Abstract
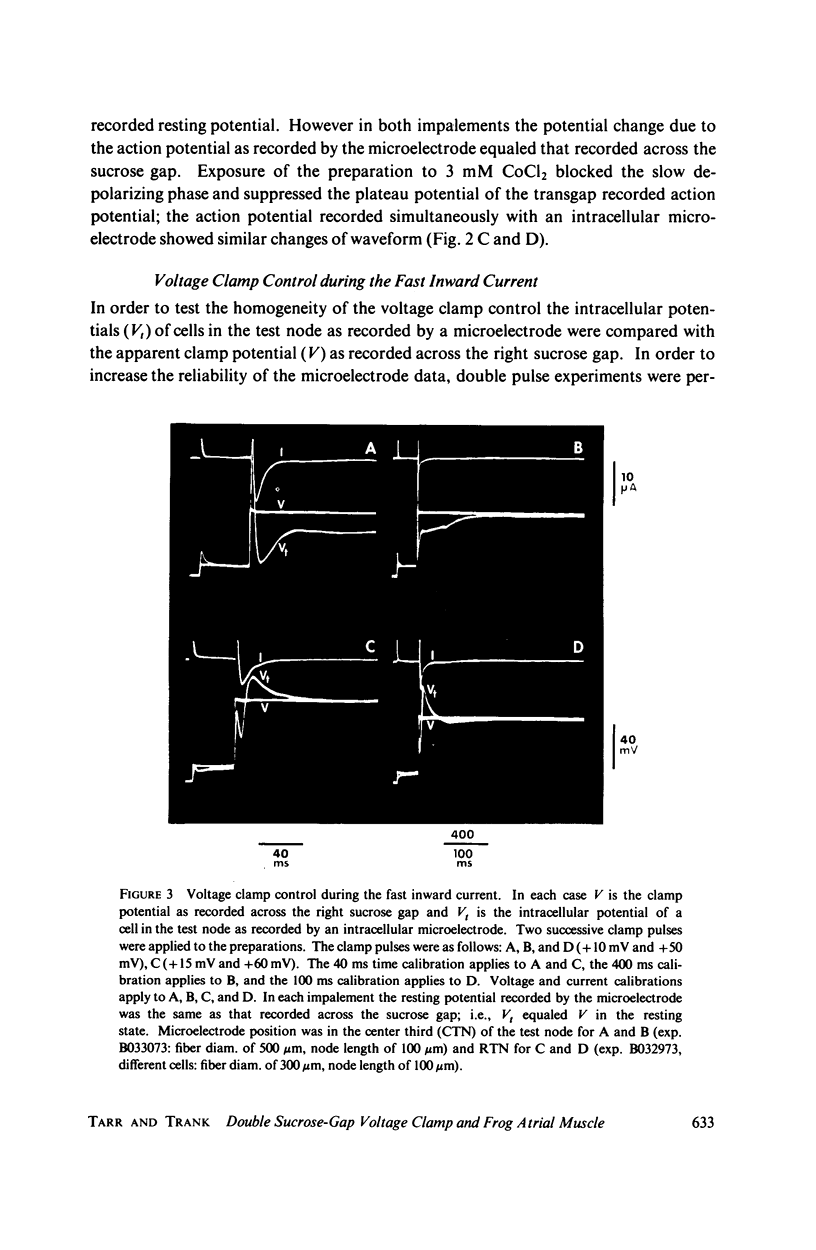
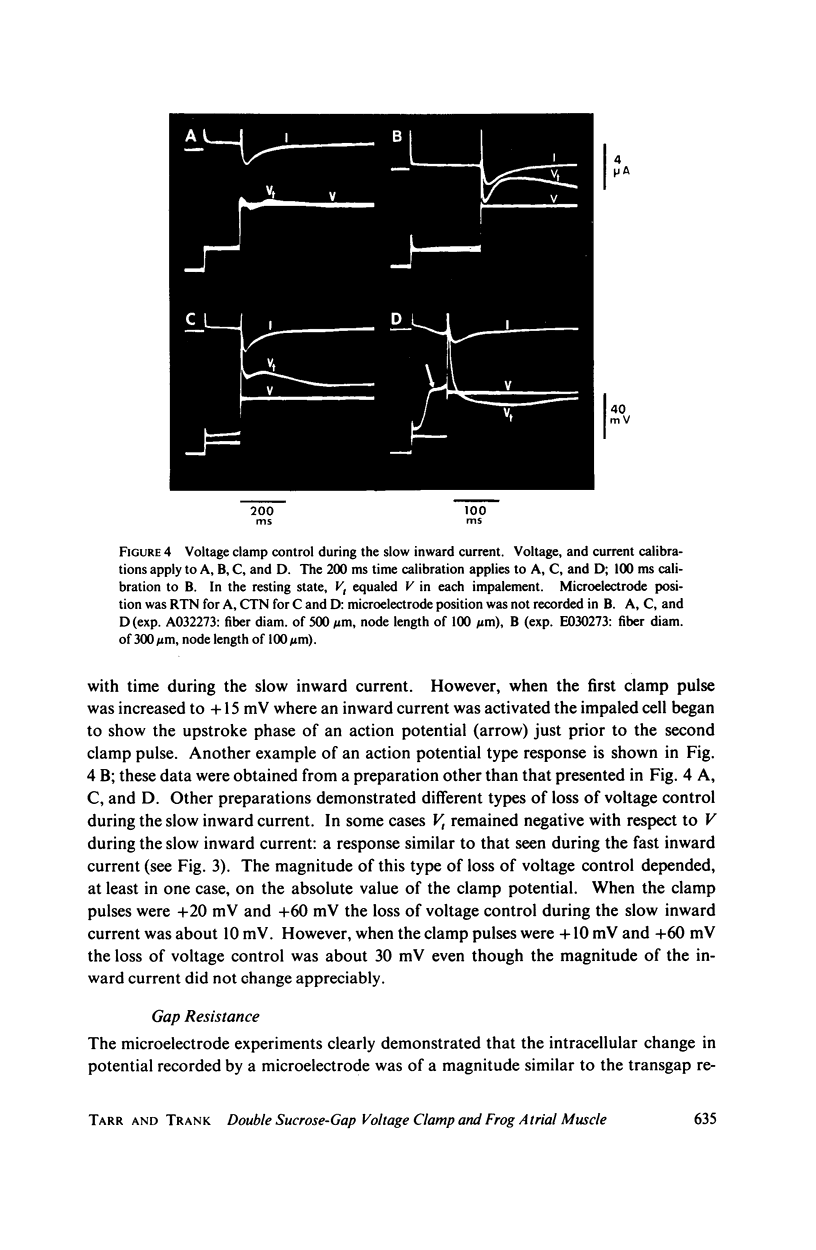
The homogeneity of voltage clamp control in small bundles of frog atrial tissue under double sucrose-gap voltage clamp conditions was assessed by intracellular microelectrode potential measurements from cells in the test node region. The microelectrode potential measurements demonstrated that (1) good voltage control of the impaled cell existed in the absence of the excitatory inward currents (e.g., during small depolarizing clamp pulses of 10-15 mV), (2) voltage control of the impaled cell was lost during either the fast or slow excitatory inward currents, and (3) voltage control of the impaled cell was regained following the inward excitatory currents. Under nonvoltage clamp conditions the transgap recorded action potential had a magnitude and waveform similar to the intracellular microelectrode recorded action potentials from cells in the test node. Transgap impedance measured with a sine-wave voltage of 1,000 Hz was about 63% of that measured either by a sine-wave voltage of 10 Hz or by an action potential method used to determine the longitudinal resistance through the sucrose-gap region. The action potential data in conjunction with the impedance data indicate that the extracellular resistance (Rs) through the sucrose gap is very large with respect to the longitudinal intracellular resistance (Ri); the frequency dependence of the transgap impedance suggests that at least part of the intracellular resistance is paralleled by a capacitance. The severe loss of spatial voltage control during the excitatory inward current raises serious doubts concerning the use of the double sucrose-gap technique to voltage clamp frog atrial muscle.
Full text
PDF


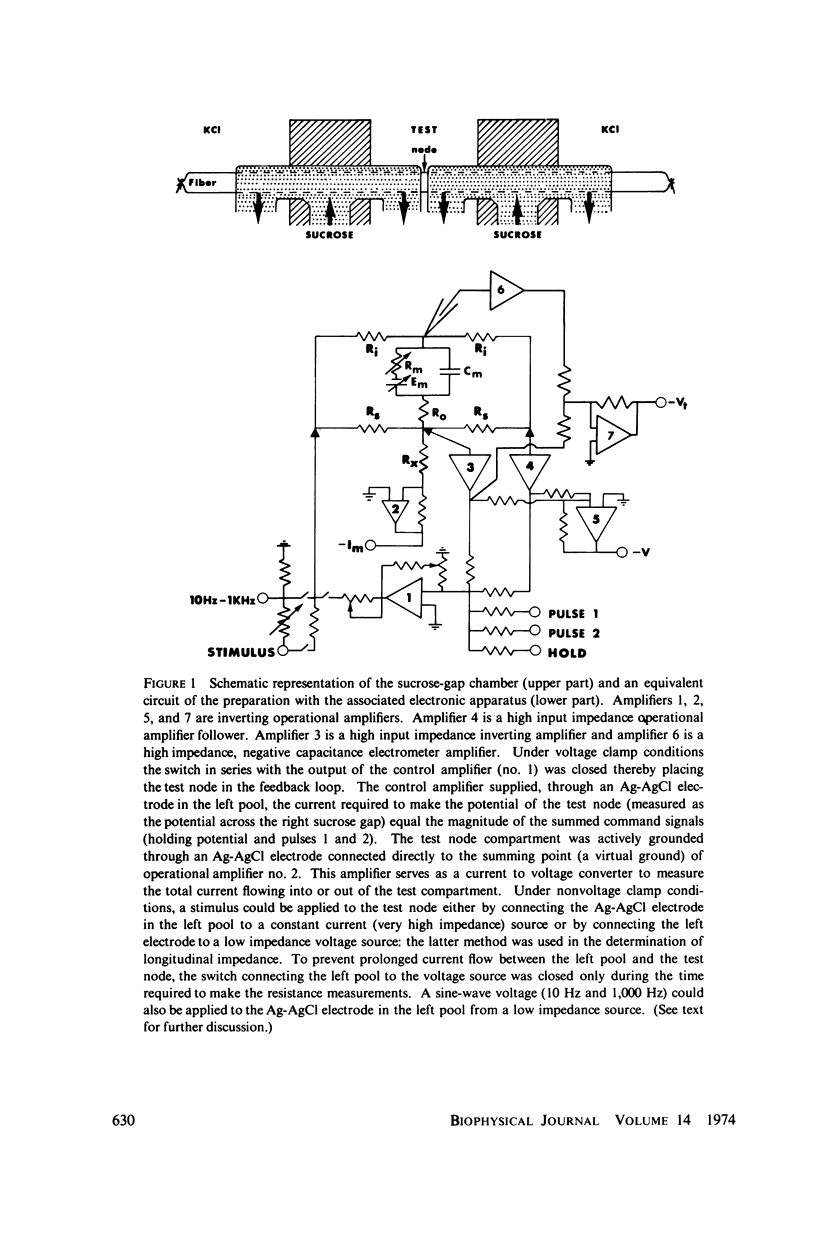











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Voltage clamp experiments on ventricular myocarial fibres. J Physiol. 1970 Mar;207(1):165–190. doi: 10.1113/jphysiol.1970.sp009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einwächter H. M., Haas H. G., Kern R. Membrane current and contraction in frog atrial fibres. J Physiol. 1972 Dec;227(1):141–171. doi: 10.1113/jphysiol.1972.sp010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freygang W. H., Trautwein W. The structural implications of the linear electrical properties of cardiac Purkinje strands. J Gen Physiol. 1970 Apr;55(4):524–547. doi: 10.1085/jgp.55.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L., Johnson E. A. Voltage clamp of cardiac muscle in a double sucrose gap. A feasibility study. Biophys J. 1973 Jul;13(7):626–647. doi: 10.1016/S0006-3495(73)86013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Differentiation of the transmembrane Na and Ca channels in mammalian cardiac fibres by the use of specific inhibitors. Pflugers Arch. 1972;335(4):309–322. doi: 10.1007/BF00586221. [DOI] [PubMed] [Google Scholar]
- Kootsey J. M., Johnson E. A. Voltage clamp of cardiac muscle. A theoretical analysis of early currents in the single sucrose gap. Biophys J. 1972 Nov;12(11):1496–1508. doi: 10.1016/S0006-3495(72)86177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New W., Trautwein W. Inward membrane currents in mammalian myocardium. Pflugers Arch. 1972;334(1):1–23. doi: 10.1007/BF00585997. [DOI] [PubMed] [Google Scholar]
- SPERELAKIS N., HOSHIKO T. Electrical impedance of cardiac muscle. Circ Res. 1961 Nov;9:1280–1283. doi: 10.1161/01.res.9.6.1280. [DOI] [PubMed] [Google Scholar]
- TAYLOR R. E., MOORE J. W., COLE K. S. Analysis of certain errors in squid axon voltage clamp measurements. Biophys J. 1960 Nov;1:161–202. doi: 10.1016/s0006-3495(60)86882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr M., Luckstead E. F., Jurewicz P. A., Haas H. G. Effect of propranolol on the fast inward sodium current in frog atrial muscle. J Pharmacol Exp Ther. 1973 Mar;184(3):599–610. [PubMed] [Google Scholar]
- Tarr M., Trank J. Equivalent circuit of frog atrial tissue as determined by voltage clamp-unclamp experiments. J Gen Physiol. 1971 Nov;58(5):511–522. doi: 10.1085/jgp.58.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr M. Two inward currents in frog atrial muscle. J Gen Physiol. 1971 Nov;58(5):523–543. doi: 10.1085/jgp.58.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. H., Narahashi T. Mechanism of action of propranolol on squid axon membranes. J Pharmacol Exp Ther. 1973 Jan;184(1):155–162. [PubMed] [Google Scholar]


