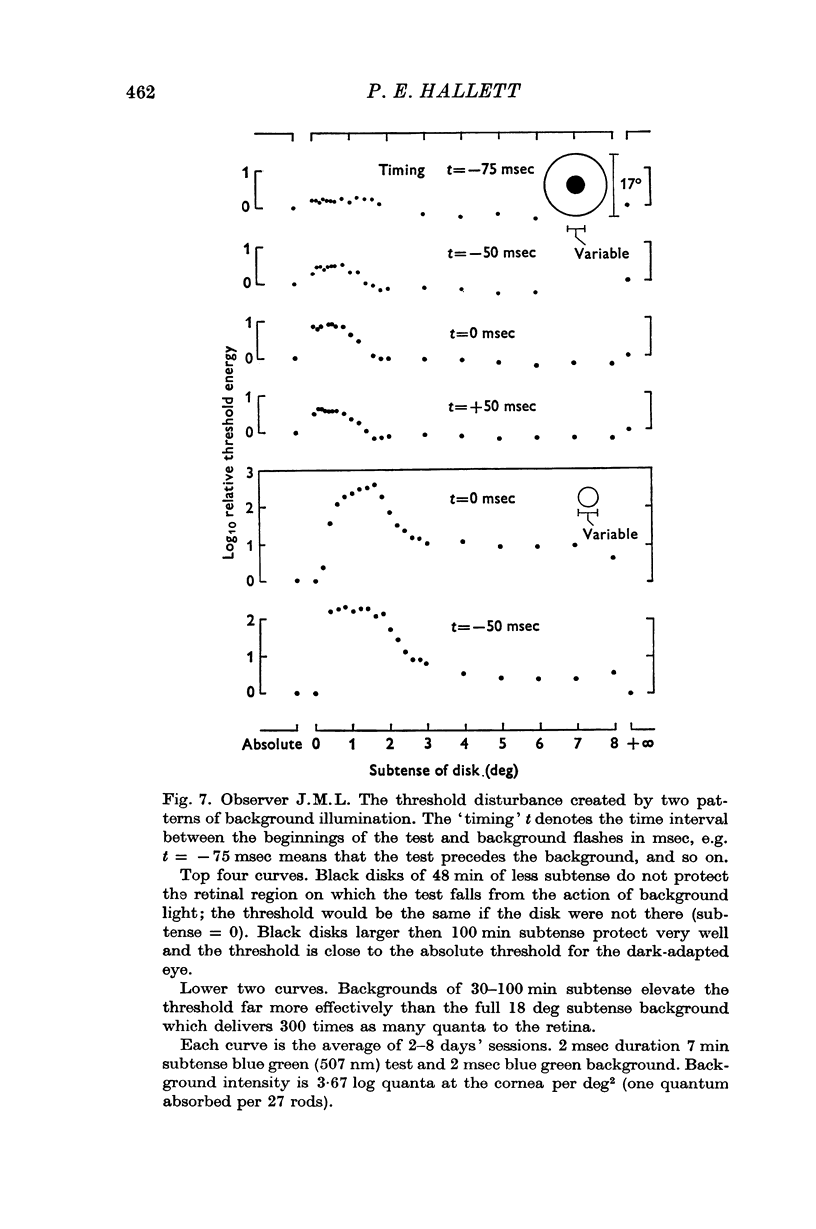
4. If the threshold is measured at the centre of a black disk presented in a briefly lit surround then (a) the dependence of threshold on time interval between test and surround suggests that the threshold elevation is due to a non-optical effect which is not `metacontrast'; (b) the dependence of threshold on black disk diameter is consistent with the notion that the spatial threshold disturbance is progressively sharpened as the separation of luminous edges increases.
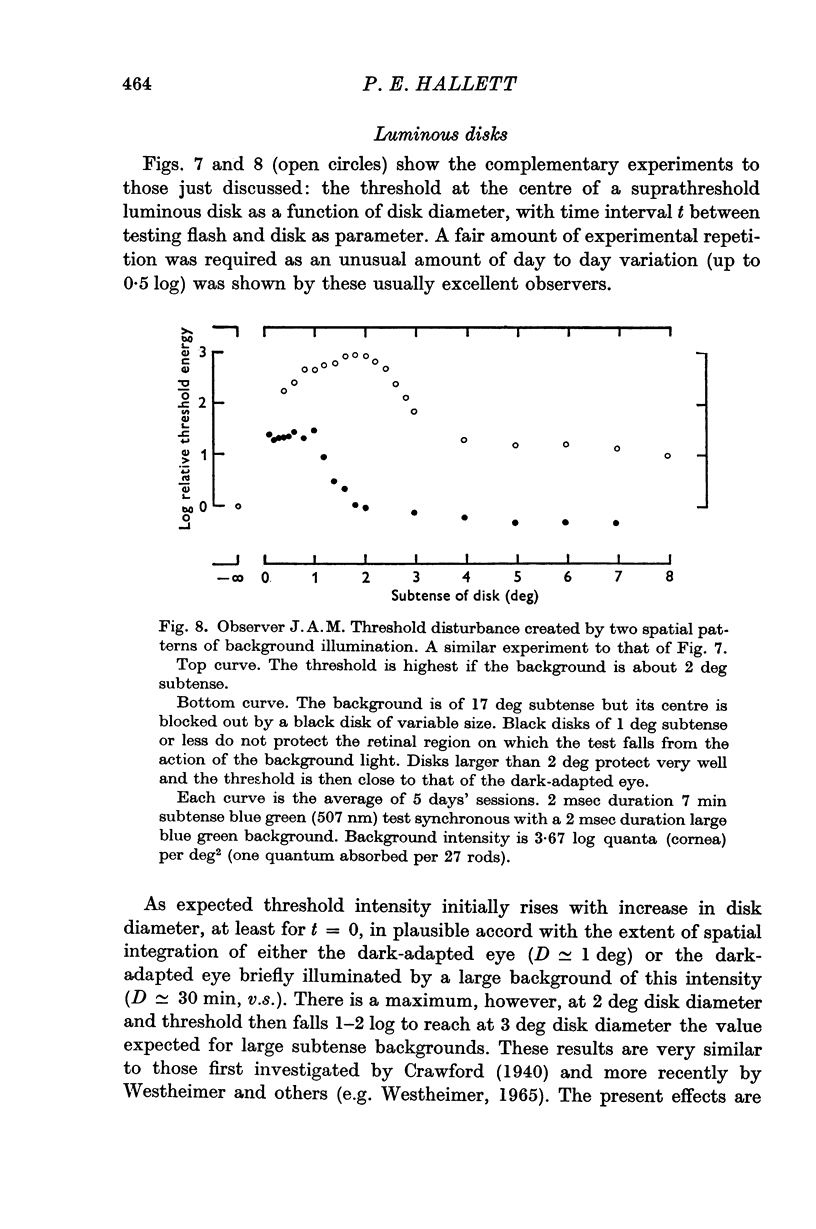
5. If the threshold is measured at the centre of briefly exposed luminous disks of various diameters one obtains the same evidence for an `antagonistic centre-surround' system as that produced by other workers (e.g. Westheimer, 1965) for the steadily light-adapted eye.
6. The previous paper (Hallett, 1971) showed that brief illumination of the otherwise dark-adapted eye can rapidly and substantially change the extent of spatial integration. The present paper shows that brief illumination leads to substantial `inhibitory' effects.
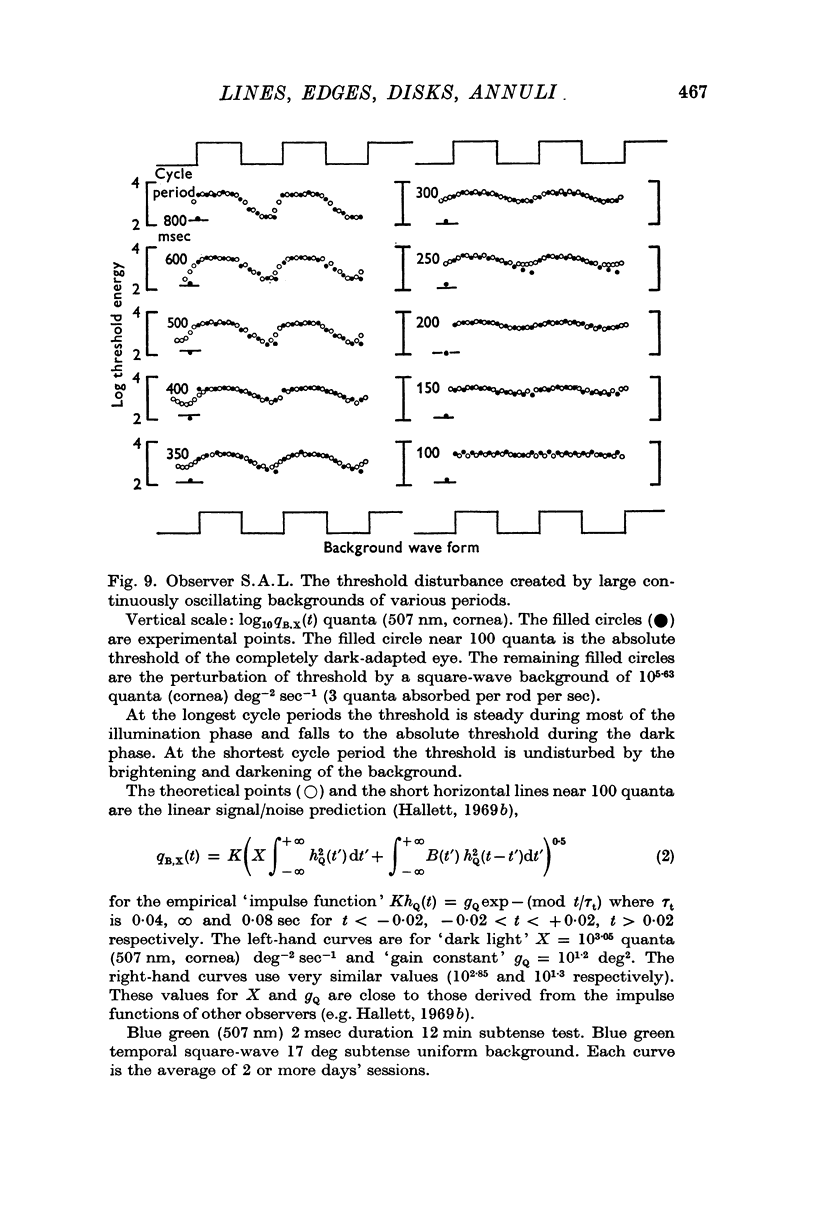
7. Earlier approaches are reviewed: (a) the linear system signal/noise theory of the time course of threshold disturbances (Hallett, 1969b) is illustrated by the case of a small subtense flash superimposed on a large oscillatory background; (b) the spatial weighting functions of some other authors are given.
8. A possible non-linear model is briefly described: the line weighting function for the receptive field centre is taken to be a single Gaussian, as is customary, but the line weighting function for the inhibitory surround is bimodal.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPERN M. Metacontrast. J Opt Soc Am. 1953 Aug;43(8):648–657. doi: 10.1364/josa.43.000648. [DOI] [PubMed] [Google Scholar]
- Alpern M., Rushton W. A., Torii S. The size of rod signals. J Physiol. 1970 Jan;206(1):193–208. doi: 10.1113/jphysiol.1970.sp009006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER H. D. Initial stages of dark and light adaptation. J Opt Soc Am. 1963 Jan;53:98–103. doi: 10.1364/josa.53.000098. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Dark adaptation, absolute threshold and Purkinje shift in single units of the cat's retina. J Physiol. 1957 Aug 6;137(3):327–337. doi: 10.1113/jphysiol.1957.sp005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Increment thresholds at low intensities considered as signal/noise discriminations. J Physiol. 1957 May 23;136(3):469–488. doi: 10.1113/jphysiol.1957.sp005774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Temporal and spatial summation in human vision at different background intensities. J Physiol. 1958 Apr 30;141(2):337–350. doi: 10.1113/jphysiol.1958.sp005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O. Central nervous system: afferent mechanisms and perception. Annu Rev Physiol. 1967;29:427–484. doi: 10.1146/annurev.ph.29.030167.002235. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Gubisch R. W. Optical quality of the human eye. J Physiol. 1966 Oct;186(3):558–578. doi: 10.1113/jphysiol.1966.sp008056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daitch J. M., Green D. G. Contrast sensitivity of the human peripheral retina. Vision Res. 1969 Aug;9(8):947–952. doi: 10.1016/0042-6989(69)90100-x. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini A., Maffei L. Perceptual correlates of inhibitory and facilitatory spatial interactions in the visual system. Vision Res. 1968 Sep;8(9):1195–1203. doi: 10.1016/0042-6989(68)90027-8. [DOI] [PubMed] [Google Scholar]
- HALLETT P. E., MARRIOTT F. H., RODGER F. C. The relationship of visual threshold to retinal position and area. J Physiol. 1962 Feb;160:364–373. doi: 10.1113/jphysiol.1962.sp006851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLINE H. K., RATLIFF F. Spatial summation of inhibitory influences in the eye of Limulus, and the mutual interaction of receptor units. J Gen Physiol. 1958 May 20;41(5):1049–1066. doi: 10.1085/jgp.41.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett P. E. Rapid changes and hysteresis in spatial integration for human rod vision. J Physiol. 1971 Jun;215(2):433–447. doi: 10.1113/jphysiol.1971.sp009478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett P. E. Rod increment thresholds on steady and flashed backgrounds. J Physiol. 1969 Jun;202(2):355–377. doi: 10.1113/jphysiol.1969.sp008816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett P. E. The variations in visual threshold measurement. J Physiol. 1969 Jun;202(2):403–419. doi: 10.1113/jphysiol.1969.sp008818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kelly D. H. Diffusion model of linear flicker responses. J Opt Soc Am. 1969 Dec;59(12):1665–1670. doi: 10.1364/josa.59.001665. [DOI] [PubMed] [Google Scholar]
- McKee S. P., Westheimer G. Specificity of cone mechanisms in lateral interaction. J Physiol. 1970 Jan;206(1):117–128. doi: 10.1113/jphysiol.1970.sp009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVAK S., SPERLING G. VISUAL THRESHOLDS NEAR A CONTINUOUSLY VISIBLE OR A BRIEFLY PRESENTED LIGHT-DARK BOUNDARY. Opt Acta (Lond) 1963 Apr;10:187–191. doi: 10.1080/713817782. [DOI] [PubMed] [Google Scholar]
- Norton A. L., Spekreijse H., Wagner H. G., Wolbarsht M. L. Responses to directional stimuli in retinal preganglionic units. J Physiol. 1970 Jan;206(1):93–107. doi: 10.1113/jphysiol.1970.sp008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIGGS L. A., RATLIFF F., KEESEY U. T. Appearance of Mach bands with a motionless retinal image. J Opt Soc Am. 1961 Jun;51:702–703. doi: 10.1364/josa.51.0702_1. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. The rhodopsin density in the human rods. J Physiol. 1956 Oct 29;134(1):30–46. doi: 10.1113/jphysiol.1956.sp005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff F., Knight B. W., Graham N. On tuning and amplification by lateral inhibition. Proc Natl Acad Sci U S A. 1969 Mar;62(3):733–740. doi: 10.1073/pnas.62.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res. 1965 Dec;5(11):583–601. doi: 10.1016/0042-6989(65)90033-7. [DOI] [PubMed] [Google Scholar]
- SCHADE O. H., Sr Optical and photoelectric analog of the eye. J Opt Soc Am. 1956 Sep;46(9):721–739. doi: 10.1364/josa.46.000721. [DOI] [PubMed] [Google Scholar]
- Scheibner H., Baumgardt E. Sur l'emploi en optique physiologique des grandeurs scotopiques. Vision Res. 1967 Jan;7(1):59–63. doi: 10.1016/0042-6989(67)90026-0. [DOI] [PubMed] [Google Scholar]
- Sperling G., Sondhi M. M. Model for visual luminance discrimination and flicker detection. J Opt Soc Am. 1968 Aug;58(8):1133–1145. doi: 10.1364/josa.58.001133. [DOI] [PubMed] [Google Scholar]
- WESTHEIMER G., CAMPBELL F. W. Light distribution in the image formed by the living human eye. J Opt Soc Am. 1962 Sep;52:1040–1045. doi: 10.1364/josa.52.001040. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Response of retinal cells to moving spots: intracellular recording in Necturus maculosus. J Neurophysiol. 1970 May;33(3):342–350. doi: 10.1152/jn.1970.33.3.342. [DOI] [PubMed] [Google Scholar]
- Westheimer G. Spatial interaction in the human retina during scotopic vision. J Physiol. 1965 Dec;181(4):881–894. doi: 10.1113/jphysiol.1965.sp007803. [DOI] [PMC free article] [PubMed] [Google Scholar]


