Abstract
1. End-plate potentials (e.p.p.s) and miniature end-plate potentials (m.e.p.p.s) were intracellularly recorded from rat diaphragm phrenic nerve preparations in vitro at temperatures between 7° and 40°C.
2. The quantal content of e.p.p.s and the frequency of m.e.p.p.s showed broadly similar relationships with temperature, with maxima about 20° and above 39°C.
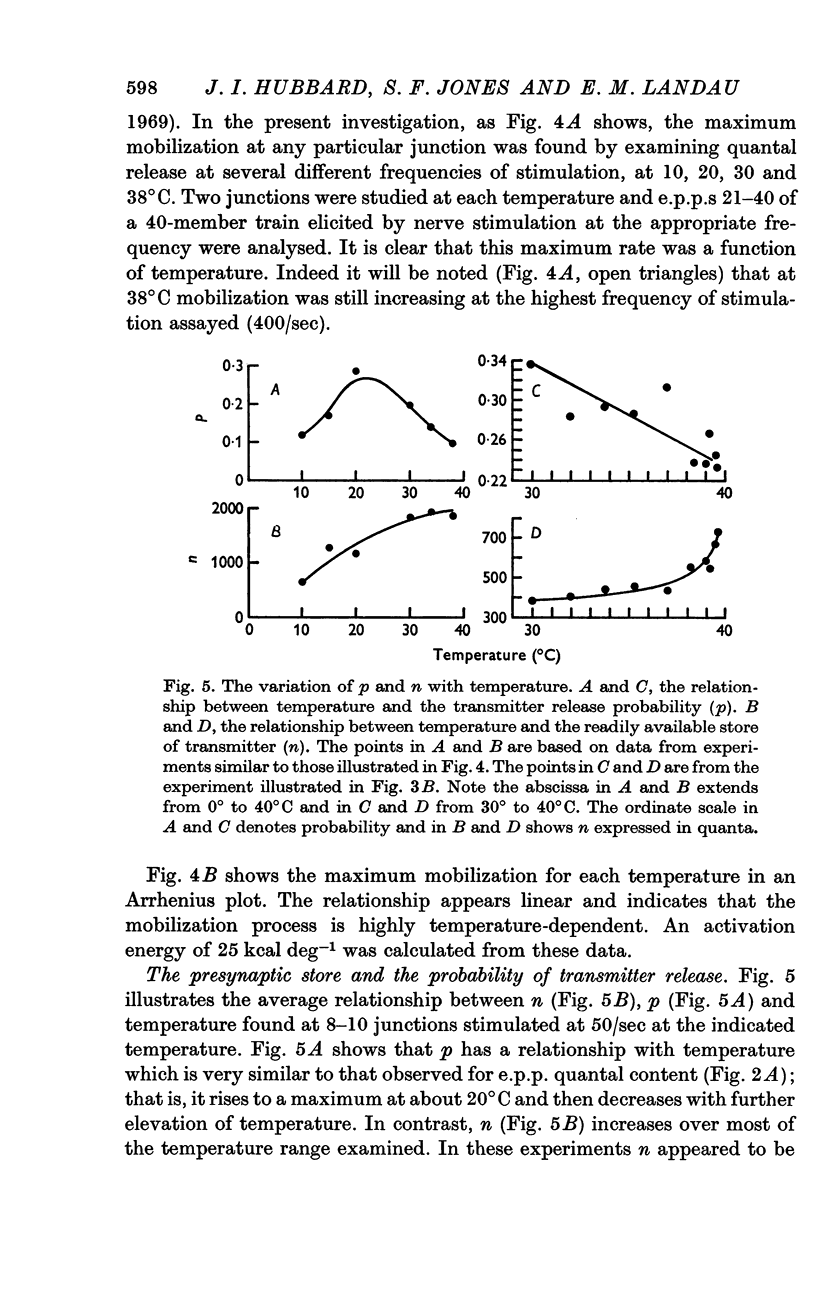
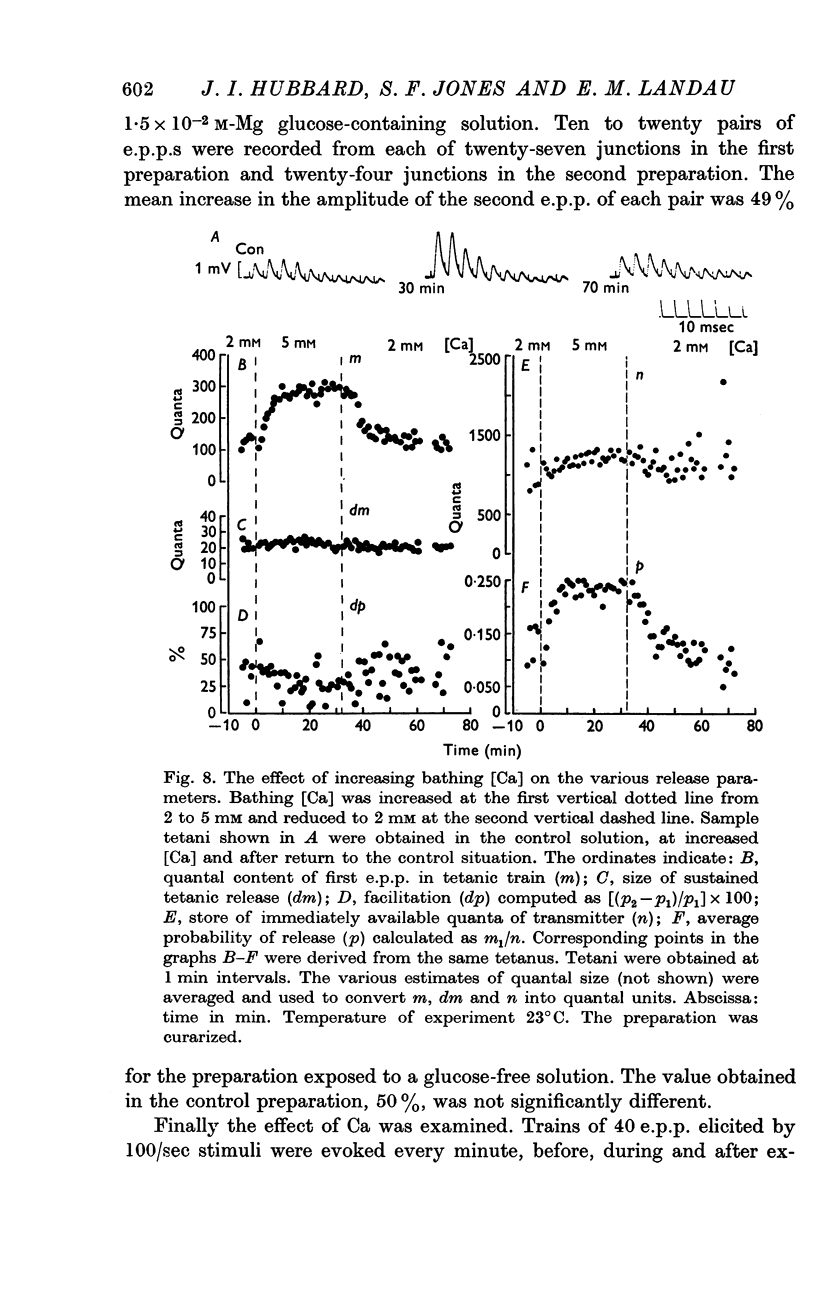
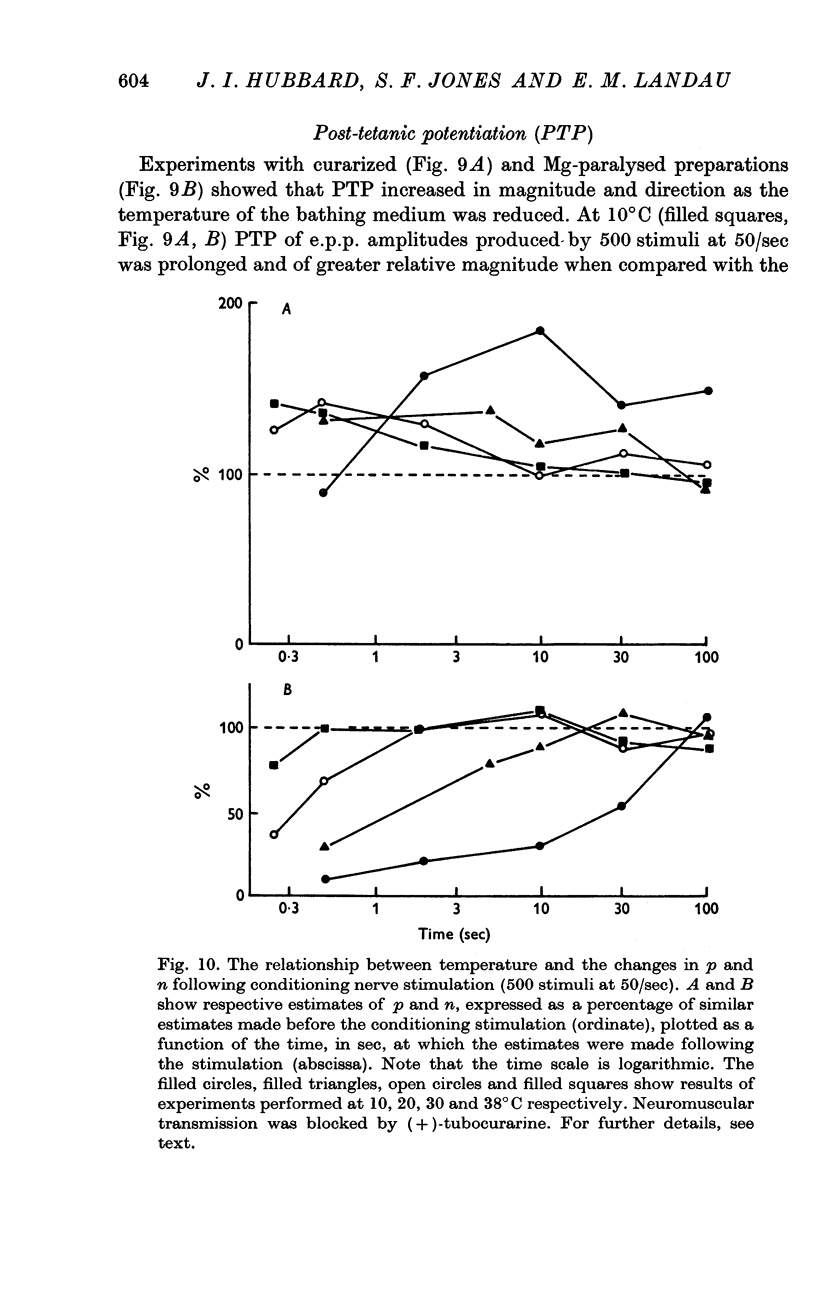
3. Analysis of the change in e.p.p. quantal content showed that the maximum about 20°C was accompanied by a similar maximum of p, the probability of release of quanta. The maximum above 39°C was associated with a rise in n, a presynaptic store of material needed for release.
4. The rate at which transmitter could be mobilized was linear in an Arrhenius plot with an apparent activation energy of 25 kcal deg-1.
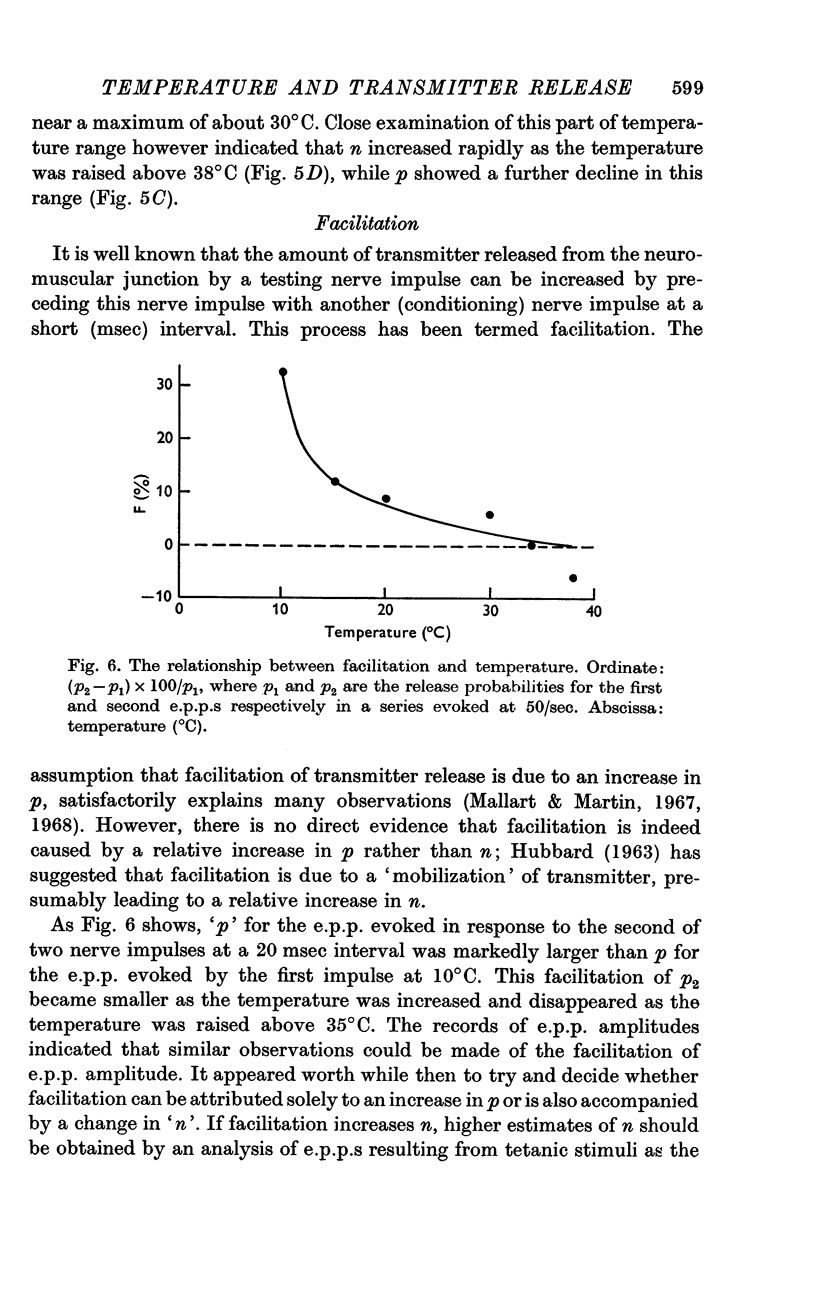
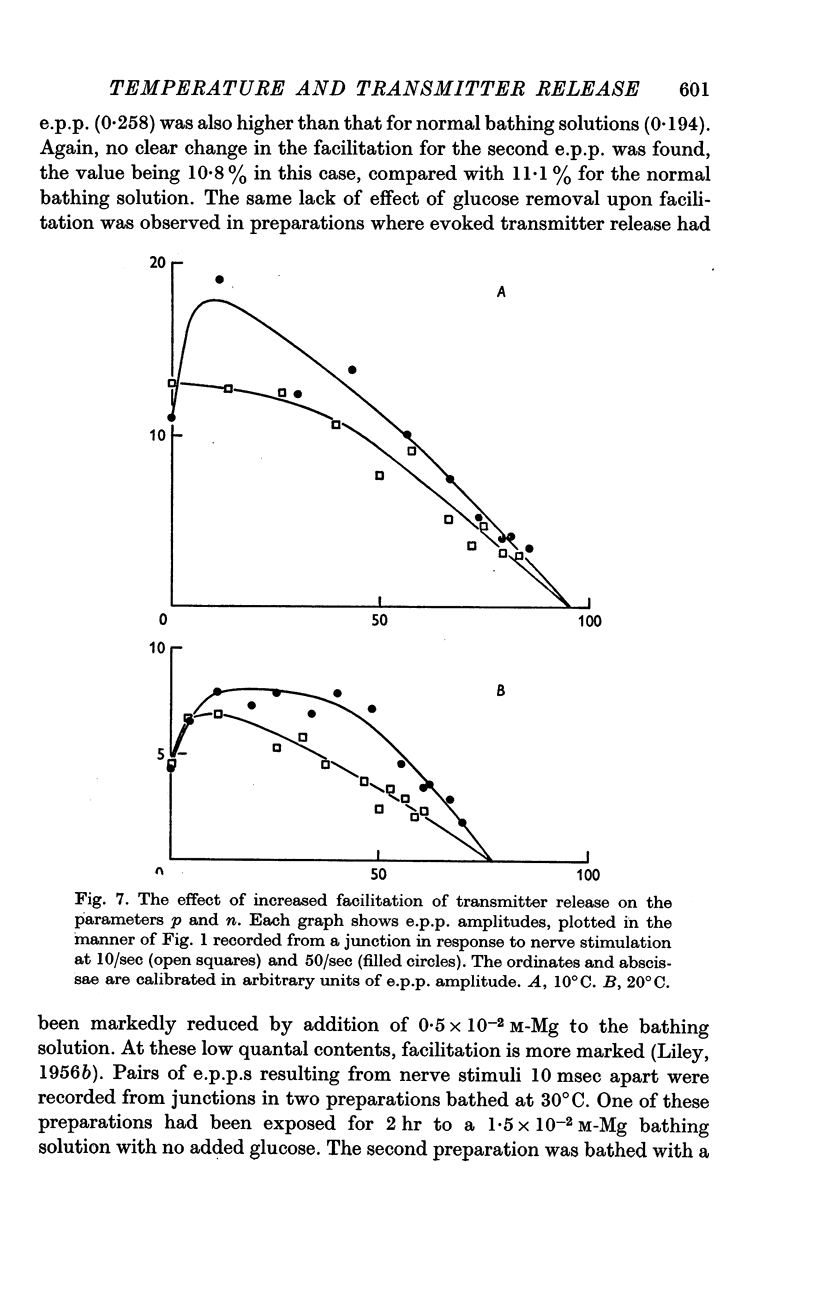
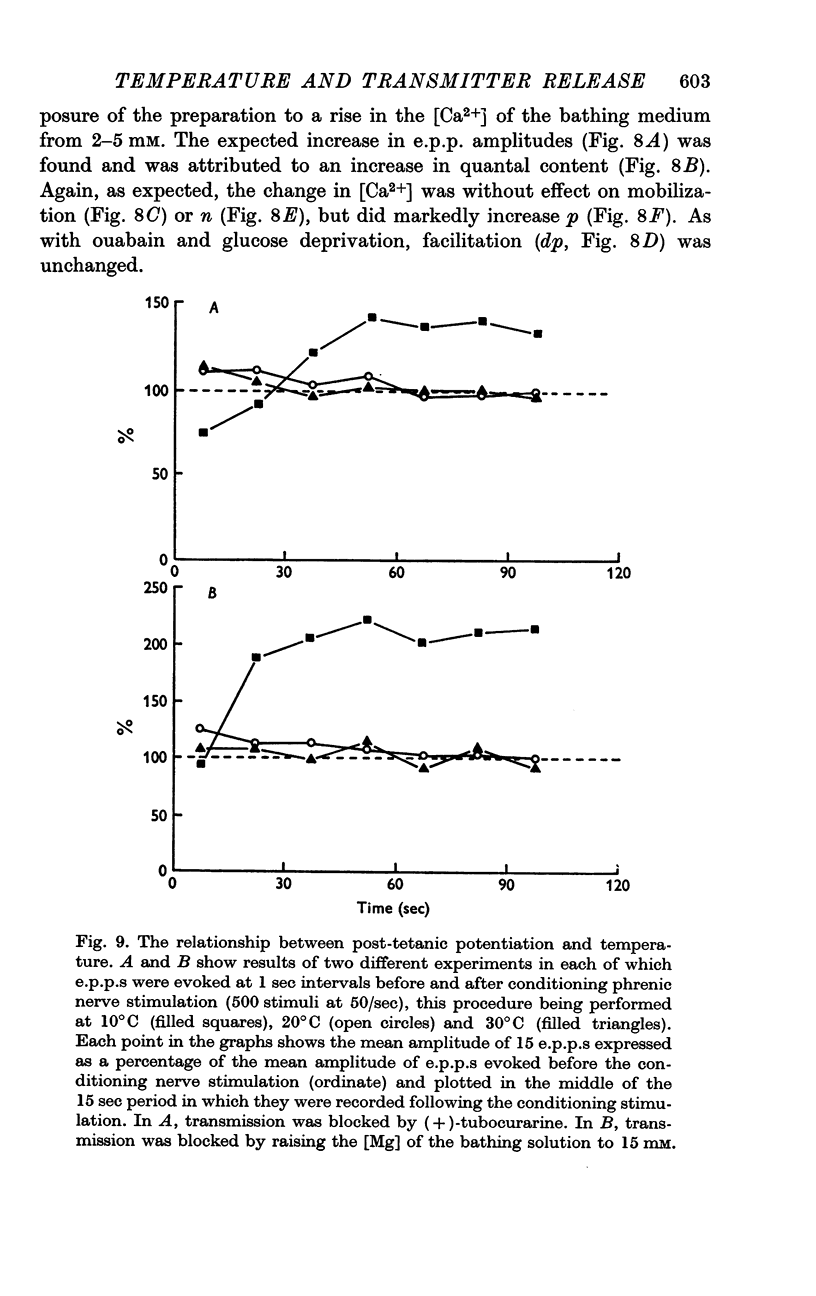
5. Facilitation and post-tetanic potentiation (PTP) were shown to be entirely attributable to changes in p.
6. It is suggested that facilitation and PTP have a common basis and that the (temperature-dependent) rate of Ca removal from intracellular sites at which it exerts its action is as important a determinant of the magnitude of quantal release as is the amount of Ca combining with these sites.
Full text
PDF

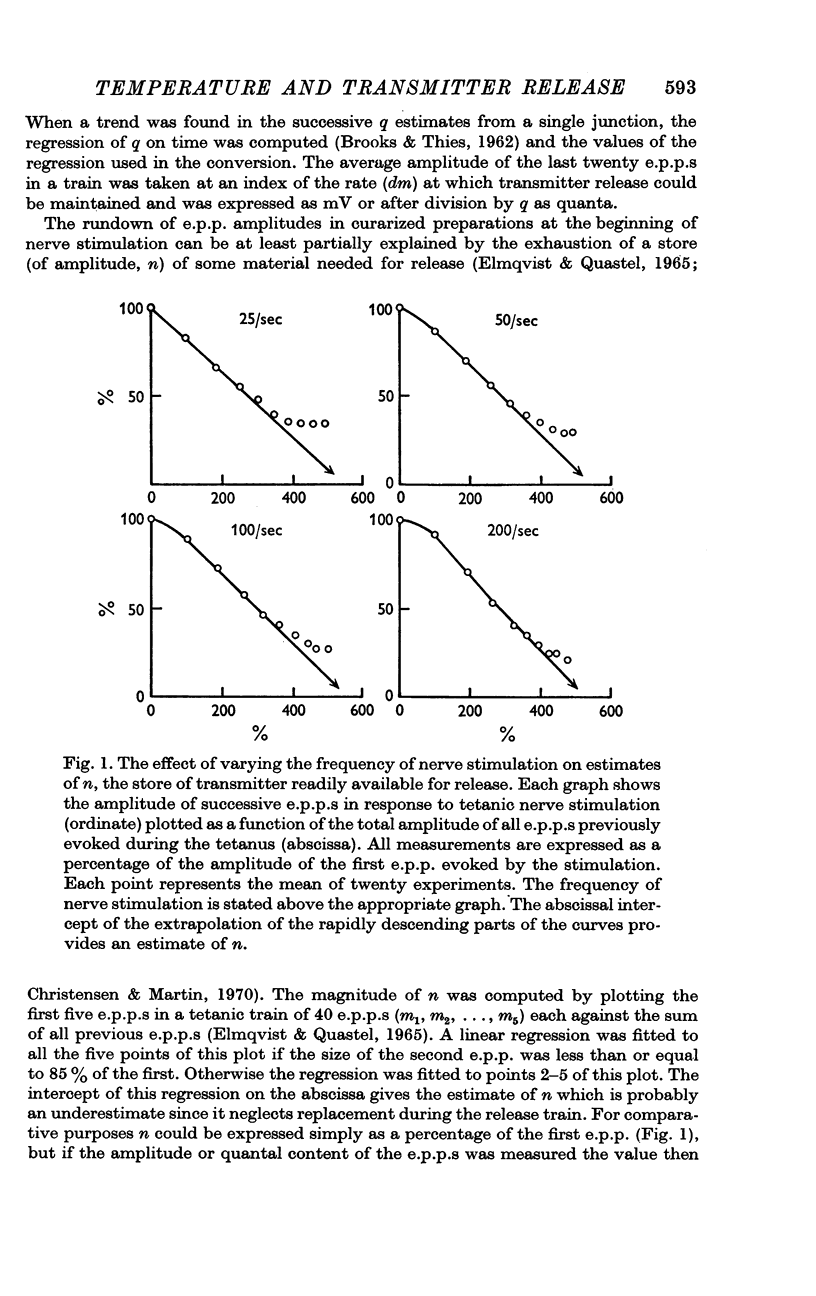

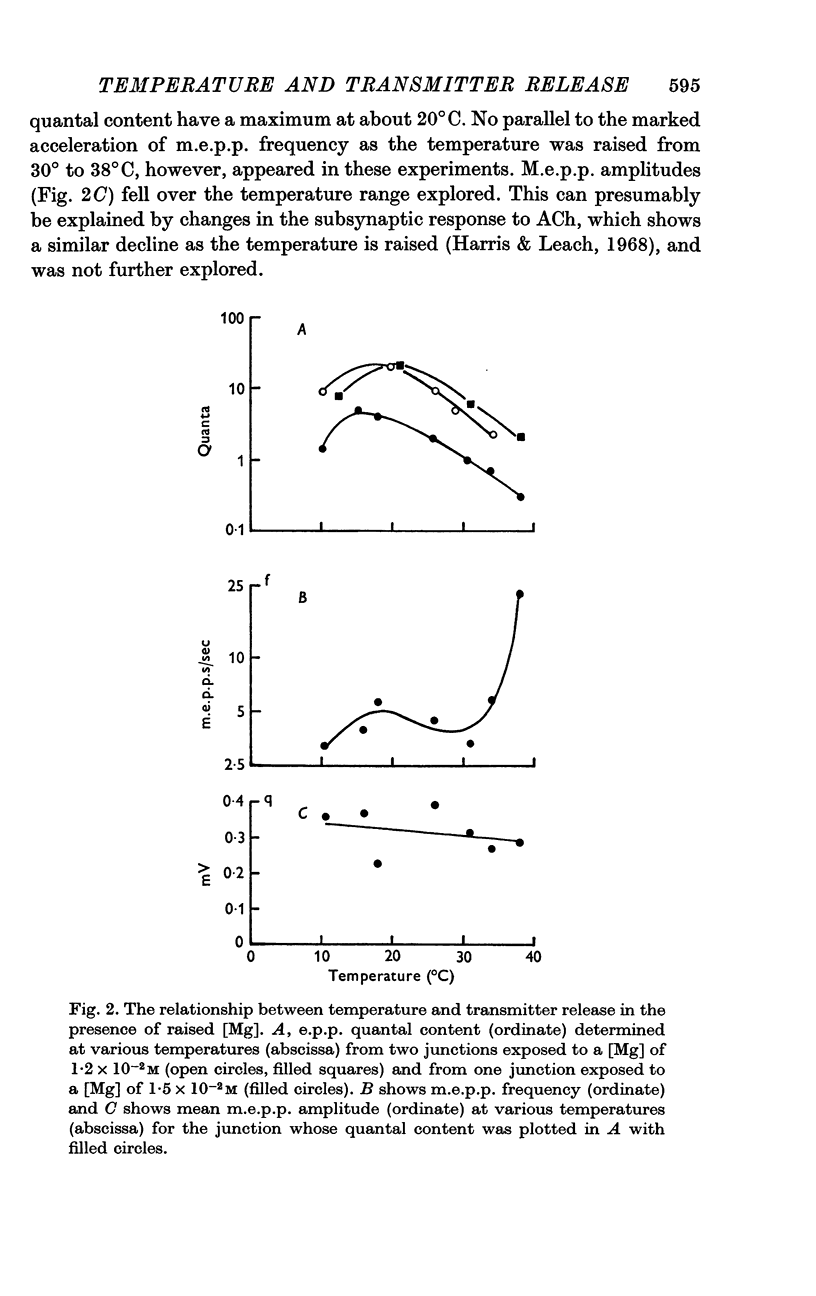
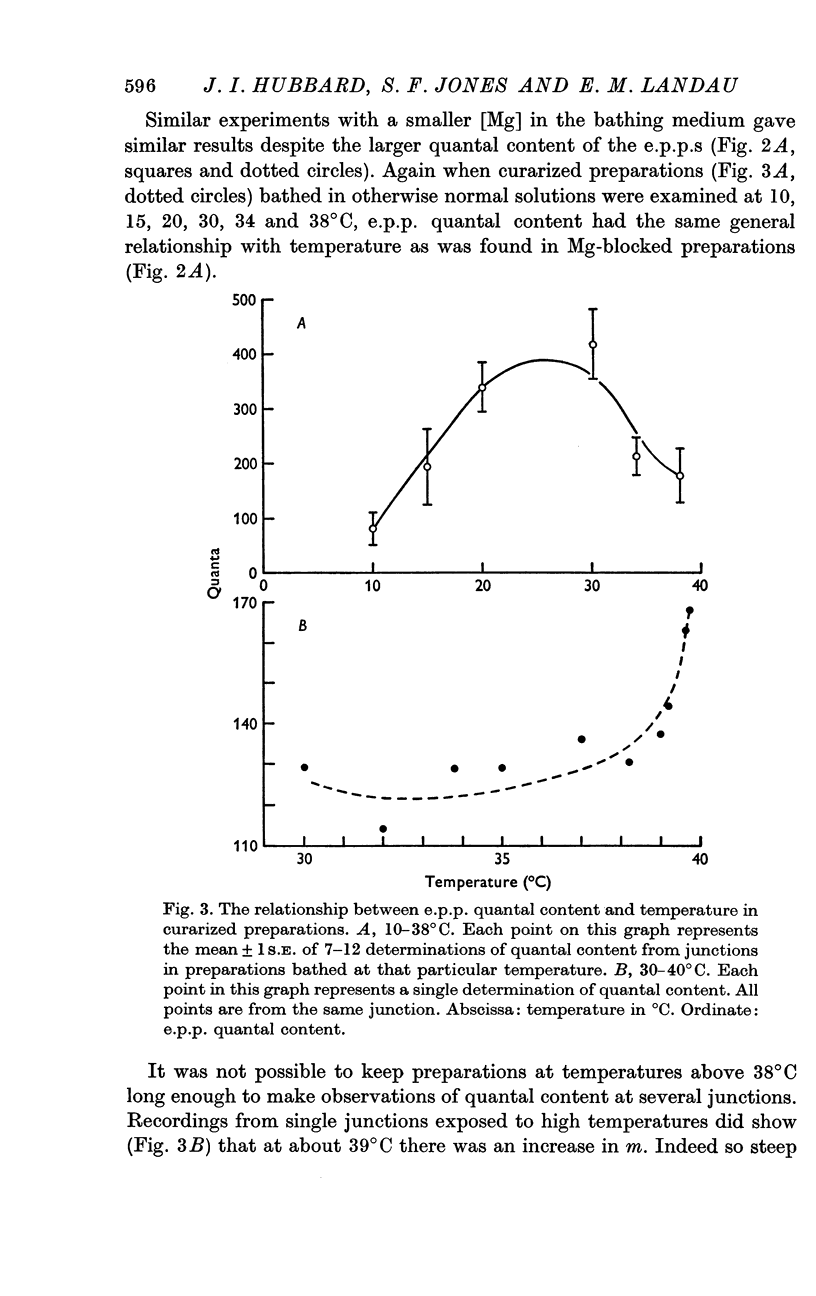
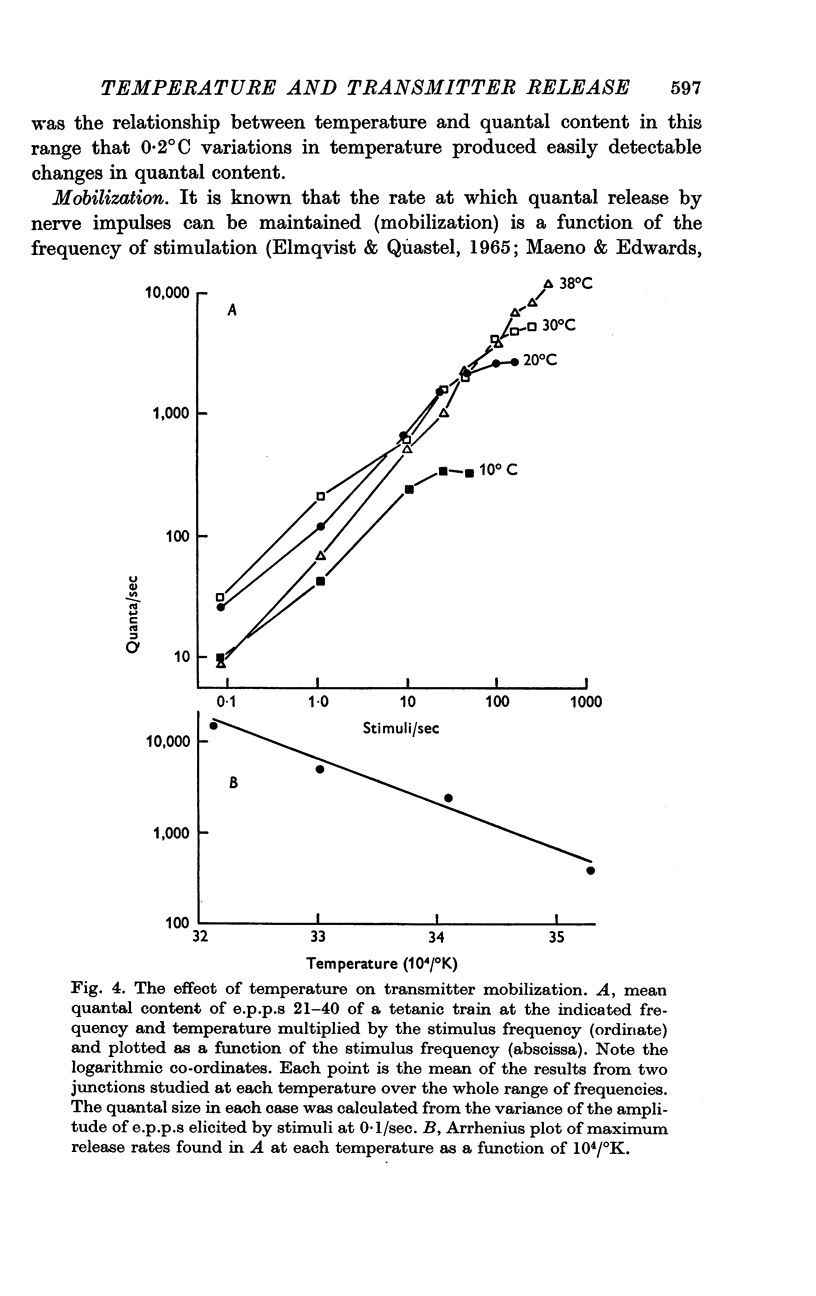






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD I. A., MARTIN A. R. Spontaneous subthreshold activity at mammalian neural muscular junctions. J Physiol. 1956 Apr 27;132(1):61–73. doi: 10.1113/jphysiol.1956.sp005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS V. B., THIES R. E. Reduction of quantum content during neuromuscular transmission. J Physiol. 1962 Jul;162:298–310. doi: 10.1113/jphysiol.1962.sp006934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balnave R. J., Gage P. W. Temperature sensitivity of the time course of facilitation of transmitter release. Brain Res. 1970 Jul 14;21(2):297–300. doi: 10.1016/0006-8993(70)90374-4. [DOI] [PubMed] [Google Scholar]
- Braun M., Schmidt R. F., Zimmermann M. Facilitation at the frog neuromuscular junction during and after repetitive stimulation. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;287(1):41–55. doi: 10.1007/BF00362453. [DOI] [PubMed] [Google Scholar]
- Christensen B. N., Martin A. R. Estimates of probability of transmitter release at the mammalian neuromuscular junction. J Physiol. 1970 Nov;210(4):933–945. doi: 10.1113/jphysiol.1970.sp009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Quastel D. M. A quantitative study of end-plate potentials in isolated human muscle. J Physiol. 1965 Jun;178(3):505–529. doi: 10.1113/jphysiol.1965.sp007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FEIGEN G. A., PETERSON N. S., HOFMANN W. W., GENTHER G. H., VANHEYNINGEN W. E. THE EFFECT OF IMPURE TETANUS TOXIN ON THE FREQUENCY OF MINIATURE END-PLATE POTENTIALS. J Gen Microbiol. 1963 Dec;33:489–495. doi: 10.1099/00221287-33-3-489. [DOI] [PubMed] [Google Scholar]
- GAGE P. W. EFFECT OF CARDIAC GLYCOSIDES ON NEUROMUSCULAR TRANSMISSION. Nature. 1965 Jan 2;205:84–85. doi: 10.1038/205084a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of temperature on the electrical activity of the giant axon of the squid. J Physiol. 1949 Aug;109(1-2):240–249. doi: 10.1113/jphysiol.1949.sp004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. REPETITIVE STIMULATION AT THE MAMMALIAN NEUROMUSCULAR JUNCTION, AND THE MOBILIZATION OF TRANSMITTER. J Physiol. 1963 Dec;169:641–662. doi: 10.1113/jphysiol.1963.sp007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Leach G. D. The effect of temperature on end-plate depolarization of the rat diaphragm produced by suxamethonium and acetylcholine. J Pharm Pharmacol. 1968 Mar;20(3):194–198. doi: 10.1111/j.2042-7158.1968.tb09720.x. [DOI] [PubMed] [Google Scholar]
- Hofmann W. W., Parsons R. L., Feigen G. A. Effects of temperature and drugs on mammalian motor nerve terminals. Am J Physiol. 1966 Jul;211(1):135–140. doi: 10.1152/ajplegacy.1966.211.1.135. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. The relationship between the state of nerve-terminal polarization and liberation of acetylcholine. Ann N Y Acad Sci. 1967 Oct 31;144(2):459–470. doi: 10.1111/j.1749-6632.1967.tb53787.x. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I. Mechanism of transmitter release. Prog Biophys Mol Biol. 1970;21:33–124. [PubMed] [Google Scholar]
- Hubbard J. I., Willis W. D. The effects of depolarization of motor nerve terminals upon the release of transmitter by nerve impulses. J Physiol. 1968 Feb;194(2):381–405. doi: 10.1113/jphysiol.1968.sp008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Release of acetylcholine from a nerve terminal by electric pulses of variable strength and duration. Nature. 1965 Sep 4;207(5001):1097–1098. doi: 10.1038/2071097a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The release of acetylcholine from nerve endings by graded electric pulses. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):23–38. doi: 10.1098/rspb.1967.0011. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C. L. Effect of cooling on neuromuscular transmission in the rat. Am J Physiol. 1958 Jul;194(1):200–206. doi: 10.1152/ajplegacy.1958.194.1.200. [DOI] [PubMed] [Google Scholar]
- LI C. L., GOURAS P. Effect of cooling on neuromuscular transmission in the frog. Am J Physiol. 1958 Mar;192(3):464–470. doi: 10.1152/ajplegacy.1958.192.3.464. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W., NORTH K. A. An electrical investigation of effects of repetitive stimulation on mammalian neuromuscular junction. J Neurophysiol. 1953 Sep;16(5):509–527. doi: 10.1152/jn.1953.16.5.509. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. The quantal components of the mammalian end-plate potential. J Physiol. 1956 Sep 27;133(3):571–587. doi: 10.1113/jphysiol.1956.sp005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno T., Edwards C. Neuromuscular facilitation with low-frequency stimulation and effects of some drugs. J Neurophysiol. 1969 Sep;32(5):785–792. doi: 10.1152/jn.1969.32.5.785. [DOI] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. The relation between quantum content and facilitation at the neuromuscular junction of the frog. J Physiol. 1968 Jun;196(3):593–604. doi: 10.1113/jphysiol.1968.sp008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmansohn D. Proteins in excitable membranes: their properties and function in bioelectricity are discussed. Science. 1970 May 29;168(3935):1059–1066. doi: 10.1126/science.168.3935.1059. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J. Post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1969 Jul;203(1):121–133. doi: 10.1113/jphysiol.1969.sp008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI N. The effect of temperature on the neuromuscular junction of the frog. Jpn J Physiol. 1958 Dec 20;8(4):391–404. doi: 10.2170/jjphysiol.8.391. [DOI] [PubMed] [Google Scholar]
- THIES R. E. NEUROMUSCULAR DEPRESSION AND THE APPARENT DEPLETION OF TRANSMITTER IN MAMMALIAN MUSCLE. J Neurophysiol. 1965 May;28:428–442. doi: 10.1152/jn.1965.28.3.427. [DOI] [PubMed] [Google Scholar]


