Abstract
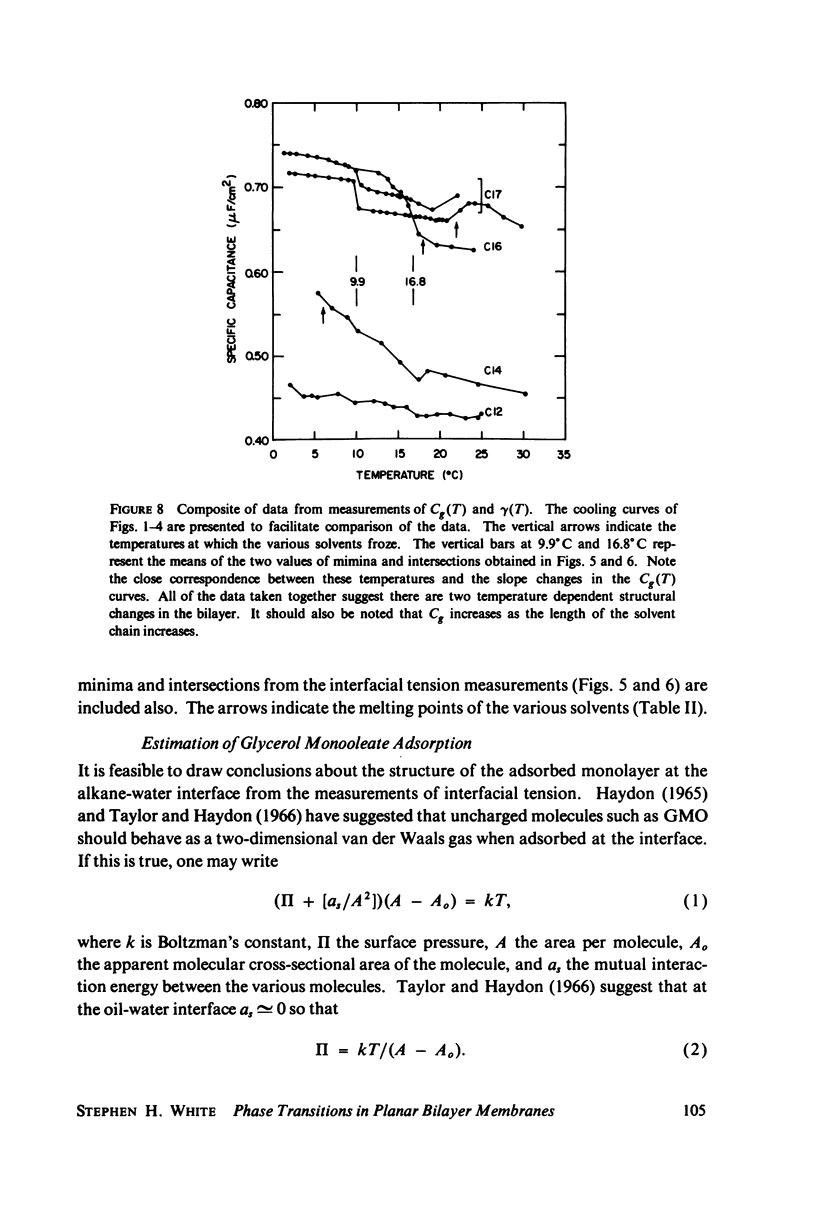
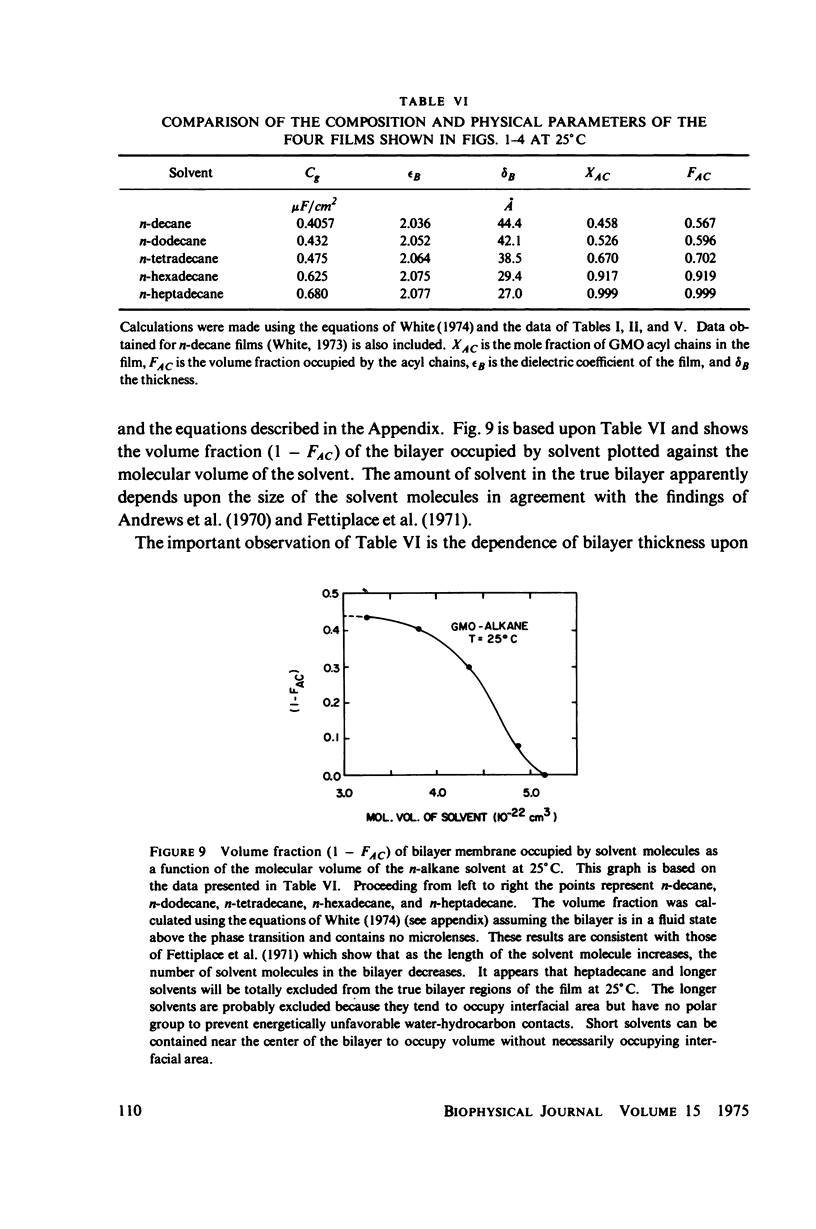
Temperature-dependent structural changes in planar bilayer membranes formed from glycerol monooleate (GMO) dispersed in various n-alkane solvents (C12-C17) have been studied using precise measurements of specific geometric capacitance (Cg). Cg generally increases as temperature (T) decreases. A change in the slope of Cg(T) occurs between 15 and 18 degrees C for all solvent systems examined. Measurements of the interfacial tension (gamma) of the bulk GMO-alkane dispersions against 0.1 M NaCl show that gamma generally decreases with decreasing temperature. The data can be fitted with two straight lines of different slope which intersect on the average at 17 degrees C. Pagano et al. (1973, Science (Wash. D.C.). 181:557) have shown using calorimetry that GMO has a phase transition at about 15 degrees C. Thus, the changes in Cg and gamma with temperature are likely to result from a GMO phase transition. A second structural change is observed to occur between 5 and 10 degrees C which has not been detected calorimetrically. Calculations of Cg based on various estimates of the hydrocarbon dielectric coefficient (epsilon-b) and/or hydrocarbon thickness (delta-b) leads to models for the structure of the bilayer above and below the phase transition temperature.
Full text
PDF



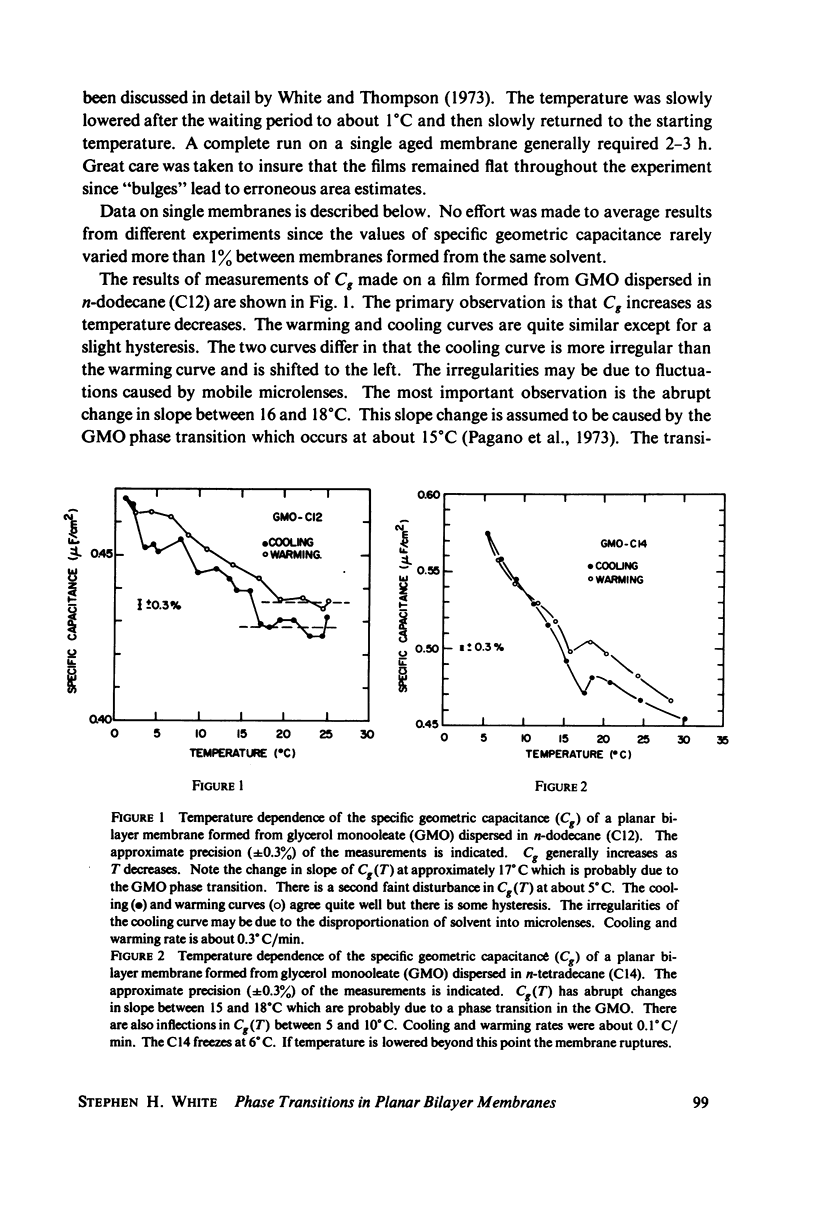

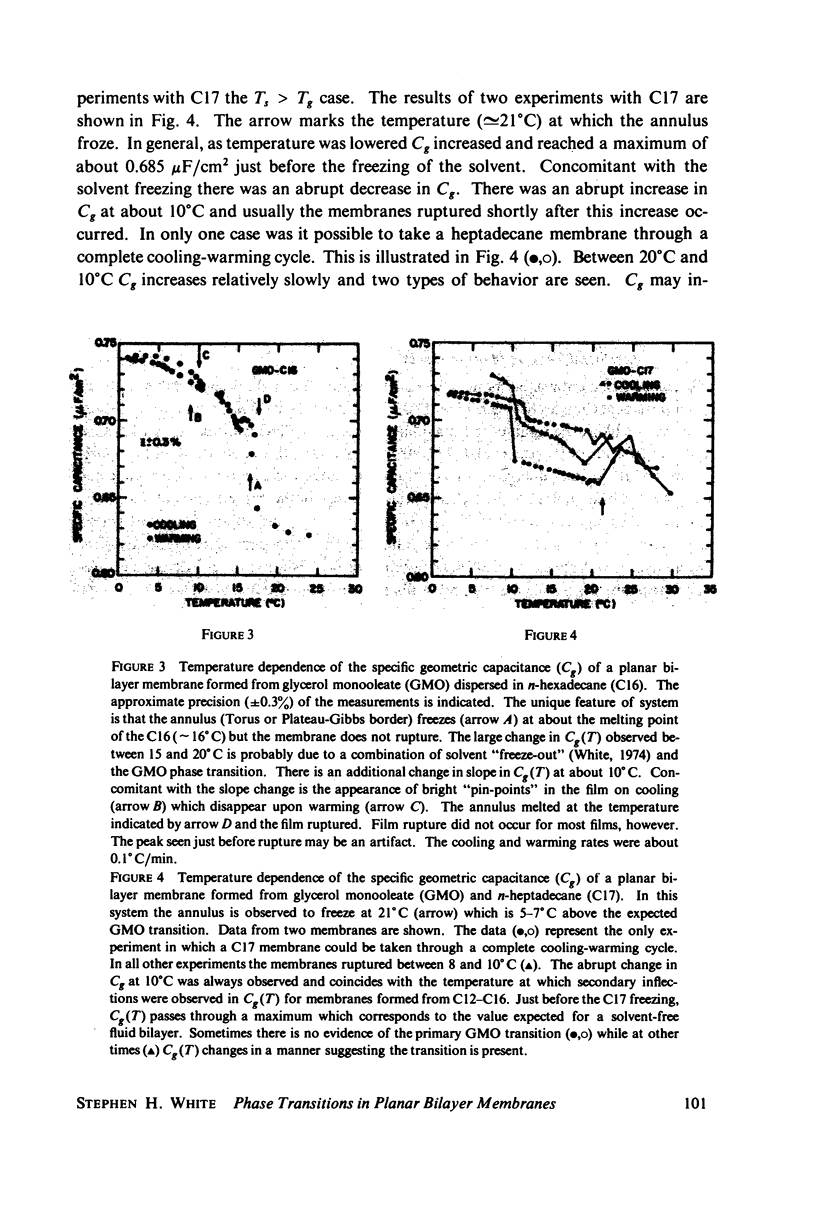

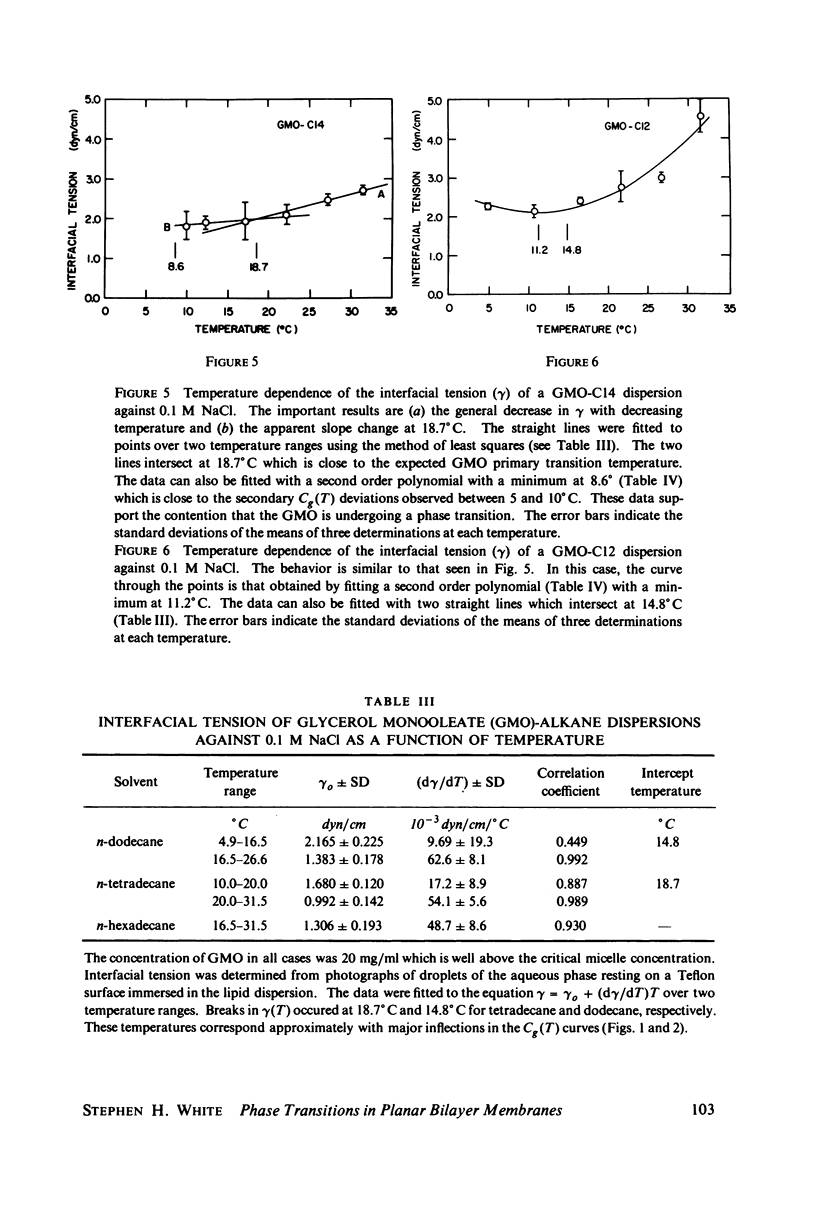
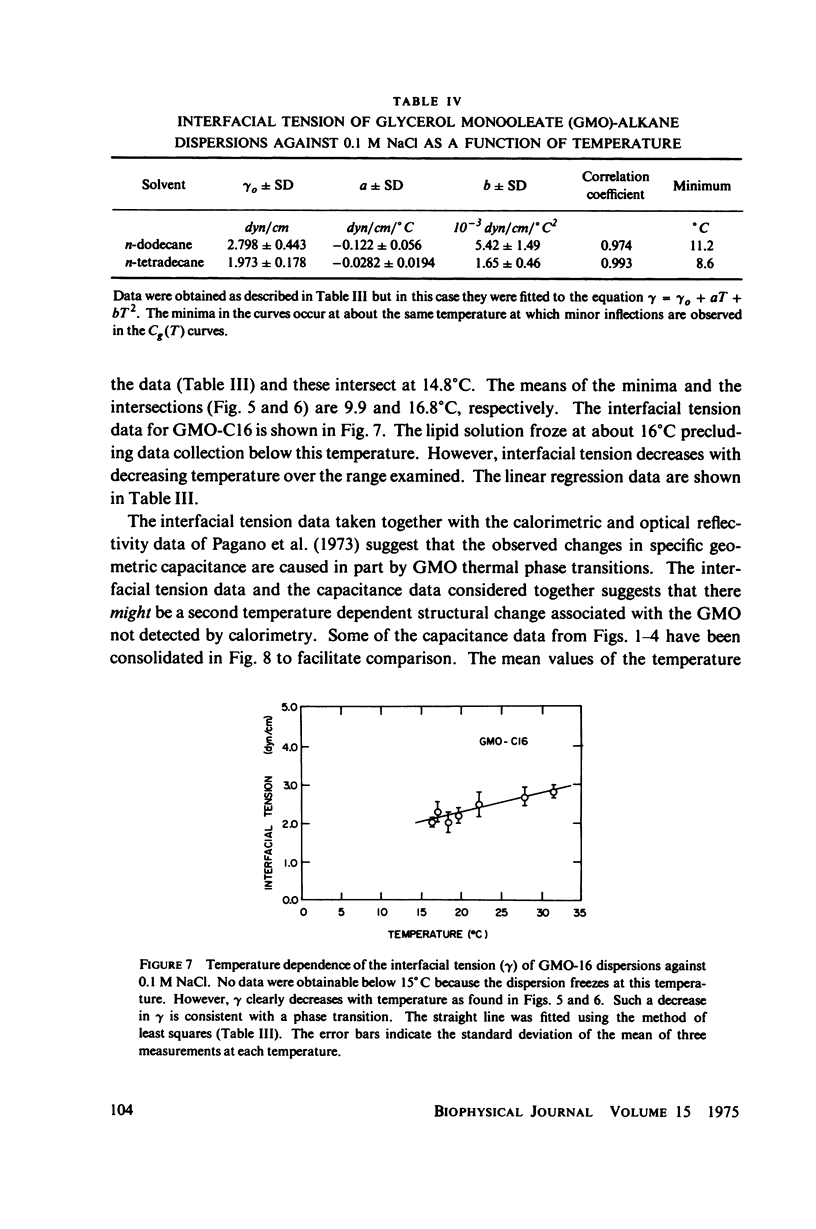











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews D. M., Haydon D. A. Electron microscope studies of lipid bilayer membranes. J Mol Biol. 1968 Feb 28;32(1):149–150. doi: 10.1016/0022-2836(68)90152-6. [DOI] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry R. J., Chapman D. Optical properties of black lecithin films. J Mol Biol. 1969 Feb 28;40(1):19–32. doi: 10.1016/0022-2836(69)90293-9. [DOI] [PubMed] [Google Scholar]
- Eletr S., Zakim D., Vessey D. A. A spin-label study of the role of phospholipids in the regulation of membrane-bound microsomal enzymes. J Mol Biol. 1973 Aug 5;78(2):351–362. doi: 10.1016/0022-2836(73)90121-6. [DOI] [PubMed] [Google Scholar]
- Freeman C. P., West D. Complete separation of lipid classes on a single thin-layer plate. J Lipid Res. 1966 Mar;7(2):324–327. [PubMed] [Google Scholar]
- Godici P. E., Landsberger F. R. The dynamic structure of lipid membranes. A 13C nuclear magnetic resonance study using spin labels. Biochemistry. 1974 Jan 15;13(2):362–368. doi: 10.1021/bi00699a022. [DOI] [PubMed] [Google Scholar]
- Henn F. A., Thompson T. E. Properties of lipid bilayer membranes separating two aqueous phases: composition studies. J Mol Biol. 1968 Jan 28;31(2):227–235. doi: 10.1016/0022-2836(68)90441-5. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric studies of dilute aqueous suspensions of bilayers formed from synthetic L- -lecithins. J Biol Chem. 1972 Oct 10;247(19):6071–6075. [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Orientation and motion of amphiphilic spin labels in membranes. Proc Natl Acad Sci U S A. 1969 Sep;64(1):20–27. doi: 10.1073/pnas.64.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasne S., Eisenman G., Szabo G. Freezing and melting of lipid bilayers and the mode of action of nonactin, valinomycin, and gramicidin. Science. 1971 Oct 22;174(4007):412–415. doi: 10.1126/science.174.4007.412. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Chapman D. Thermal analysis of lipids, proteins and biological membranes. A review and summary of some recent studies. Chem Phys Lipids. 1969 Dec;3(4):304–356. doi: 10.1016/0009-3084(69)90040-1. [DOI] [PubMed] [Google Scholar]
- Levine Y. K., Birdsall N. J., Lee A. G., Metcalfe J. C. 13 C nuclear magnetic resonance relaxation measurements of synthetic lecithins and the effect of spin-labeled lipids. Biochemistry. 1972 Apr 11;11(8):1416–1421. doi: 10.1021/bi00758a014. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Peticolas W. L. Laser Raman investigation of the effect of cholesterol on conformational changes in dipalmitoyl lecithin multilayers. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1572–1576. doi: 10.1073/pnas.68.7.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland B. G., McConnell H. M. Bent fatty acid chains in lecithin bilayers. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1274–1278. doi: 10.1073/pnas.68.6.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior D. L., Morowitz H. J. Dilatometry of dilute suspensions of synthetic lecithin aggregates. Biochemistry. 1972 Nov 21;11(24):4558–4562. doi: 10.1021/bi00774a020. [DOI] [PubMed] [Google Scholar]
- Mendelsohn R. Laser-Raman spectroscopic study of egg lecithin and egg lecithin-cholesterol mixtures. Biochim Biophys Acta. 1972 Dec 1;290(1):15–21. doi: 10.1016/0005-2736(72)90047-8. [DOI] [PubMed] [Google Scholar]
- Overath P., Hill F. F., Lamnek-Hirsch I. Biogenesis of E. coli membrane: evidence for randomization of lipid phase. Nat New Biol. 1971 Dec 29;234(52):264–267. doi: 10.1038/newbio234264a0. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Cherry R. J., Chapman D. Phase transitions and heterogeneity in lipid bilayers. Science. 1973 Aug 10;181(4099):557–559. doi: 10.1126/science.181.4099.557. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Ruysschaert J. M., Miller I. R. The molecular composition of some lipid bilayer membranes in aqueous solution. J Membr Biol. 1972;10(1):11–30. doi: 10.1007/BF01867845. [DOI] [PubMed] [Google Scholar]
- Reiss-Husson F. Structure des phases liquide-cristallines de différents phospholipides, monoglycérides, sphingolipides, anhydres ou en présence d'eau. J Mol Biol. 1967 May 14;25(3):363–382. doi: 10.1016/0022-2836(67)90192-1. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Engelman D. M. Molecular mechanism for the interaction of phospholipid with cholesterol. Nat New Biol. 1972 May 10;237(71):42–44. doi: 10.1038/newbio237042a0. [DOI] [PubMed] [Google Scholar]
- Stark G., Benz R., Pohl G. W., Janko K. Valinomycin as a probe for the study of structural changes of black lipid membranes. Biochim Biophys Acta. 1972 Jun 20;266(3):603–612. doi: 10.1016/0006-3002(72)90004-2. [DOI] [PubMed] [Google Scholar]
- Steim J. M., Tourtellotte M. E., Reinert J. C., McElhaney R. N., Rader R. L. Calorimetric evidence for the liquid-crystalline state of lipids in a biomembrane. Proc Natl Acad Sci U S A. 1969 May;63(1):104–109. doi: 10.1073/pnas.63.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- White S. H. Temperature-dependent structural changes in planar bilayer membranes: solvent "freeze-out". Biochim Biophys Acta. 1974 Jul 12;356(1):8–16. doi: 10.1016/0005-2736(74)90289-2. [DOI] [PubMed] [Google Scholar]
- White S. H. The surface charge and double layers of thin lipid films formed from neutral lipids. Biochim Biophys Acta. 1973 Oct 25;323(3):343–350. doi: 10.1016/0005-2736(73)90180-6. [DOI] [PubMed] [Google Scholar]
- White S. H. Thickness changes in lipid bilayer membranes. Biochim Biophys Acta. 1970;196(2):354–357. doi: 10.1016/0005-2736(70)90023-4. [DOI] [PubMed] [Google Scholar]
- White S. H., Thompson T. E. Capacitance, area, and thickness variations in thin lipid films. Biochim Biophys Acta. 1973 Sep 27;323(1):7–22. doi: 10.1016/0005-2736(73)90428-8. [DOI] [PubMed] [Google Scholar]
- Wilkins M. H., Blaurock A. E., Engelman D. M. Bilayer structure in membranes. Nat New Biol. 1971 Mar 17;230(11):72–76. doi: 10.1038/newbio230072a0. [DOI] [PubMed] [Google Scholar]


