Abstract
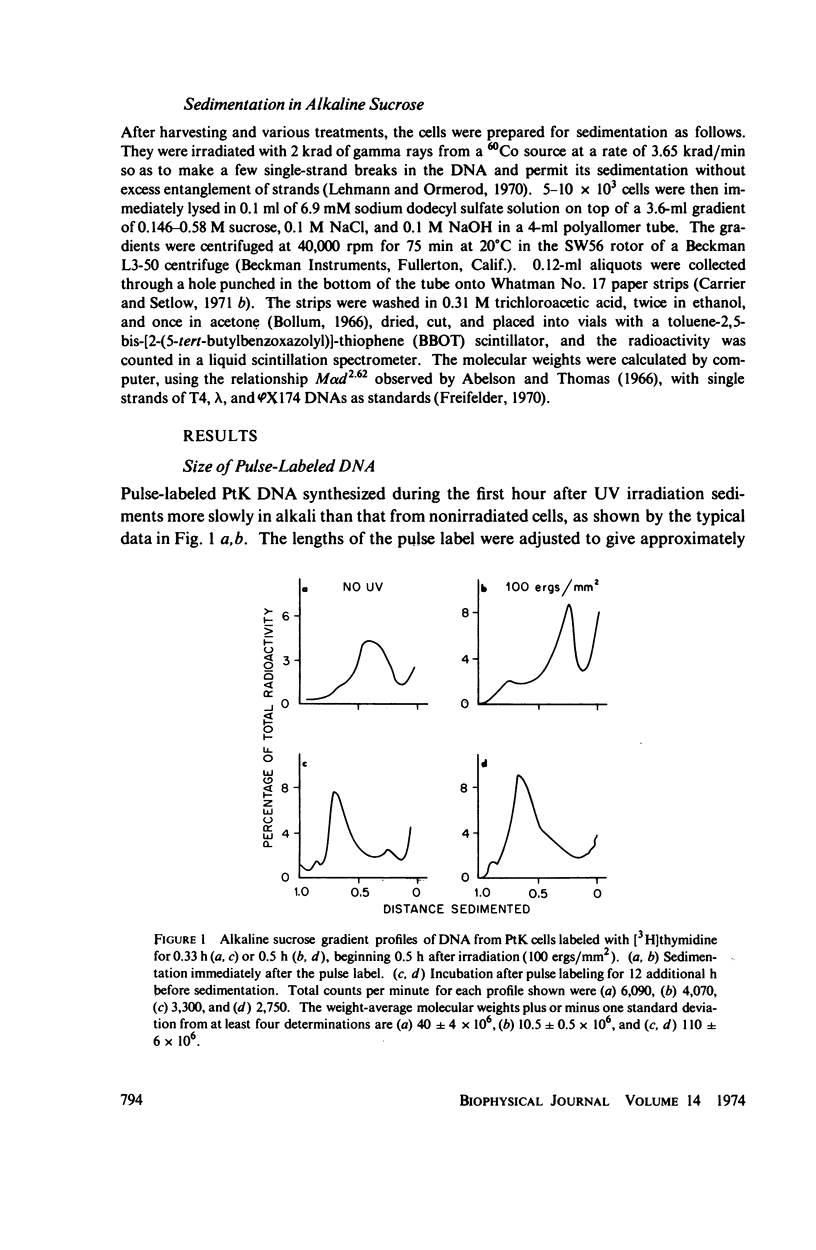
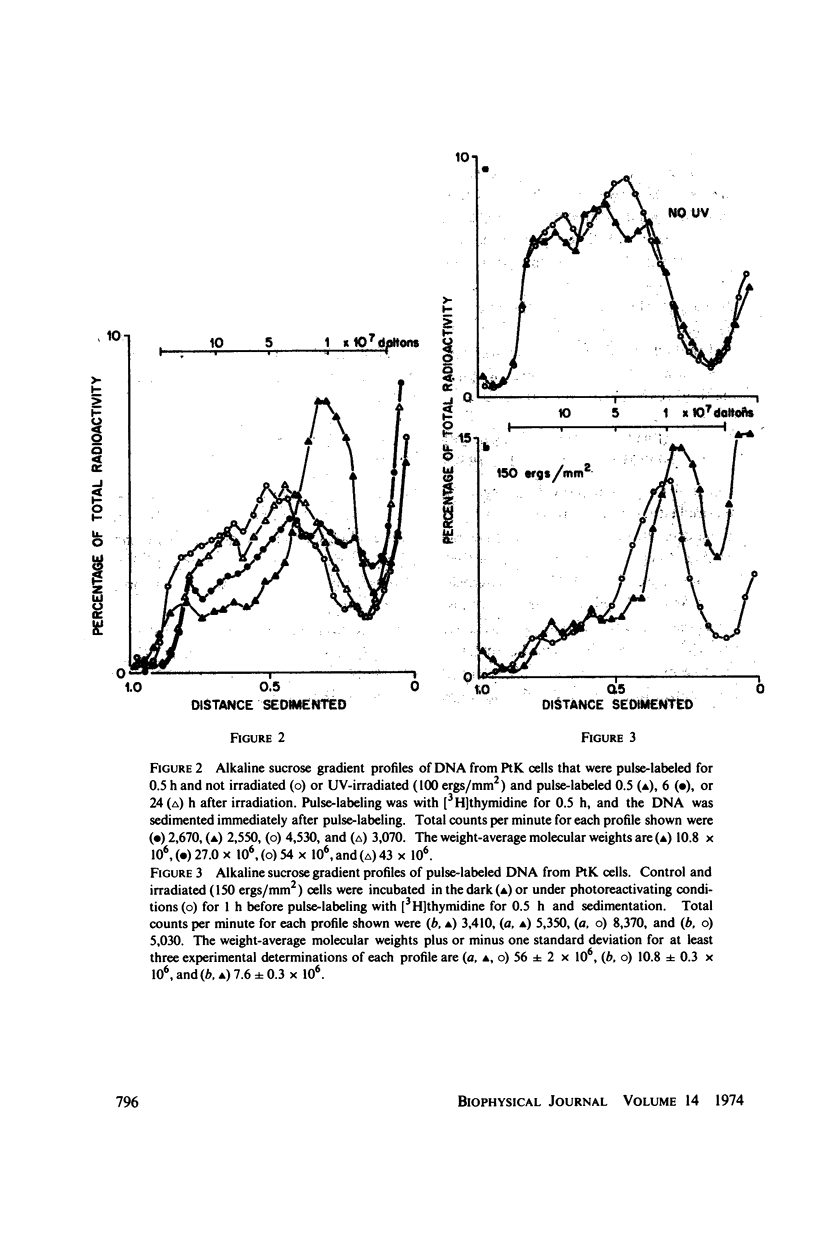
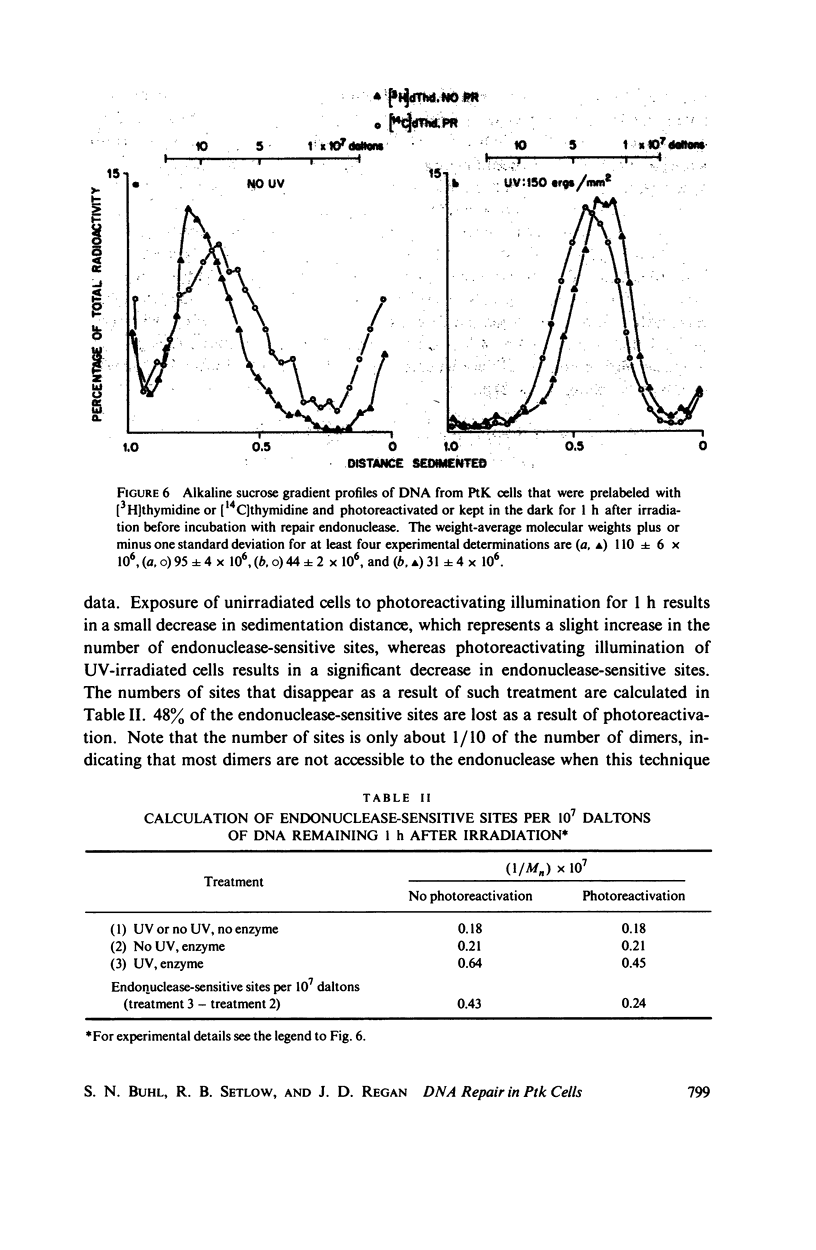
The DNA synthesized shortly after ultraviolet (UV) irradiation of Potorous tridactylis (PtK) cells sediments more slowly in alkali than that made by nonirradiated cells. The size of the single-strand segments is approximately equal to the average distance between 1 or 2 cyclobutyl pyrimidine dimers in the parental DNA. These data support the notion that dimers are the photoproducts which interrupt normal DNA replication. Upon incubation of irradiated cells the small segments are enlarged to form high molecular weight DNA as in nonirradiated cells. DNA synthesized at long times (∼ 24 h) after irradiation is made in segments approximately equal to those synthesized by nonirradiated cells, although only 10-15% of the dimers have been removed by excision repair. These data imply that dimers are not the lesions which initially interrupt normal DNA replication in irradiated cells. In an attempt to resolve these conflicting interpretations, PtK cells were exposed to photoreactivating light after irradiation and before pulse-labeling, since photoreactivation repair is specific for only one type of UV lesion. After 1 h of exposure ∼ 35% of the pyrimidine dimers have been monomerized, and the reduction in the percentage of dimers correlates with an increased size for the DNA synthesized by irradiated cells. Therefore, we conclude that the dimers are the lesions which initially interrupt DNA replication in irradiated PtK cells. The monomerization of pyrimidine dimers correlates with a disappearance of repair endonuclease-sensitive sites, as measured in vivo immediately after 1 h of photoreactivation, indicating that some of the sites sensitive to the repair endonuclease (from Micrococcus luteus) are pyrimidine dimers. However, at 24 h after irradiation and 1 h of photoreactivation there are no endonuclease-sensitive sites, even though ∼ 50% of the pyrimidine dimers remain in the DNA. These data indicate that not all pyrimidine dimers are accessible to the repair endonuclease. The observation that at long times after irradiation DNA is made in segments equal to those synthesized by nonirradiated cells although only a small percentage of the dimers have been removed suggests that an additional repair system alters dimers so that they no longer interrupt DNA replication.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buhl S. N., Stillman R. M., Setlow R. B., Regan J. D. DNA chain elongation and joining in normal human and xeroderma pigmentosum cells after ultraviolet irradiation. Biophys J. 1972 Sep;12(9):1183–1191. doi: 10.1016/S0006-3495(72)86154-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. F., Rauth A. M. Nascent DNA synthesis in ultraviolet light-irradiated mouse L cells. Biochim Biophys Acta. 1972 Jan 31;259(2):164–174. doi: 10.1016/0005-2787(72)90056-1. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968 May 18;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H. Single strand interruptions in DNA and the effects of caffeine in Chinese hamster cells irradiated with ultraviolet light. Biochem Biophys Res Commun. 1969 Jul 23;36(2):203–208. doi: 10.1016/0006-291x(69)90315-5. [DOI] [PubMed] [Google Scholar]
- Cook J. S. Photoreactivation in animal cells. Photophysiology. 1970;5:191–233. [PubMed] [Google Scholar]
- Cook J. S., Regan J. D. Photoreactivation and photoreactivating enzyme activity in an order of mammals (Marsupialia). Nature. 1969 Sep 6;223(5210):1066–1067. doi: 10.1038/2231066a0. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Kondo T. Caffeine-sensitive repair of ultraviolet light-damaged DNA of mouse L cells. Biochem Biophys Res Commun. 1972 May 12;47(3):557–564. doi: 10.1016/0006-291x(72)90915-1. [DOI] [PubMed] [Google Scholar]
- Iyer V. N., Rupp W. D. Usefulness of benzoylated naphthoylated DEAE-cellulose to distinguish and fractionate double-stranded DNA bearing different extents of single-stranded regions. Biochim Biophys Acta. 1971 Jan 1;228(1):117–126. doi: 10.1016/0005-2787(71)90551-x. [DOI] [PubMed] [Google Scholar]
- Krishnan D., Painter R. B. Photoreactivation and repair replication in rat kangaroo cells. Mutat Res. 1973 Feb;17(2):213–222. doi: 10.1016/0027-5107(73)90169-3. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Ormerod M. G. The replication of DNA in murine lymphoma cells (L5178Y). I. Rate of replication. Biochim Biophys Acta. 1970 Mar 19;204(1):128–143. doi: 10.1016/0005-2787(70)90496-x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Meyn R. E., Humphrey R. M. Deoxyribonucleic acid synthesis in ultraviolet-light-irradiated Chinese hamster cells. Biophys J. 1971 Mar;11(3):295–301. doi: 10.1016/S0006-3495(71)86215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick M. H., Harm H. Substrate specificity of a bacterial UV endonuclease and the overlap with in vitro photoenzymatic repair. Photochem Photobiol. 1973 Nov;18(5):371–386. doi: 10.1111/j.1751-1097.1973.tb06437.x. [DOI] [PubMed] [Google Scholar]
- Rauth A. M., Tammemagi M., Hunter G. Nascent DNA synthesis in ultraviolet light-irradiated mouse, human and Chinese hamster cells. Biophys J. 1974 Mar;14(3):209–220. doi: 10.1016/S0006-3495(74)85908-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B., Ley R. D. Normal and defective repair of damaged DNA in human cells: a sensitive assay utilizing the photolysis of bromodeoxyuridine. Proc Natl Acad Sci U S A. 1971 Apr;68(4):708–712. doi: 10.1073/pnas.68.4.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J. D., Trosko J. E., Carrier W. L. Evidence for excision of ultraviolet-induced pyrimidine dimers from the DNA of human cells in vitro. Biophys J. 1968 Mar;8(3):319–325. doi: 10.1016/S0006-3495(68)86490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Setlow J. K. Photorepair of biological systems. Res Prog Org Biol Med Chem. 1972;3(Pt 1):335–355. [PubMed] [Google Scholar]
- Setlow R. B., Regan J. D., German J., Carrier W. L. Evidence that xeroderma pigmentosum cells do not perform the first step in the repair of ultraviolet damage to their DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1035–1041. doi: 10.1073/pnas.64.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B. The photochemistry, photobiology, and repair of polynucleotides. Prog Nucleic Acid Res Mol Biol. 1968;8:257–295. doi: 10.1016/s0079-6603(08)60548-6. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Meun D. H. Repair of radiation-induced damage in Escherichia coli. I. Effect of rec mutations on post-replication repair of damage due to ultraviolet radiation. J Mol Biol. 1970 Aug;51(3):459–472. doi: 10.1016/0022-2836(70)90001-x. [DOI] [PubMed] [Google Scholar]


