Abstract
The pulsed-gradient spin echo nuclear magnetic resonance (PGSENMR) technique was used to measure restricted diffusion of water in three types of animal tissue: human blood plasma and red cells; rat and rabbit heart; rat and rabbit liver. Characteristic lengths (L) for restriction of diffusion are estimated from dependence on the measuring time. Limitations on the range of observable restrictive lengths (1.5-15 μm) are discussed.
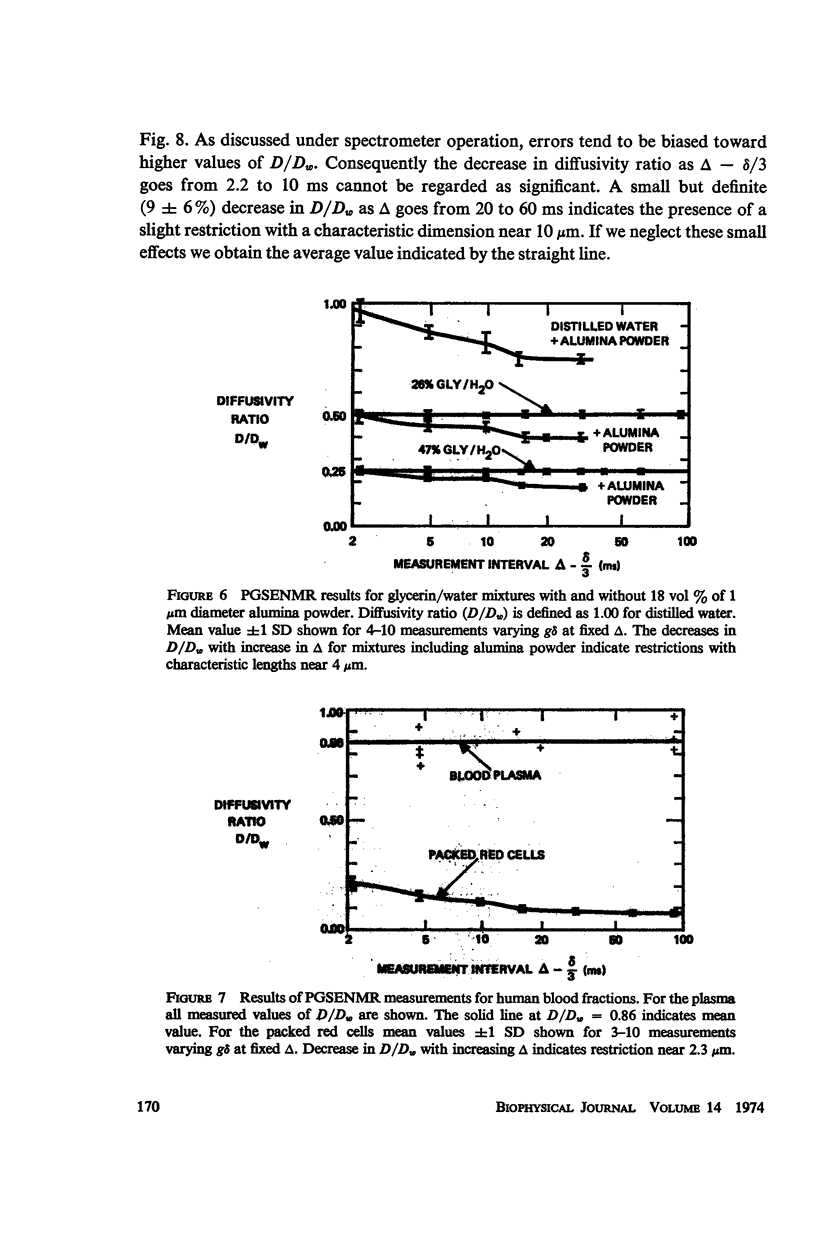
The decrease in diffusivity due to 1 μm alumina powder (volume fraction = 0.18) in glycerin/water mixtures agrees with the Wang theory assuming spherical particles and no hydration. The characteristic length (L ≃ 4 μm) is larger than the particle size (1 μm) or separation (1.8 μm). Comparison of the diffusivities in tissues at short diffusion times with the Wang theory indicates some bound or trapped water.
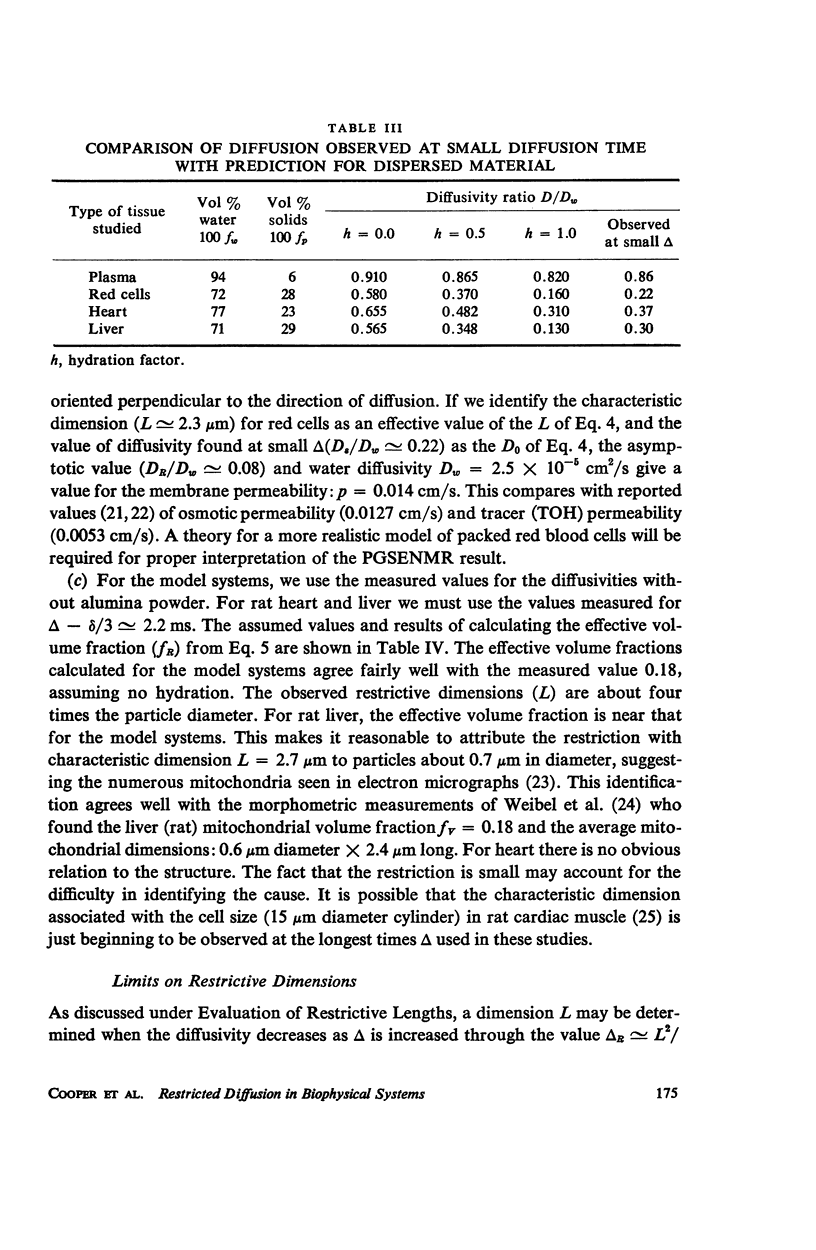
For packed red blood cells, a restriction (L ≃ 2.3 μm) was attributed tothe red cell membrane. A permeability p ≃ 0.014 cm/s may be estimated from the decrease in diffusivity. Average values of diffusivity ratio in heart were: 0.36 ± 0.02 for rat; and 0.26 ± 0.03 for rabbit; and in liver: 0.25 ± 0.01 for rat; 0.25 ± .04 for 10-day old rabbit; and 0.195 ± 0.03 for 2-yr old rabbit. A restriction (L ≃ 2.7 μm) in rat liver probably results from the mitochondria.
Full text
PDF


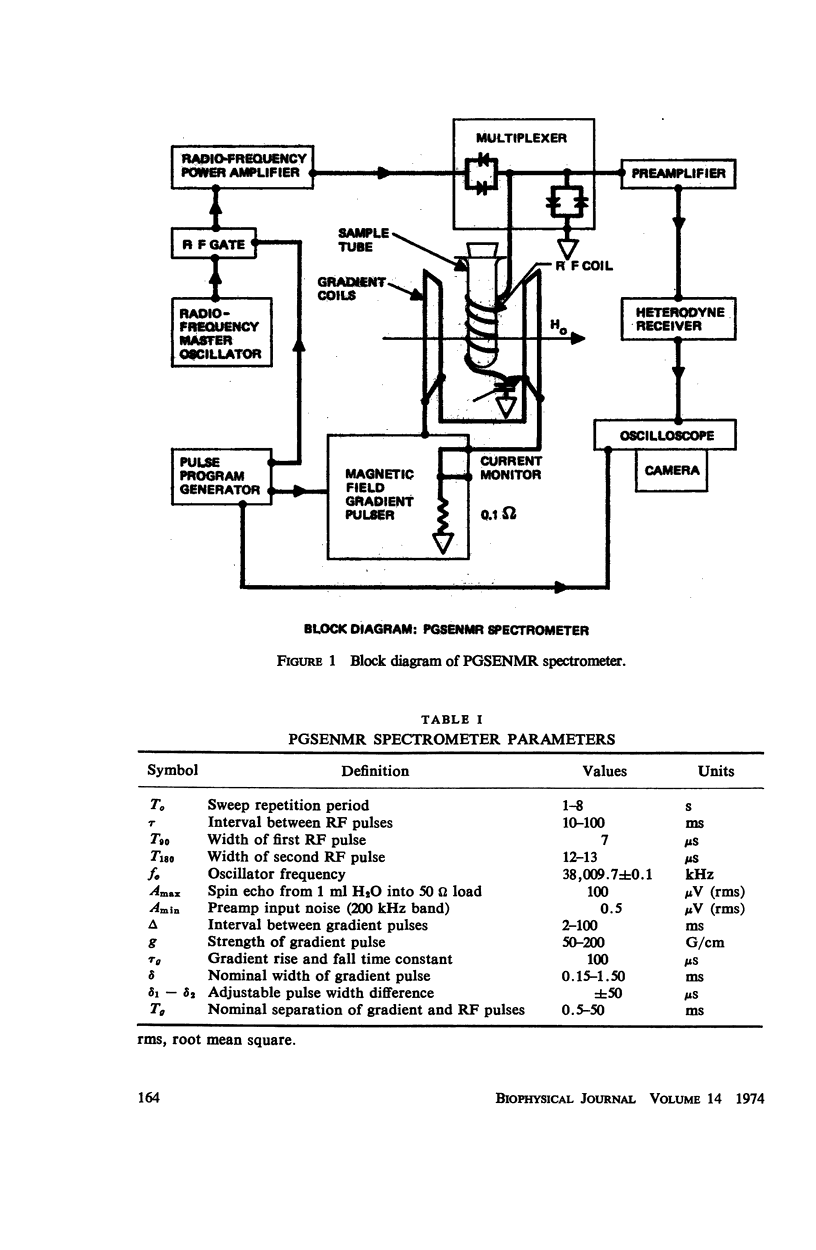
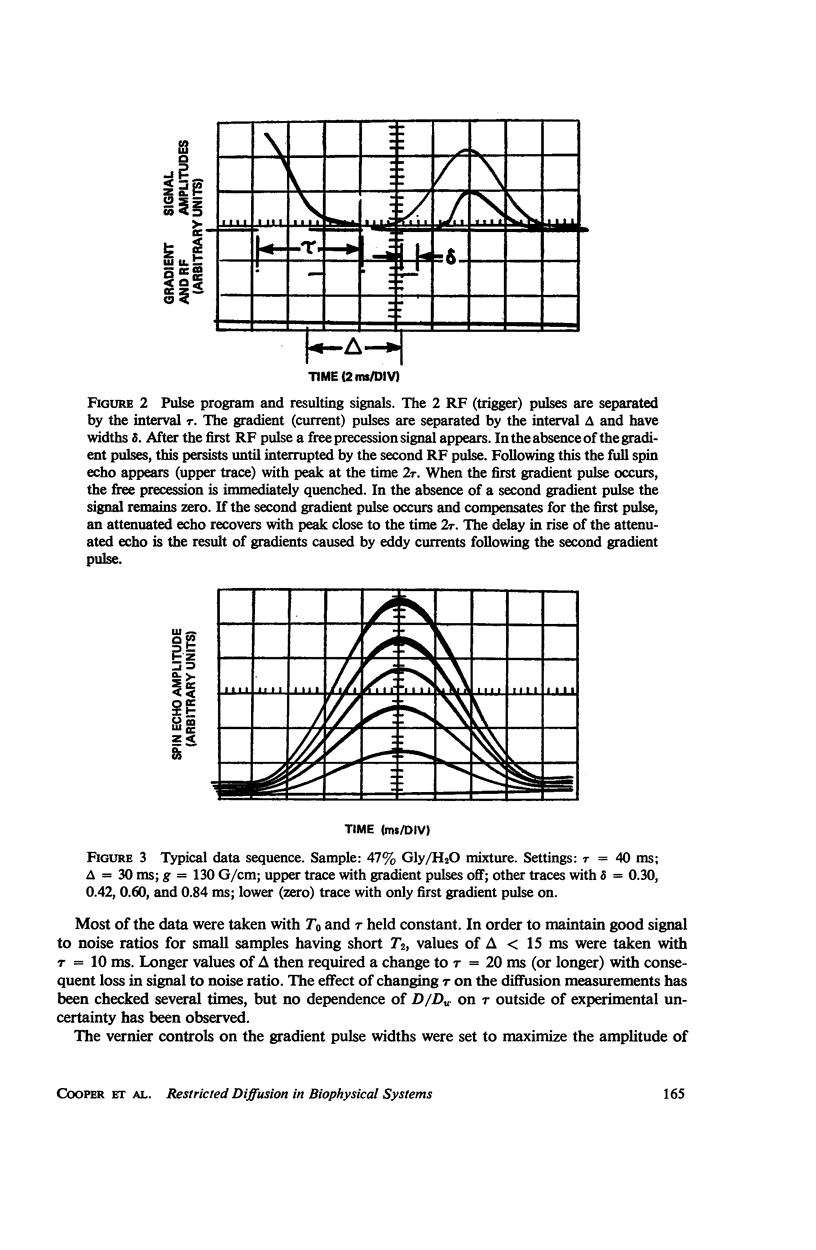

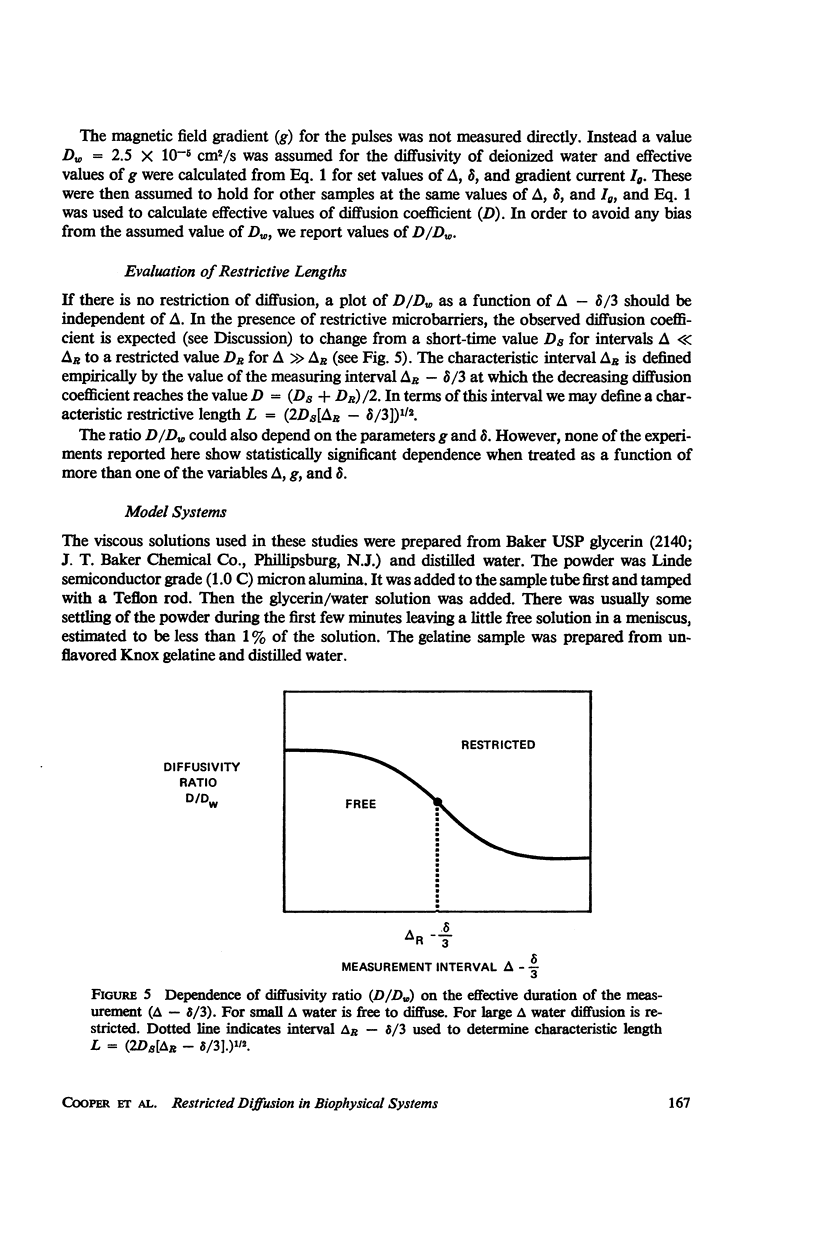

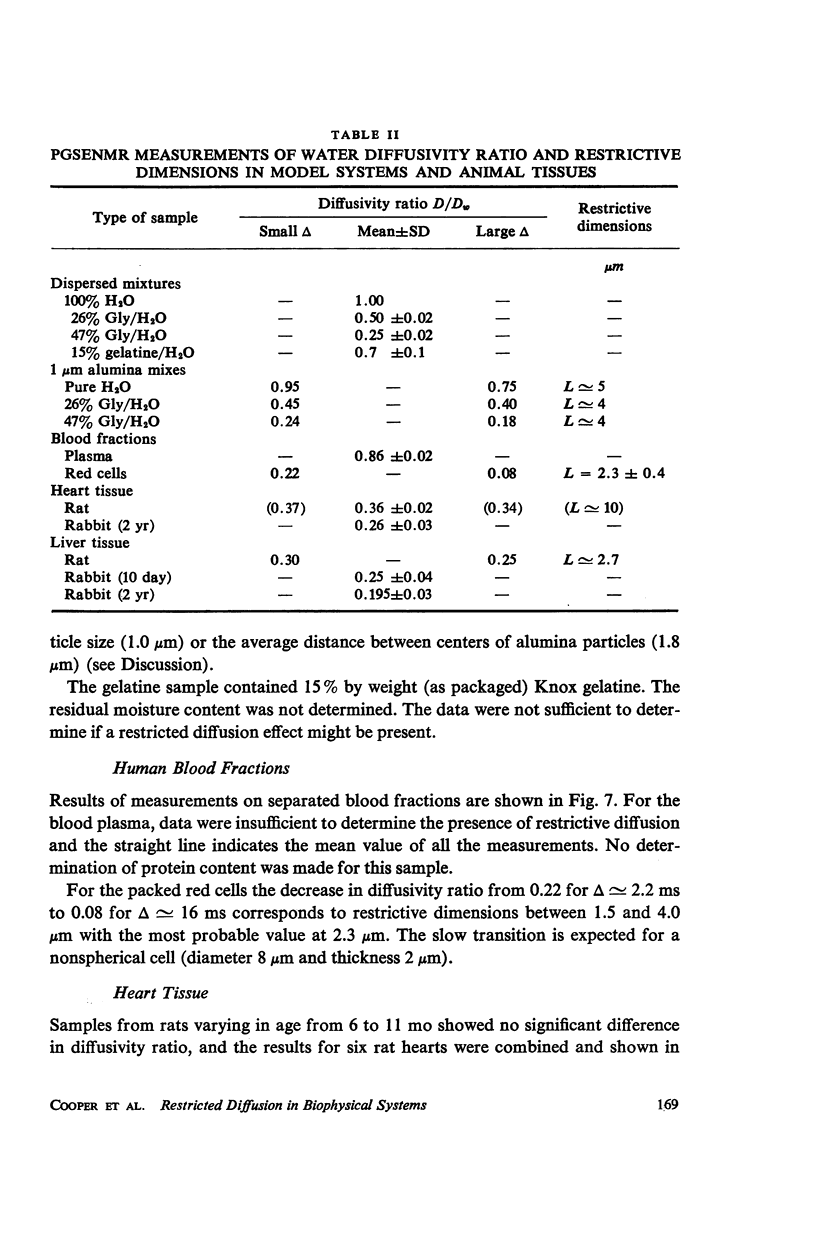






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang D. C., Hazlewood C. F., Nichols B. L., Rorschach H. E. Spin echo studies on cellular water. Nature. 1972 Jan 21;235(5334):170–171. doi: 10.1038/235170a0. [DOI] [PubMed] [Google Scholar]
- Crick F. Diffusion in embryogenesis. Nature. 1970 Jan 31;225(5231):420–422. doi: 10.1038/225420a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Excitation-contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemmas. Calcium-dependent cyclic and tonic contractions. Circ Res. 1972 Sep;31(3):293–307. doi: 10.1161/01.res.31.3.293. [DOI] [PubMed] [Google Scholar]
- Finch E. D., Harmon J. F., Muller B. H. Pulsed NMR measurements of the diffusion constant of water in muscle. Arch Biochem Biophys. 1971 Nov;147(1):299–310. doi: 10.1016/0003-9861(71)90337-7. [DOI] [PubMed] [Google Scholar]
- Hansen J. R. Pulsed NMR study of water mobility in muscle and brain tissue. Biochim Biophys Acta. 1971;230(3):482–486. doi: 10.1016/0304-4165(71)90177-2. [DOI] [PubMed] [Google Scholar]
- Ling G. N. A new model for the living cell: a summary of the theory and recent experimental evidence in its support. Int Rev Cytol. 1969;26:1–61. doi: 10.1016/s0074-7696(08)61633-2. [DOI] [PubMed] [Google Scholar]
- Sha'afi R. I., Rich G. T., Sidel V. W., Bossert W., Solomon A. K. The effect of the unstirred layer on human red cell water permeability. J Gen Physiol. 1967 May;50(5):1377–1399. doi: 10.1085/jgp.50.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]


