Abstract
1. The interaction between the pressor response to electrical stimulation of the fastigial nucleus (FN), the fastigial pressor response (FPR), and the depressor response to electrical stimulation of the carotid sinus nerve (CSN) was examined in paralysed anaesthetized cats.
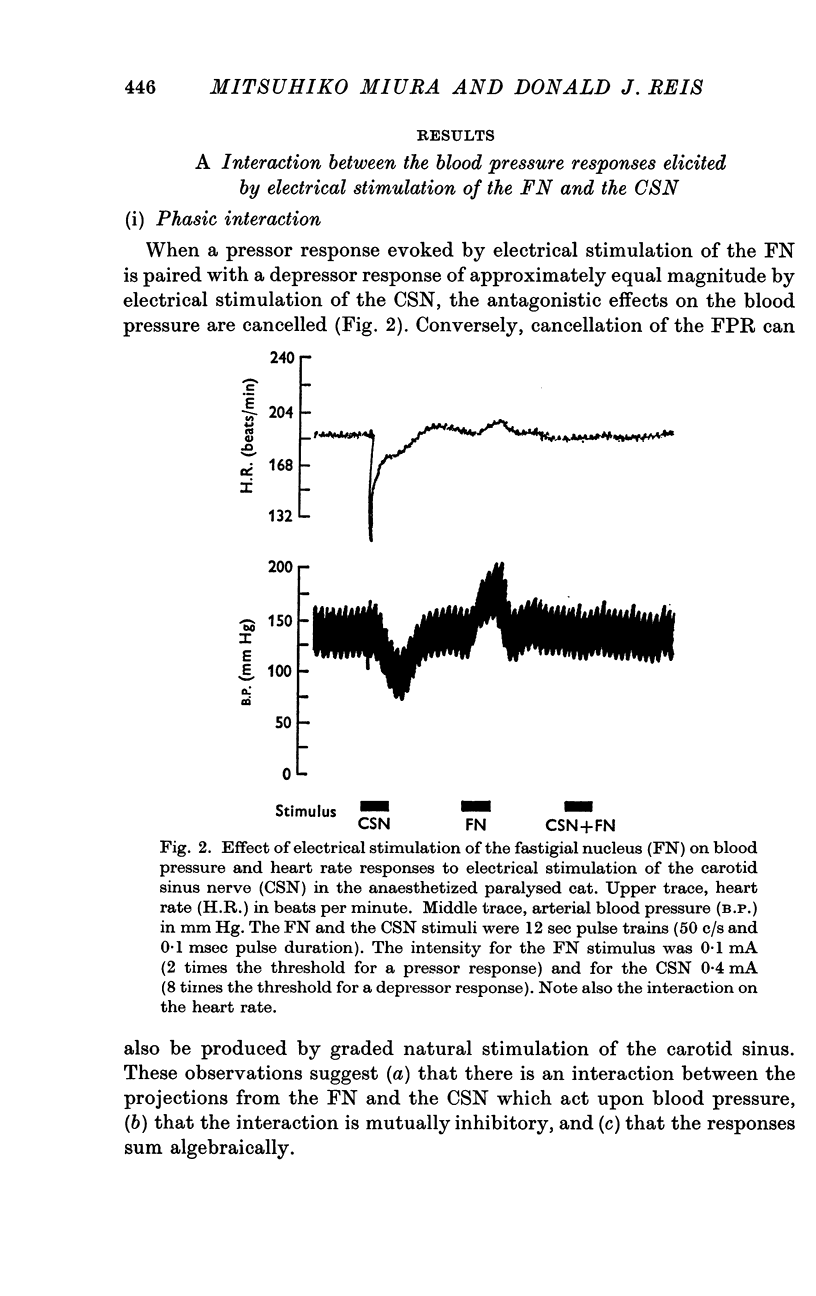
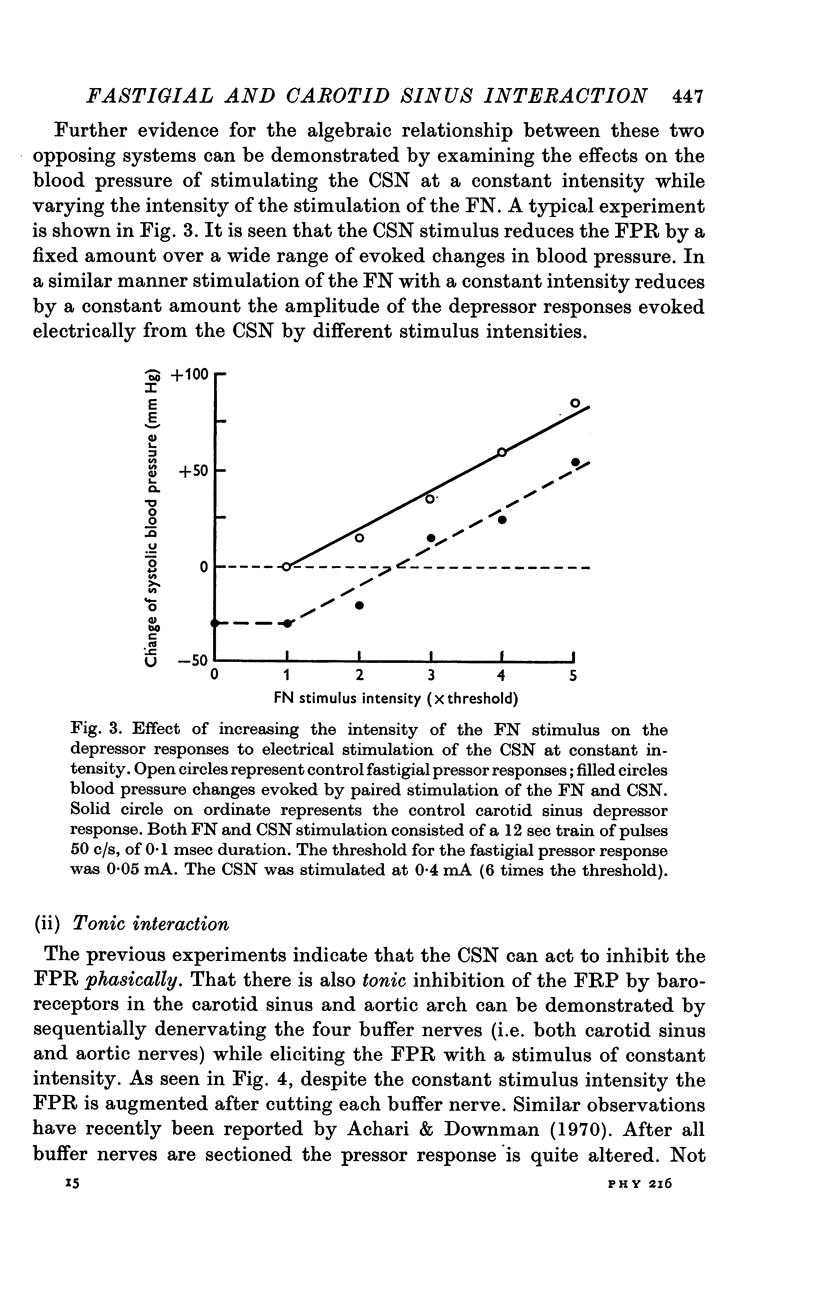
2. Blood pressure responses evoked by electrical stimulation of the FN and the CSN were mutually inhibitory and summed algebraically.
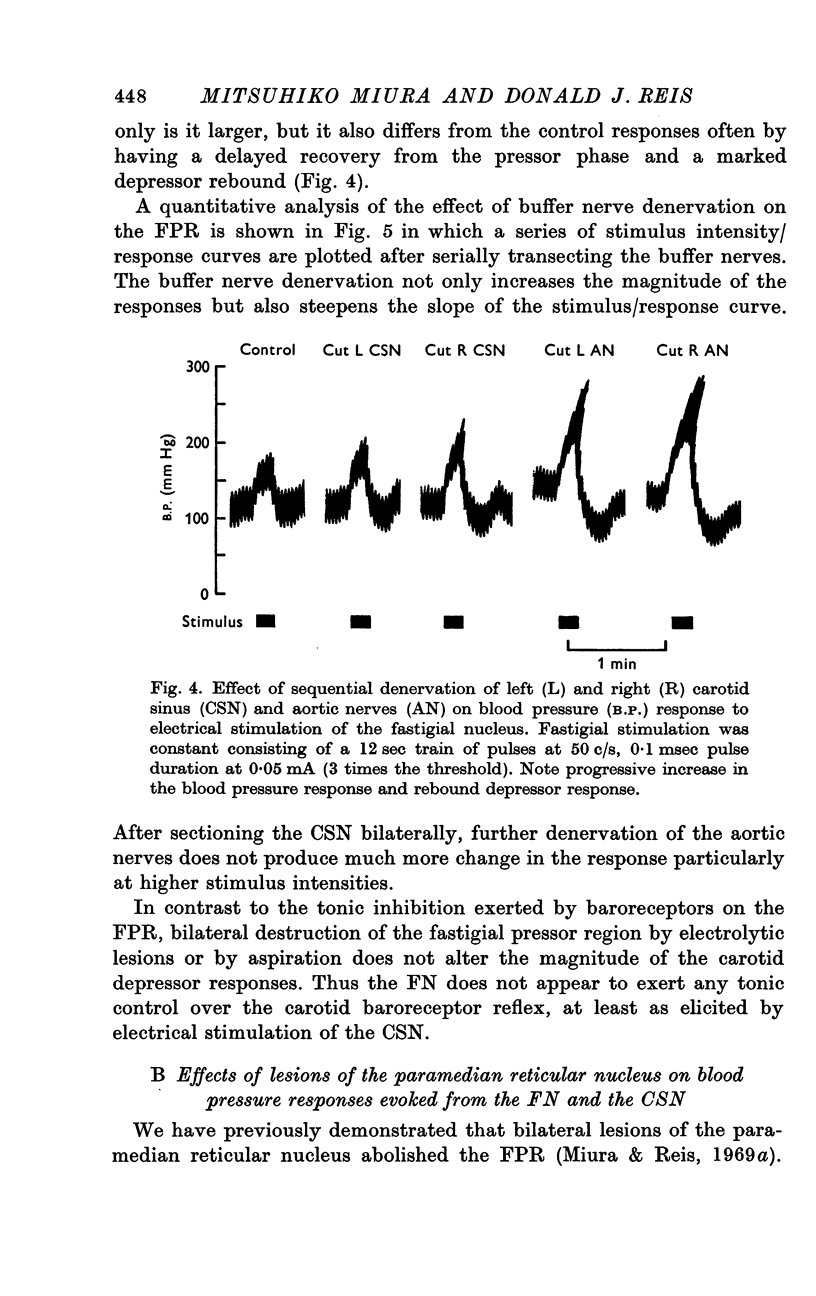
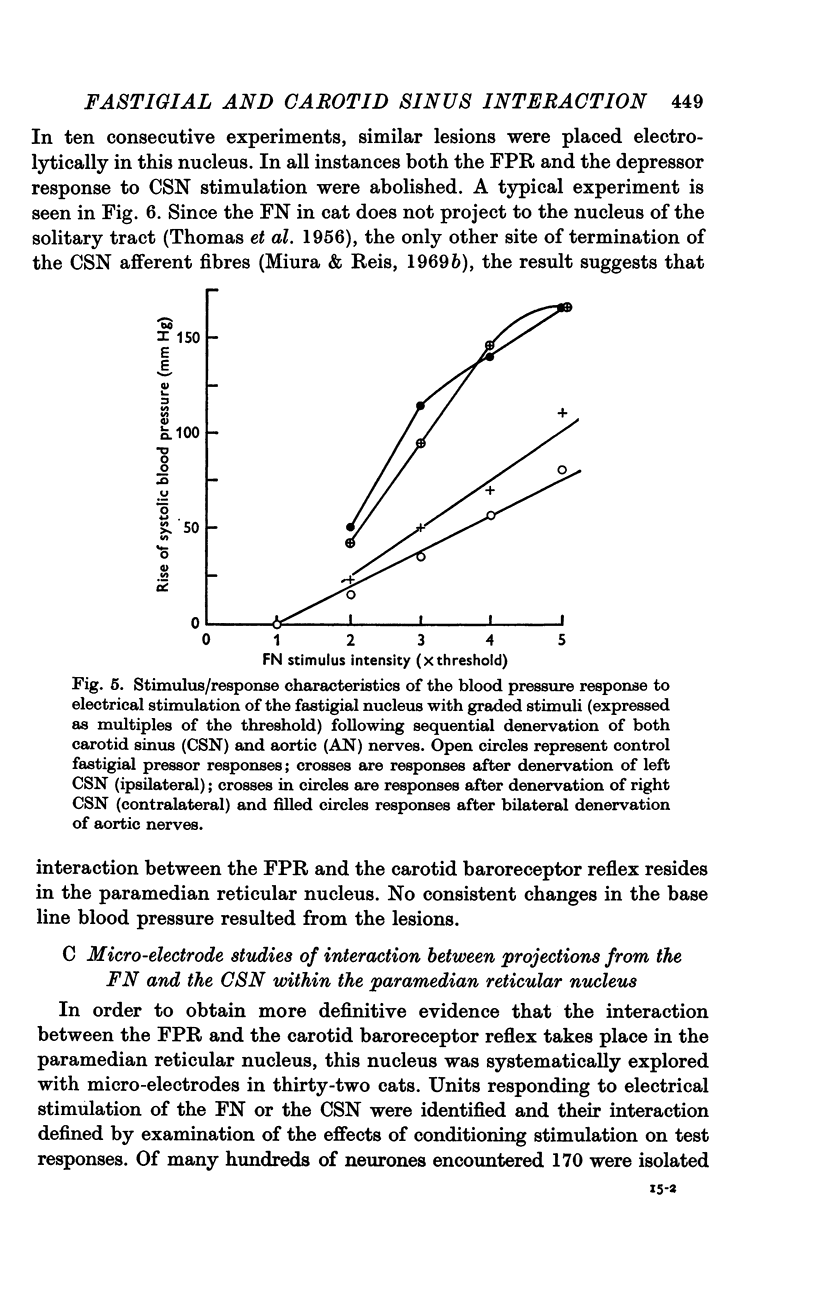
3. The FPR was augmented after denervation of buffer nerves. Lesions of the FN did not alter the depressor response to stimulation of the CSN.
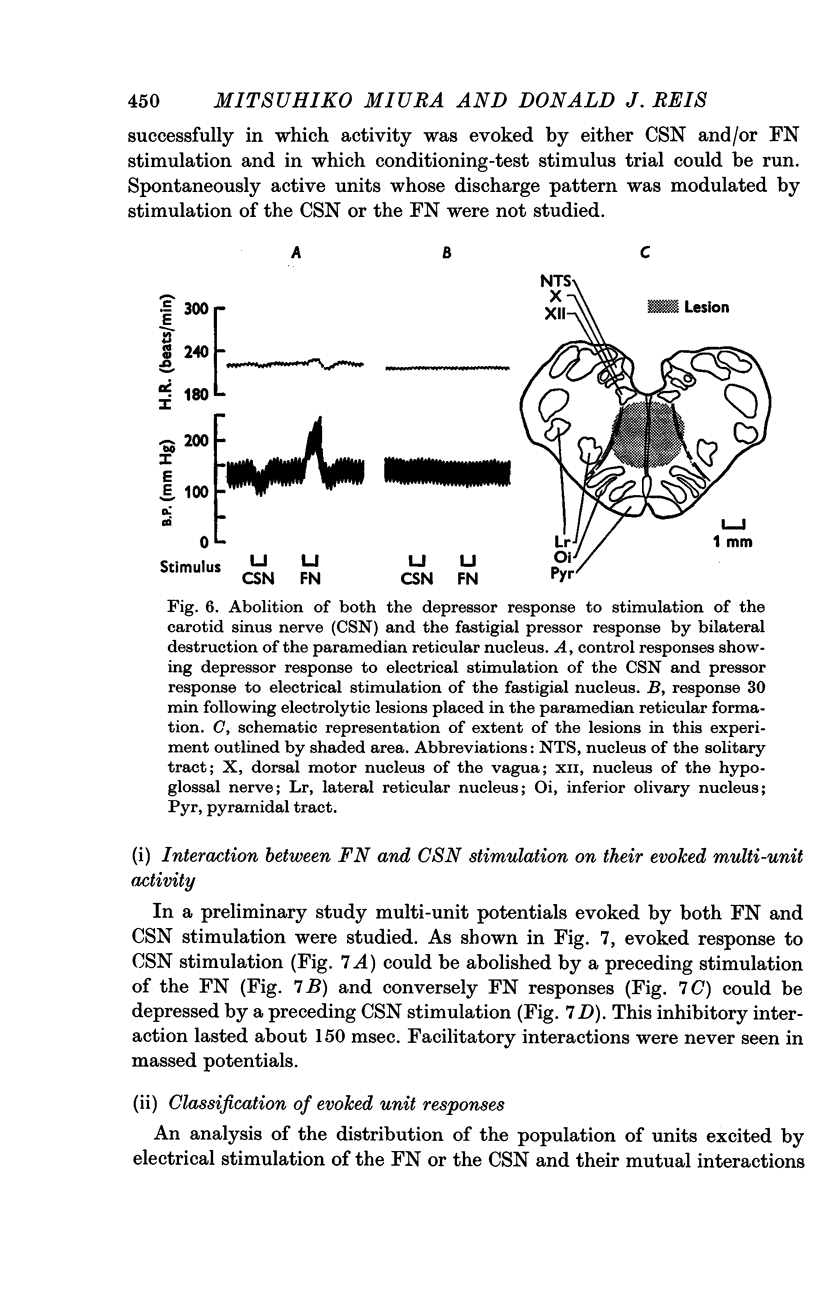
4. Bilateral electrolytic lesions of the paramedian reticular nucleus abolished both the FPR and the CSN depressor response without altering base line pressure.
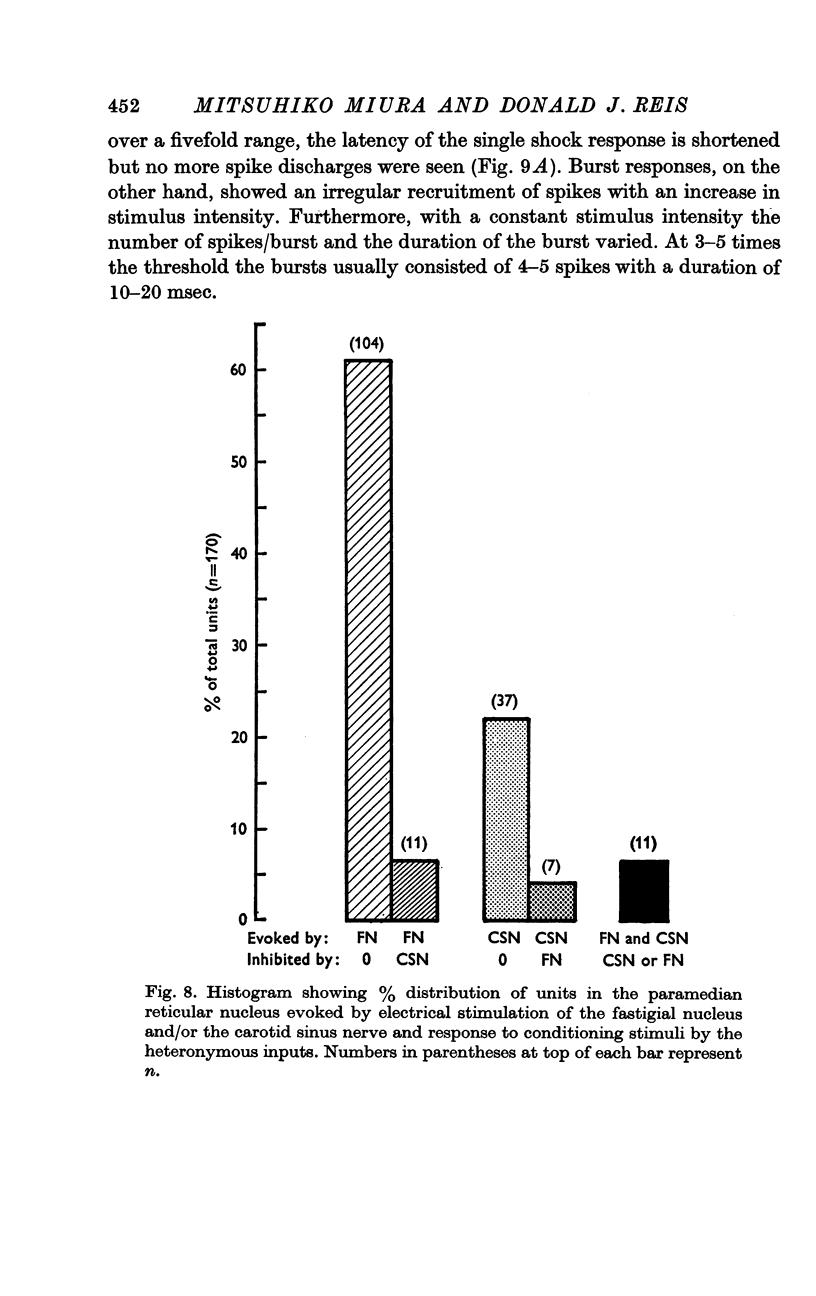
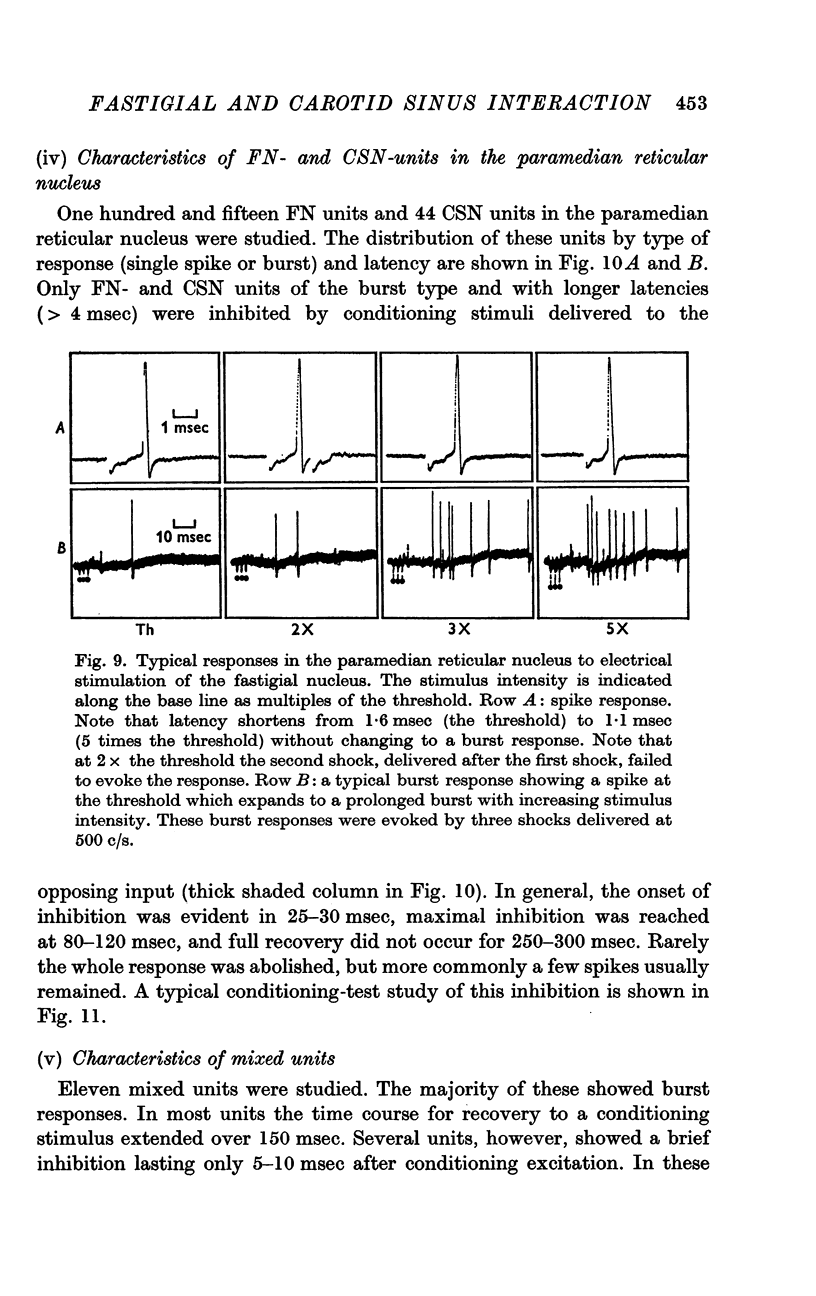
5. With micro-electrode recording neurones were discovered within the paramedian reticular nucleus which responded to electrical stimulation of the FN or the CSN. These neurones were polysynaptically excited by stimulation of either the FN or the CSN but rarely from both, and could be further subdivided into cells responding with either a single spike or a burst discharge.
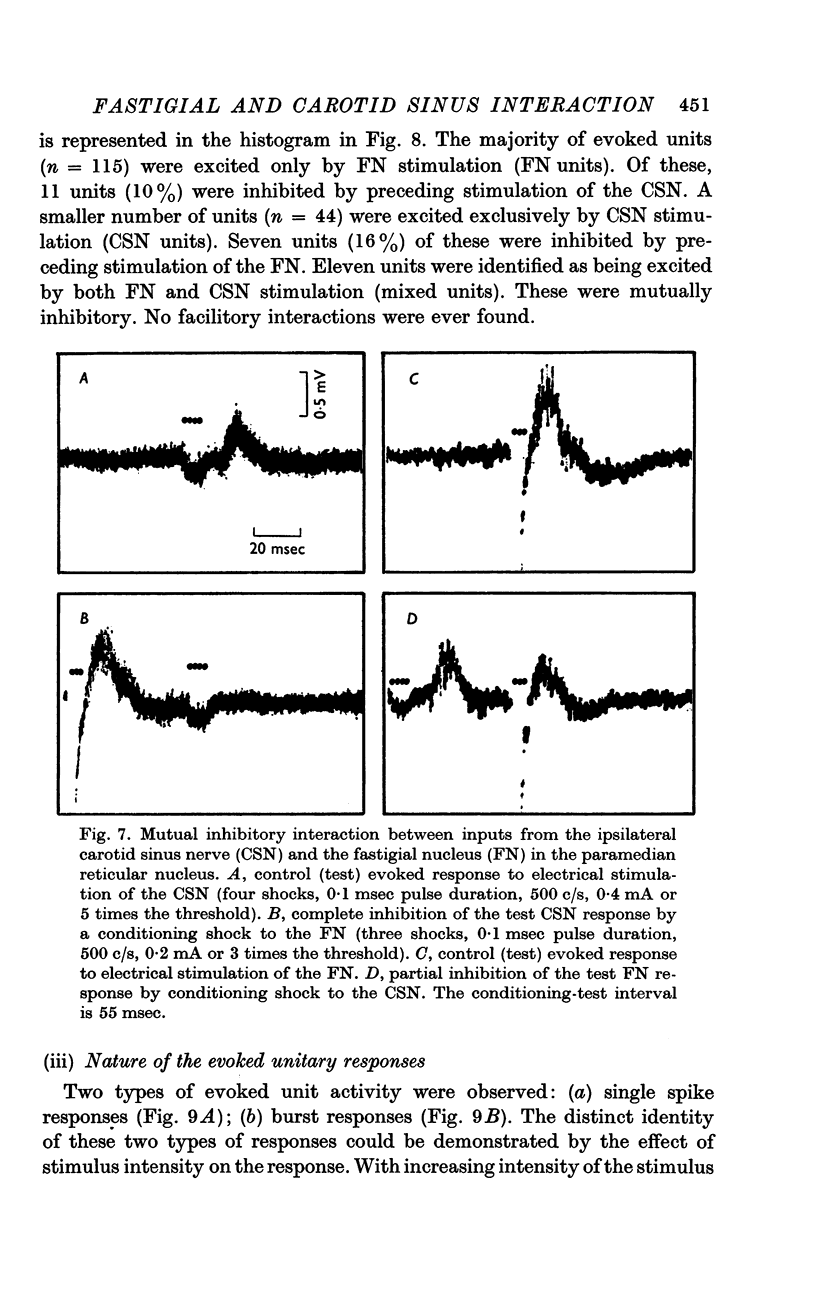
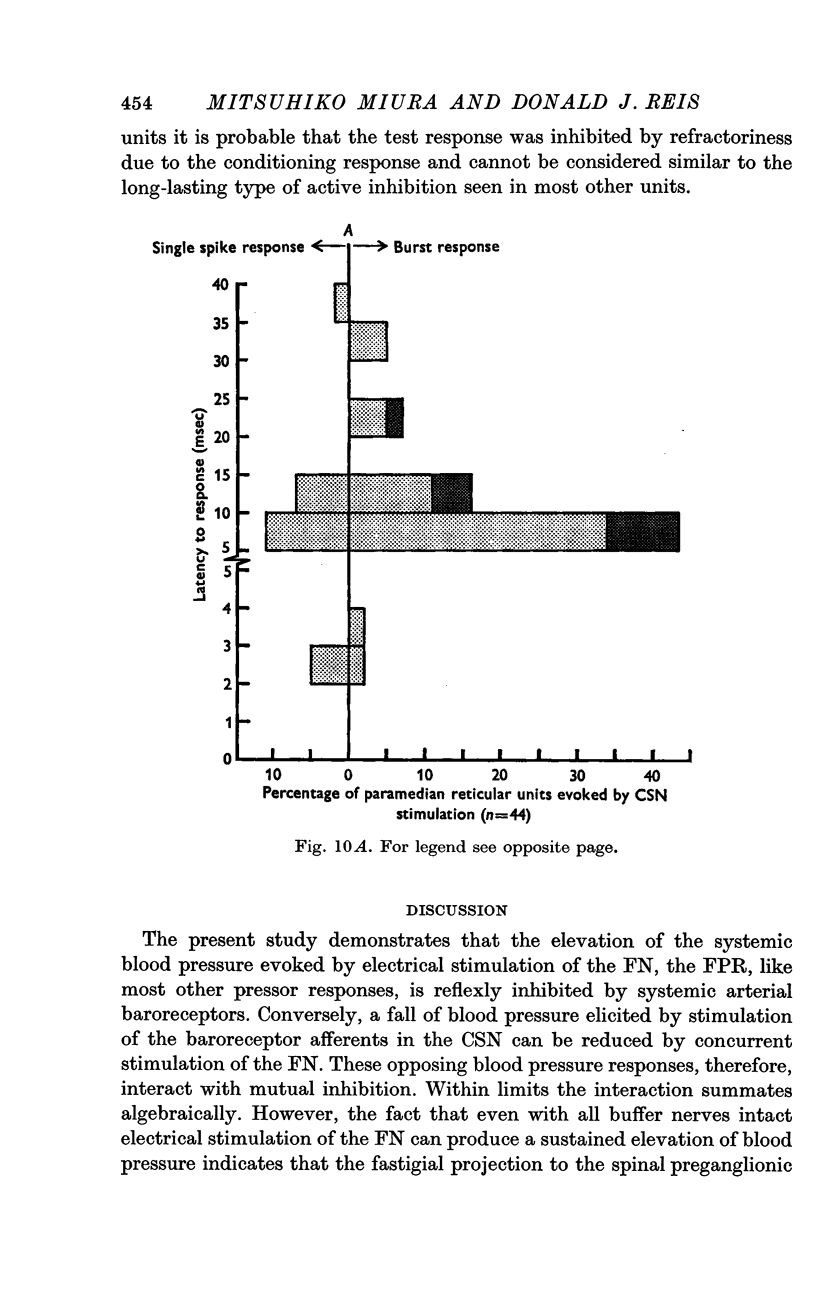
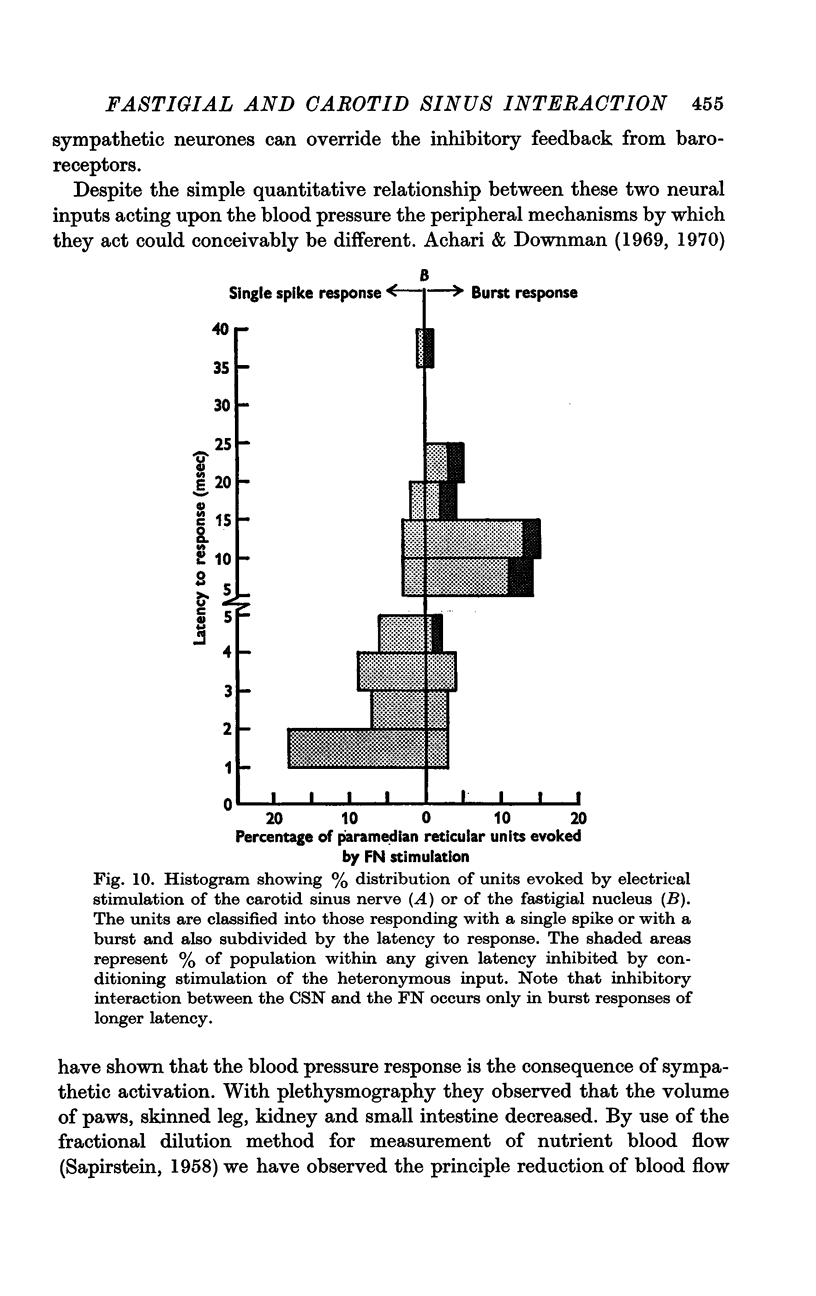
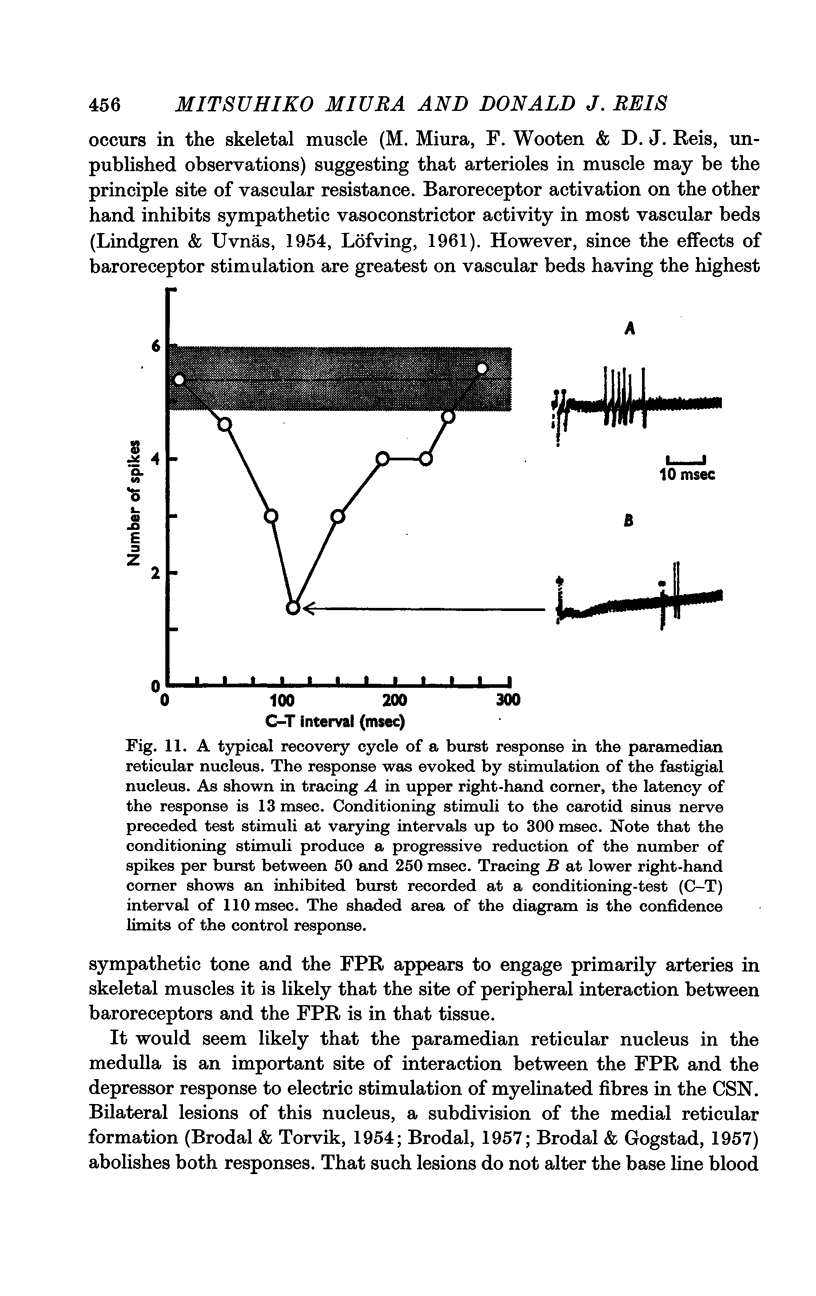
6. The interaction between the FN and the CSN projections to the paramedian reticular nucleus was examined by conditioning-test studies. Eleven per cent of FN- and CSN-units were inhibited by conditioning stimulation of the heteronymous input. The interaction was exclusively inhibitory and observed only in units with latencies > 4 msec and having burst responses. The latency for inhibition was > 20 msec, peaked around 100 msec and lasted up to 300 msec.
7. We conclude that the FRP is buffered by baroreceptors and that there is a mutually inhibitory interaction between projections from the FN and the CSN acting on sympathetic vasomotor neurones. The paramedian reticular nucleus appears to be an important site for the interaction.
8. The findings support the view that interneurones mediating pressor and depressor responses are intermixed within the medial reticular formation of the medulla.
Full text
PDF



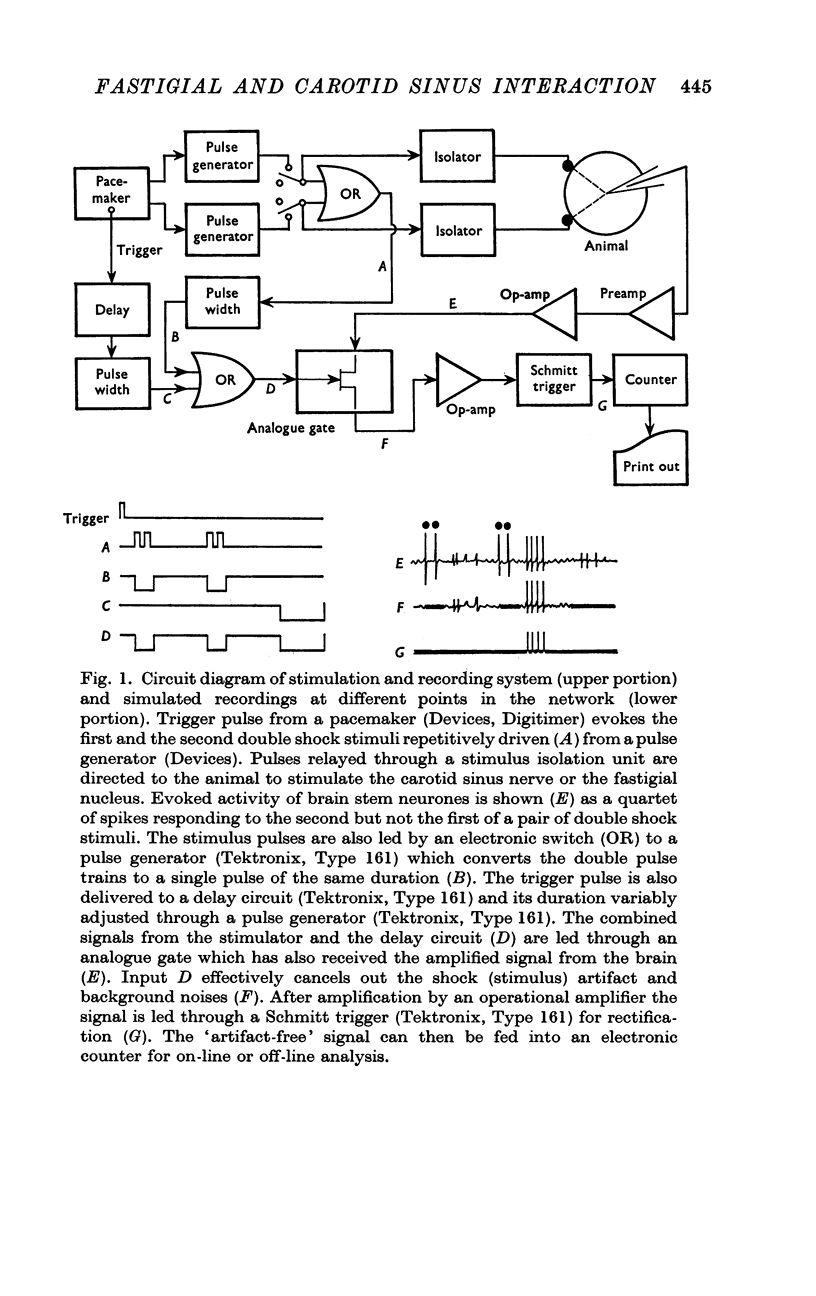




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achari N. K., Downman C. B. Autonomic effector responses to stimulation of nucleus fastigius. J Physiol. 1970 Oct;210(3):637–650. doi: 10.1113/jphysiol.1970.sp009232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achari N. K., Downman C. B. Autonomic responses evoked by stimulation of fastigial nuclei in the anaesthetized cat. J Physiol. 1969 Oct;204(2):130P+–130P+. [PubMed] [Google Scholar]
- BACH L. M. N. Relationships between bulbar respiratory, vasomotor and somatic facilitatory and inhibitory areas. Am J Physiol. 1952 Nov;171(2):417–435. doi: 10.1152/ajplegacy.1952.171.2.417. [DOI] [PubMed] [Google Scholar]
- BRODAL A., GOGSTAD A. C. Afferent connexions of the paramedian reticular nucleus of the medulla oblongata in the cat; an experimental study. Acta Anat (Basel) 1957;30(1-4):133–151. doi: 10.1159/000141194. [DOI] [PubMed] [Google Scholar]
- BRODAL A., TORVIK A. Cerebellar projection of paramedian reticular nucleus of medulla oblongata in cat. J Neurophysiol. 1954 Sep;17(5):484–495. doi: 10.1152/jn.1954.17.5.484. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. Field potentials evoked in the brain stem of the cat by stimulation of the carotid sinus, glossopharyngeal, aortic and superior laryngeal nerves. J Physiol. 1970 Aug;209(2):341–358. doi: 10.1113/jphysiol.1970.sp009168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. Responses of cells in the brain stem of the cat to stimulation of the sinus, glossopharyngeal, aortic and superior laryngeal nerves. J Physiol. 1970 Aug;209(2):359–373. doi: 10.1113/jphysiol.1970.sp009169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAI C. Y., SHARE N. N., WANG S. C. CENTRAL CONTROL OF SYMPATHETIC CARDIAC AUGMENTATION IN LOWER BRAIN STEM OF THE CAT. Am J Physiol. 1963 Oct;205:749–753. doi: 10.1152/ajplegacy.1963.205.4.749. [DOI] [PubMed] [Google Scholar]
- CHAI C. Y., WANG S. C. Localization of central cardiovascular control mechanism in lower brain stem of the cat. Am J Physiol. 1962 Jan;202:25–30. doi: 10.1152/ajplegacy.1962.202.1.25. [DOI] [PubMed] [Google Scholar]
- COTTLE M. K. DEGENERATION STUDIES OF PRIMARY AFFERENTS OF IXTH AND XTH CRANIAL NERVES IN THE CAT. J Comp Neurol. 1964 Jun;122:329–345. doi: 10.1002/cne.901220304. [DOI] [PubMed] [Google Scholar]
- Calaresu F. R., Henry J. L. The mechanism of the cardio-acceleration elicited by electrical stimulation of the parahypoglossal area in the cat. J Physiol. 1970 Sep;210(1):107–120. doi: 10.1113/jphysiol.1970.sp009198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill W. E., Reis D. J. Distribution of carotid sinus and depressor nerves in cat brain stem. Am J Physiol. 1968 Feb;214(2):269–276. doi: 10.1152/ajplegacy.1968.214.2.269. [DOI] [PubMed] [Google Scholar]
- Homma S., Miura M., Reis D. J. Intracellular recording from paramedian reticular neurons monosynaptically excited by stimulation of the carotid sinus nerve. Brain Res. 1970 Feb 17;18(1):185–188. doi: 10.1016/0006-8993(70)90467-1. [DOI] [PubMed] [Google Scholar]
- Ito M., Udo M., Mano N., Kawai N. Synaptic action of the fastigiobulbar impulses upon neurones in the medullary reticular formation and vestibular nuclei. Exp Brain Res. 1970;11(1):29–47. doi: 10.1007/BF00234200. [DOI] [PubMed] [Google Scholar]
- KERR F. W. Facial, vagal and glossopharyngeal nerves in the cat. Afferent connections. Arch Neurol. 1962 Apr;6:264–281. doi: 10.1001/archneur.1962.00450220006003. [DOI] [PubMed] [Google Scholar]
- LINDGREN P., UVNAS B. Postulated vasodilator center in the medulla oblongata. Am J Physiol. 1954 Jan;176(1):68–76. doi: 10.1152/ajplegacy.1953.176.1.68. [DOI] [PubMed] [Google Scholar]
- LOFVING B. Cardiovascular adjustments induced from the rostral cingulate gyrus with special reference to sympatho-inhibitory mechanisms. Acta Physiol Scand Suppl. 1961;53(184):1–82. [PubMed] [Google Scholar]
- Miura M., Reis D. J. A blood pressure response from fastigial nucleus and its relay pathway in brainstem. Am J Physiol. 1970 Nov;219(5):1330–1336. doi: 10.1152/ajplegacy.1970.219.5.1330. [DOI] [PubMed] [Google Scholar]
- Miura M., Reis D. J. Cerebellum: a pressor response elicited from the fastigial nucleus and its efferent pathway in brainstem. Brain Res. 1969 May;13(3):595–599. doi: 10.1016/0006-8993(69)90269-8. [DOI] [PubMed] [Google Scholar]
- Miura M., Reis D. J. Electrophysiological evidence that carotid sinus nerve fibers terminated the bulbar reticular formation. Brain Res. 1968 Jul;9(2):394–397. doi: 10.1016/0006-8993(68)90247-3. [DOI] [PubMed] [Google Scholar]
- Miura M., Reis D. J. Termination and secondary projections of carotid sinus nerve in the cat brain stem. Am J Physiol. 1969 Jul;217(1):142–153. doi: 10.1152/ajplegacy.1969.217.1.142. [DOI] [PubMed] [Google Scholar]
- Reis D. J., Cuénod M. Central neural regulation of carotid baroreceptor reflexes in the cat. Am J Physiol. 1965 Dec;209(6):1267–1277. doi: 10.1152/ajplegacy.1965.209.6.1267. [DOI] [PubMed] [Google Scholar]
- SALMOIRAGHI G. C., BURNS B. D. Localization and patterns of discharge of respiratory neurones in brain-stem of cat. J Neurophysiol. 1960 Jan;23:2–13. doi: 10.1152/jn.1960.23.1.2. [DOI] [PubMed] [Google Scholar]
- SAPIRSTEIN L. A. Regional blood flow by fractional distribution of indicators. Am J Physiol. 1958 Apr;193(1):161–168. doi: 10.1152/ajplegacy.1958.193.1.161. [DOI] [PubMed] [Google Scholar]
- Seller H., Illert M. The localization of the first synapse in the carotid sinus baroreceptor reflex pathway and its alteration of the afferent input. Pflugers Arch. 1969;306(1):1–19. doi: 10.1007/BF00586608. [DOI] [PubMed] [Google Scholar]
- TERZUOLO C. A., ARAKI T. An analysis of intra- versus extracellular potential changes associated with activity of single spinal motoneurons. Ann N Y Acad Sci. 1961 Sep 6;94:547–558. doi: 10.1111/j.1749-6632.1961.tb35558.x. [DOI] [PubMed] [Google Scholar]
- THOMAS D. M., KAUFMAN R. P., SPRAGUE J. M., CHAMBERS W. W. Experimental studies of the vermal cerebellar projections in the brain stem of the cat (fastigiobulbar tract). J Anat. 1956 Jul;90(3):371–385. [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C., Wilson V. J. Precise localization of Renshaw cells with a new marking technique. Nature. 1965 Apr 10;206(980):211–213. doi: 10.1038/206211b0. [DOI] [PubMed] [Google Scholar]


