Abstract
1. Field stimulation of desheathed preparations of guinea-pig vas deferens, treated with a ganglion-blocking agent, has revealed the presence of two tetrodotoxin-susceptible components in the motor response, suggesting the existence of two sets of post-ganglionic motor nerve fibres of different excitability: one set responding maximally to pulses of 0·1-0·4 msec; the other, to pulses of 2 msec. No distinction could be made pharmacologically between the two components.
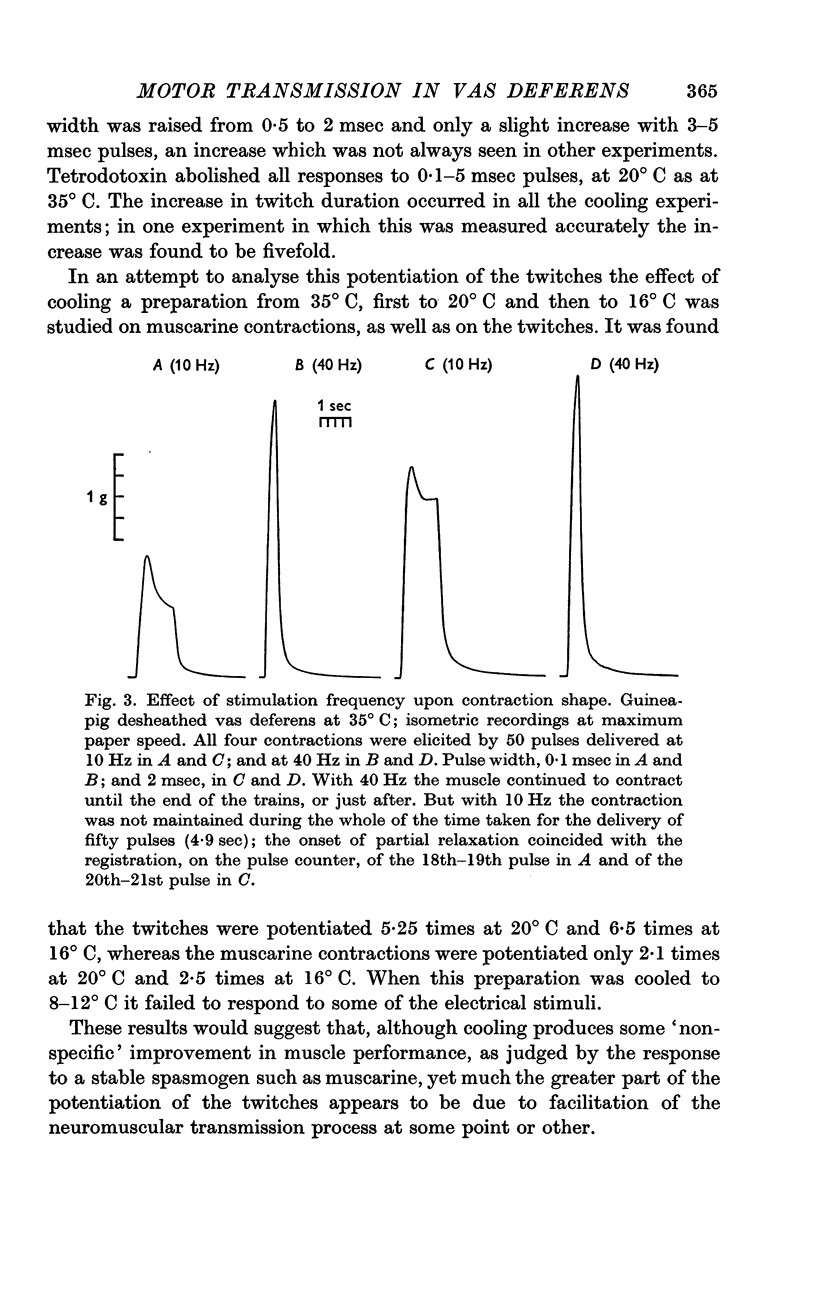
2. Cooling potentiated that component in the twitch-responses which was due to stimulation of the more excitable fibres.
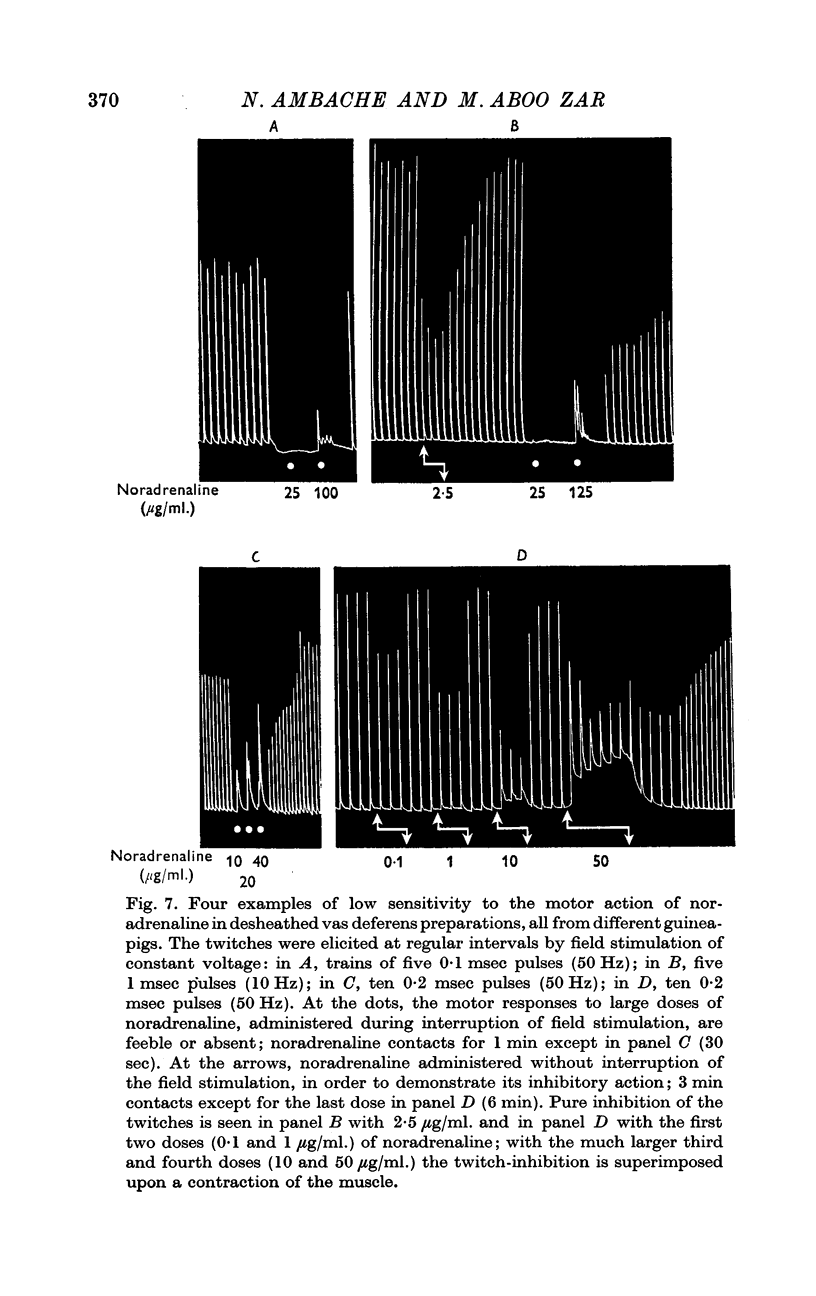
3. The sensitivity of the longitudinal muscle to the motor action of noradrenaline was low and was subject to considerable animal variation. But normal responses to post-ganglionic field stimulation were elicited in noradrenaline-insensitive preparations, in which the twitches elicited by 5 pulses could not be matched with noradrenaline, even 100-125 μg/ml.
4. In some forty experiments, small doses of noradrenaline inhibited the twitch-responses evoked by either set of motor fibres. This inhibition differed from that produced by isoprenaline in two respects. Firstly, propranolol did not antagonize the noradrenaline inhibition, thus excluding an action on β-adrenoceptors; and secondly, noradrenaline did not depress contractions elicited by muscarine or by 5-methylfurmethide.
5. Phenoxybenzamine, 10-6 g/ml., produced a thousandfold reduction in the sensitivity of the muscle to the motor action of noradrenaline, without any decrease in the height of the twitches elicited by 0·1 or 1 msec pulses.
6. The twitch-responses were not affected by combined α + β adrenoceptor blockade with phentolamine and propranolol.
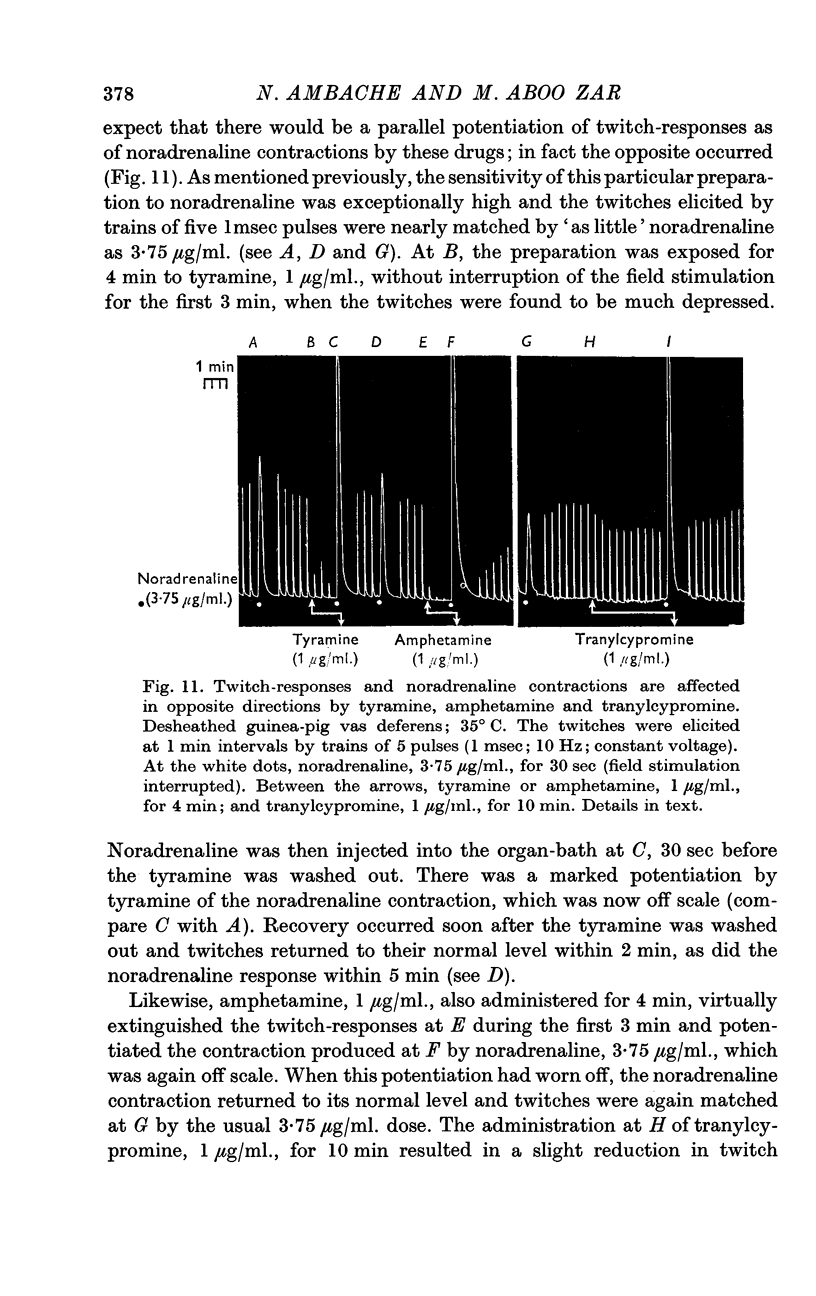
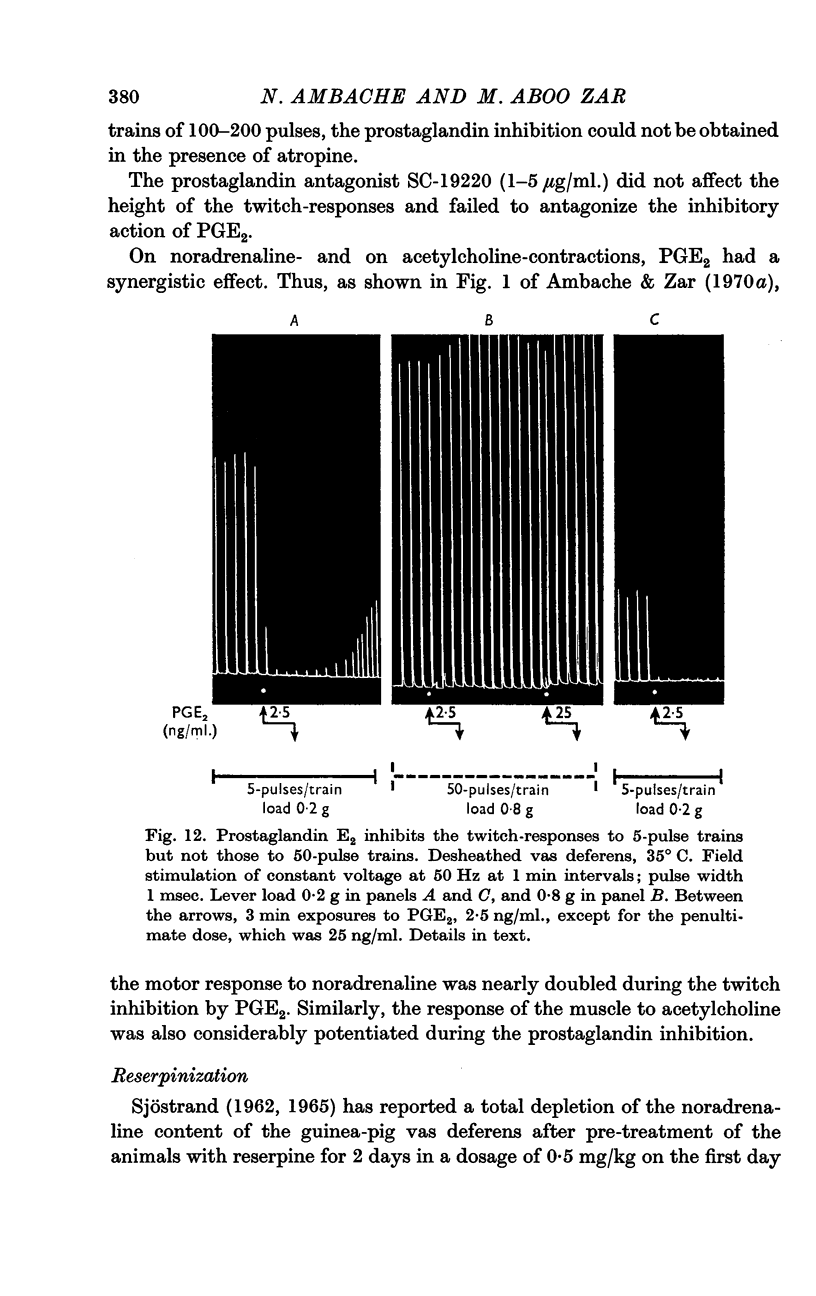
7. Tyramine, amphetamine, tranylcypromine and prostaglandin E2 inhibited the twitches but potentiated the contractile effect of noradrenaline.
8. The twitch-responses and their inhibition by noradrenaline were present in preparations taken from reserpinized animals.
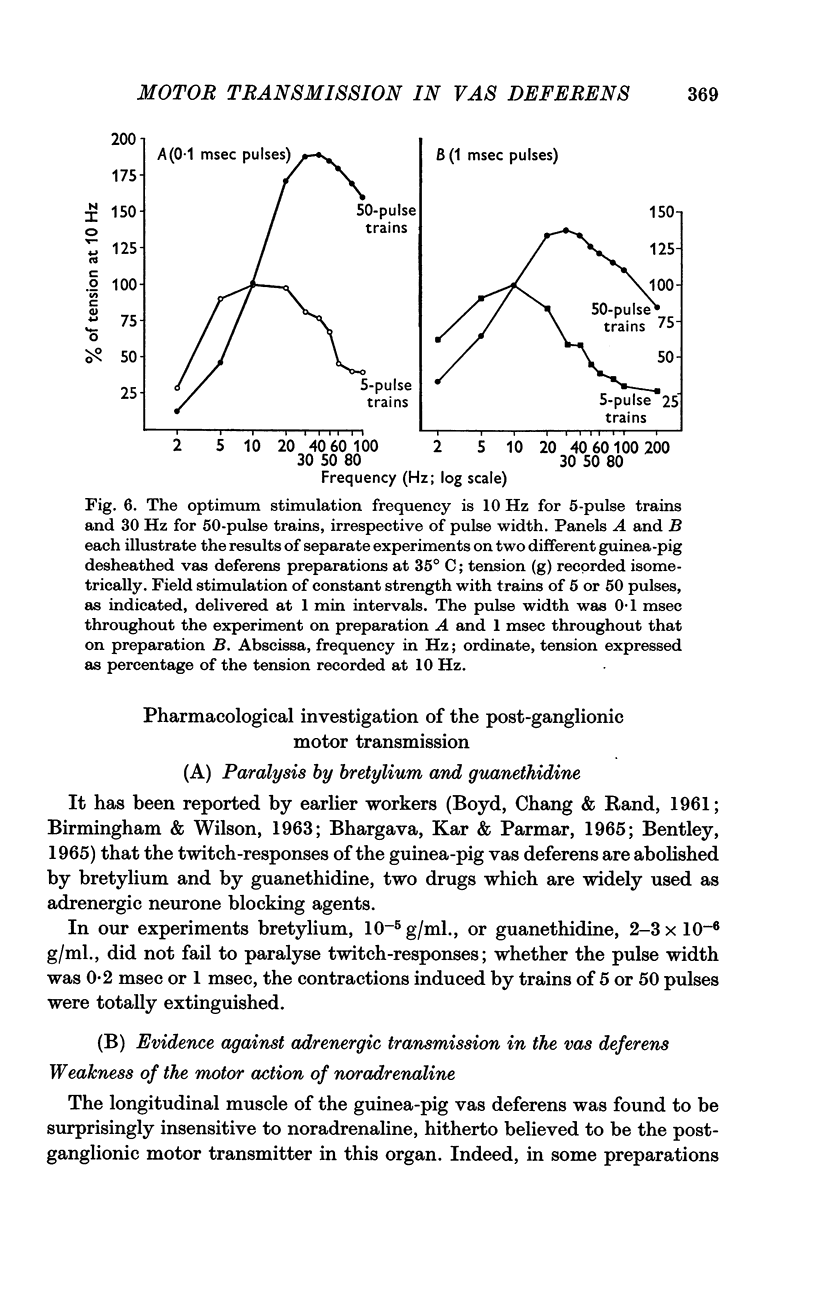
9. Although the twitch-responses could be paralysed by bretylium or guanethidine, the foregoing results excluded adrenergic transmission at the motor endings. Cholinergic transmission was also excluded by negative findings with anticholinesterases, atropine, nicotine and (+)-tubocurarine.
10. Motor transmission by histamine, 5-hydroxytryptamine, γ-aminobutyric acid or ATP was also excluded.
Full text
PDF

























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Zar M. A. Motor transmission in the vas deferens: the inhibitory action of noradrenaline. Br J Pharmacol. 1970 Nov;40(3):556P–558P. [PMC free article] [PubMed] [Google Scholar]
- Ambache N., Zar M. A. Non-cholinergic transmission by post-ganglionic motor neurones in the mammalian bladder. J Physiol. 1970 Oct;210(3):761–783. doi: 10.1113/jphysiol.1970.sp009240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHARGAVA K. P., KAR K., PARMAR S. S. INDEPENDENT CHOLINERGIC AND ADRENERGIC MECHANISMS IN THE GUINEA-PIG ISOLATED NERVE-VAS DEFERENS PREPARATION. Br J Pharmacol Chemother. 1965 Jun;24:641–650. doi: 10.1111/j.1476-5381.1965.tb01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRMINGHAM A. T., WILSON A. B. PREGANGLIONIC AND POSTGANGLIONIC STIMULATION OF THE GUINEA-PIG ISOLATED VAS DEFERENS PREPARATION. Br J Pharmacol Chemother. 1963 Dec;21:569–580. doi: 10.1111/j.1476-5381.1963.tb02024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. The transmission of excitation from autonomic nerve to smooth muscle. J Physiol. 1961 Jan;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham A. T. Sympathetic denervation of the smooth muscle of the vas deferens. J Physiol. 1970 Mar;206(3):645–661. doi: 10.1113/jphysiol.1970.sp009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley A. G., Dearnaley D. P., Harrison V. The noradrenaline content of the vas deferens of the guinea-pig. Proc R Soc Lond B Biol Sci. 1970 Jan 20;174(1037):491–502. doi: 10.1098/rspb.1970.0007. [DOI] [PubMed] [Google Scholar]
- Clementi F., Naimzada K. M., Mantegazza P. Study of the nerve endings in the vas deferens and seminal vesicle of the guinea pig. Int J Neuropharmacol. 1969 Sep;8(5):399–403. doi: 10.1016/0028-3908(69)90056-2. [DOI] [PubMed] [Google Scholar]
- DIXIT B. N., GULATI O. D., GOKHALE S. D. Action of bretylium and guanethidine at the neuromuscular junction. Br J Pharmacol Chemother. 1961 Dec;17:372–379. doi: 10.1111/j.1476-5381.1961.tb01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Bella D., Gandini A., Preti M. Influence of temperature on the responses of the guinea-pig hypogastric nerve-vas deferens preparation. J Pharm Pharmacol. 1965 May;17(5):265–273. doi: 10.1111/j.2042-7158.1965.tb07667.x. [DOI] [PubMed] [Google Scholar]
- Ferry C. B. The innervation of the vas deferens of the guinea-pig. J Physiol. 1967 Sep;192(2):463–478. doi: 10.1113/jphysiol.1967.sp008309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. D., Katib H A. L. Adrenolytic and sympatholytic properties of 2-halogenoalkylamines in the vas deferens of the guinea-pig. Br J Pharmacol Chemother. 1967 Sep;31(1):42–55. doi: 10.1111/j.1476-5381.1967.tb01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARGE B. J. SYMPATHETIC BETA-RECEPTORS AND THE GUINEA-PIG VAS DEFERENS. Br J Pharmacol Chemother. 1965 Feb;24:194–204. doi: 10.1111/j.1476-5381.1965.tb02095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEAME K. D. Uptake of L-histidine, L-proline, L-tyrosine and L-ornithine by brain, intestinal mucosa, testis, kidney, spleen, liver, heart muscle, skeletal muscle and erythrocytes of the rat in vitro. J Physiol. 1962 Jun;162:1–12. doi: 10.1113/jphysiol.1962.sp006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICKERSON M. Nonequilibrium drug antagonism. Pharmacol Rev. 1957 Jun;9(2):246–259. [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol. 1969 Jan;35(1):10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Zar M. A. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol. 1968 Jan;194(1):13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SJOSTRAND N. O. Effect of reserpine and hypogastric denervation on the noradrenaline content of the vas deferens and the seminal vesicle of the guinea-pig. Acta Physiol Scand. 1962 Nov-Dec;56:376–380. doi: 10.1111/j.1748-1716.1962.tb02513.x. [DOI] [PubMed] [Google Scholar]
- von Euler U. S., Hedqvist P. Inhibitory action of prostaglandins E1 and E2 on the neuromuscular transmission in the guinea pig vas deferens. Acta Physiol Scand. 1969 Dec;77(4):510–512. doi: 10.1111/j.1748-1716.1969.tb04593.x. [DOI] [PubMed] [Google Scholar]


