Abstract
Retrieval of recently acquired declarative memories depends on the hippocampus, but with time, retrieval is increasingly sustainable by neocortical representations alone. This process has been conceptualized as system-level consolidation. Using functional magnetic resonance imaging, we assessed over the course of three months how consolidation affects the neural correlates of memory retrieval. The duration of slow-wave sleep during a nap/rest period after the initial study session and before the first scan session on day 1 correlated positively with recognition memory performance for items studied before the nap and negatively with hippocampal activity associated with correct confident recognition. Over the course of the entire study, hippocampal activity for correct confident recognition continued to decrease, whereas activity in a ventral medial prefrontal region increased. These findings, together with data obtained in rodents, may prompt a revision of classical consolidation theory, incorporating a transfer of putative linking nodes from hippocampal to prelimbic prefrontal areas.
Keywords: hippocampus, retrograde amnesia, sleep, ventral medial prefrontal cortex, recognition
We are able to recall memories from the remote past, suggesting a high-capacity system for long-term storage of declarative memories. Lesion studies suggest that declarative memory retrieval initially depends on the hippocampus, although, with time, stored information becomes reorganized in a way that makes retrieval gradually less dependent on the hippocampus (1–3). This pattern of findings has been conceptualized as system-level consolidation, a mnemonic process that leads to a shift from the hippocampus toward distributed neocortical traces (4–8). Although memory consolidation is a fundamental mnemonic operation, its neural correlates at the human brain-system level are not well understood. In particular, it is unknown whether the hippocampus participates under normal circumstances in remote memory retrieval or whether other brain regions take over putative linking nodes created initially in the hippocampus where they are used for recent memory retrieval.
Sleep, in particular slow-wave sleep, appears to play a role in declarative memory consolidation in humans (9–15; but see ref. 16). Moreover, animal studies have shown that during sleep, hippocampal and neocortical cell-assemblies reflecting recent events exhibit spontaneous coordinated reactivation (17–20), that, thereby, may strengthen and/or refine neocortical assemblies so that they can be used for retrieval without hippocampal engagement.
The time course of declarative memory consolidation in humans is poorly defined. Estimates reach up to several decades, based on retrospective lesion and functional neuroimaging studies (1, 21). When investigated on a shorter time scale, prospective studies in rodents and nonhuman primates have revealed upper-bound estimates of a few weeks (22–25); however, it remains to be determined whether these findings are related to a similar set of processes supporting memory consolidation or whether they are related to separate operations acting on greatly differing time scales, all termed consolidation. Combined learning and sleep studies indicate that the behavioral outcome of consolidation can be observed also in humans on a shorter time scale of a couple of hours of sleep for procedural memory (26) and a night of sleep for declarative memory (12). These findings suggest that processes related to system-level reorganization, conceptualized as consolidation, do occur during the first sleep after the initial encounter.
To assess how declarative memory consolidation in humans affects the neural correlates of memory retrieval and to characterize its time course, we performed a prospective study in which subjects initially memorized a large set of visual stimuli comprising of photographs of natural landscapes. Subsequently, memory for different subsets of these stimuli was probed four times within three months (days 1, 2, 30, and 90) by recognition memory tests during which brain activity was assessed by using event-related functional magnetic resonance imaging (fMRI). During each imaging session, we assessed brain activity associated with correct confident recognition of these items (confident remote hits) with correct confident recognition of items studied just before scanning (confident recent hits). We predicted that the hippocampal activation level related to the retrieval of consolidated memories (confident remote hits) would decline progressively over time. In line with data obtained in rodents (23, 24, 27), we expected progressively stronger activations associated with the retrieval of consolidated memories in a ventral medial prefrontal region, the only known brain region in rodents where a circumscribed lesion causes a selective memory deficit for remote but not recent memories (24). We also investigated the effect of sleep by introducing a polysomnographically controlled rest after the initial study session on day 1. If slow-wave sleep promotes memory consolidation (9), we predicted that longer slow-wave sleep should go along with weaker hippocampal and/or stronger medial prefrontal activation associated with the retrieval of information learned before sleep.
Results
Recognition Memory Performance. Recognition memory performance remained above chance level for recent and remote items (probability remote hit minus probability false alarm on day 90: 16.2%, t = 7.1, P < 0.0001). Recognition memory performance for recent items remained stable over time [(F (3, 19) = 2.54, not significant (n.s.)], but it changed for remote items [F (3, 19) = 28.2, P < 0.001], whereas it did not change between day 1 and 2(t = 0.9, n.s.), it dropped from days 2 to 30 (t = 7.9, P < 0.001) and from days 30 to 90 (t = 2.2, P < 0.05). Reaction times were shorter for confident recent than confident remote hits [F (1, 21) = 96.3, P < 0.001]. Although reaction times for confident recent hits did not change over time [F (3, 19) = 1.82, n.s.], they did change for confident remote hits [F (3, 19) = 8.95, P = 0.001]. More detailed behavioral results are available in Table 1, which is available as supporting information on the PNAS web site.
Recognition-Related Activity. To confirm that the hippocampus was engaged by our experimental paradigm, we initially compared activity obtained on day 1 for recent hits (i.e., confident “old” response to pictures studied just before scanning) versus misses (i.e., confident “new” response to a previously studied item) and observed bilateral hippocampal activations (P < 0.001, corrected; Fig. 1). Other brain regions, known to be involved in declarative memory retrieval (28, 29), also showed significant activations (Table 2, which is available as supporting information on the PNAS web site). Thus, as expected, the successful recognition of the pictures encountered just before scanning engaged the hippocampus.
Fig. 1.
FMRI results: recognition effect. Contrasting confident recent hits and misses (day 1), we observed, in addition to other areas (cf. Table 2), bilateral medial temporal lobe activations centered in the hippocampus (Hi).
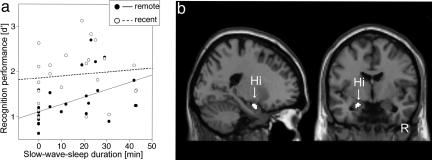
Effect of Sleep. Subjects slept on average for 90 min (see Table 3, which is available as supporting information on the PNAS web site, for details). Fifteen subjects reached slow-wave sleep (i.e., stages 3 and 4). Correlations between recognition memory performance on day 1 and the duration of each sleep stage during the nap were calculated separately for both remote and recent items. Duration of sleep stage 1 and rapid-eye movement sleep did not reveal any significant correlations with recognition memory performance, neither for remote nor recent items (max r = 0.32, n.s.). There were almost identical correlations between recognition memory performance and the duration of sleep stage 2 for both remote and recent items (remote: r = 0.50, P < 0.05; recent: r = 0.48, P < 0.05), indicating an unspecific effect of sleep stage 2. In contrast, the effect of slow-wave sleep was specific for information learned before the rest/nap (remote: r = 0.46, P < 0.05; recent: r = 0.17, n.s.; difference: Z = 1.92, P < 0.05; Fig. 2a). Because performance for remote items increased with longer slow-wave sleep duration, but performance for recent items did not, the effect on memory performance for remote items cannot be explained by a general effect of slow-wave sleep on memory retrieval. Rather, this modulating effect of slow-wave sleep seems to be specific for memory traces that have been formed before sleep. The slow-wave sleep during the nap on day 1 might have had an even longer-lasting effect, because we found a linear relationship between slow-wave sleep duration within this nap and recognition memory performance until day 30 (day 2: r = 0.39, P < 0.1; day 30: r = 0.44, P < 0.05), whereas we did not find such a correlation for recent items.
Fig. 2.
Behavioral and fMRI results: sleep effect. (a) Scatter plot of recognition memory performance (d′) on day 1 for recent and remote items related to individual slow-wave sleep duration. (b) A correlation based on weaker responses to confident remote hits as compared to confident recent hits with longer slow-wave sleep duration was observed in the left hippocampus (Hi).
To follow up the differential effect of slow-wave sleep on recognition memory performance on day 1 for items learned before and after the nap, we computed the correlation between slow-wave sleep duration and the difference in activation for confident remote and confident recent hits (i.e., the contrast image of remote vs. recent hits on day 1). Given the observed hippocampal effect in the contrast between confident recent hits and misses, we used the coordinates of local maxima in the anterior hippocampus from this contrast as centers of spherical regions of interest [radius, 8 mm; centered at (x/y/z) = (–20/–4/–24) and (20/–6/–22)]. We observed a correlation in the left anterior hippocampus based on weaker responses to confident remote hits with longer slow-wave sleep duration [cluster P = 0.036; local maximum: (–22/0/–20), P = 0.040, small volume corrected (SVC); Fig. 2b]. Hence, subjects that had longer slow-wave sleep exhibited less hippocampal activation during successful and confident retrieval of memories acquired before the nap (remote) compared with that of recent items.
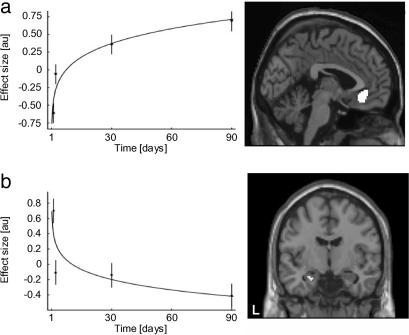
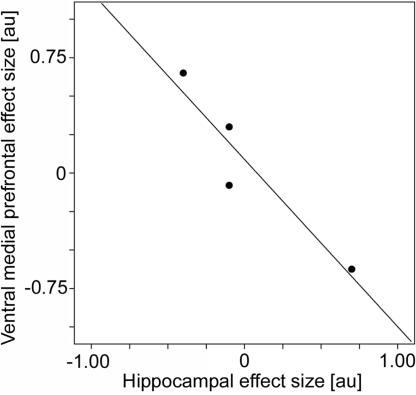
Effect of Time (Days 1 to 90). The time course of memory consolidation was assessed by examining blood oxygenation level-dependent responses to remote hits on days 1 to 90. The time by condition interaction [time x (remote hits – null events)], modulated by an approximate power law, was tested, predicting progressive but asymptotic changes in activation levels. To ensure time stability in the responses of recent hits, all voxels in recent hit trials that showed changes over time in the recent hits to baseline contrast (days 1–90, P ≤ 0.05, uncorrected) were masked out. In this way, we are able to investigate the time by condition effect of remote hits by using the baseline (null events) to control for nonspecific time effects in combination with the exclusive masking procedure to gain statistical power while, at the same time, to preserve the specificity of the time effect. The only brain regions that showed specific time by condition interactions were observed in the ventral medial prefrontal region bilaterally (cluster P = 0.028, corrected; Fig. 3a) and the hippocampus bilaterally [left: (–30/–8/–16), P = 0.022; (–32/–12/–16), P = 0.027; right: (36/–4/–22), P = 0.028; corrected; Fig. 3b]. Thus, we revealed an increase in activation over time for confident remote hits in the ventral medial prefrontal region and a decrease over time for confident remote hits in the hippocampus. To illustrate the close relationship between the effects, we plotted activity levels obtained from the local maxima at the four time points for these two regions. This plot revealed a negative correlation, suggesting an orchestrated effect acting on both regions at the same time (r = –0.94, P < 0.05, one-tailed; Fig. 4).
Fig. 3.
FMRI results: time effect (day 1 to day 90). (a) The ventral medial prefrontal region showed an increase in activity related to confident remote hits over time. (b) In contrast, the hippocampus showed a decline in activity related to confident remote hits over time. Note that we fitted power laws [i.e., Y(t) = a + b × tc] to the observations depicted in Left for descriptive purposes only (error bars = standard errors of the mean).
Fig. 4.
FMRI results: correlation between hippocampal and ventral medial prefrontal time effects (day 1 to day 90). Scatter plot with a linear regression line of parameter estimates (arbitrary units) derived from local maxima of the time effects (day 1 to day 90) in the hippocampus and the ventral medial prefrontal cortex (see Results for details).
To further substantiate these time effects, we investigated day-to-day changes within spherical regions of interests in a ventral medial prefrontal region [radius, 8 mm; centered at (–2/32/–10)] and the hippocampus [radius, 8 mm; centered at (–32/–10/–18) and (32/–4/–26)]. With respect to the left hippocampus, significant changes occurred between days 1 and 2 (cluster P = 0.009, SVC) and between days 30 and 90 (cluster P = 0.018, SVC). For the right hippocampus, only the days 1–2 comparison was significant (cluster P = 0.05, SVC). The analysis on the ventral medial prefrontal region revealed a trend (cluster P < 0.1, SVC) for the days-1–2 comparison and a significant effect (cluster P = 0.05, SVC) for the days 2–30 comparison.
Spatial Overlap of Sleep and Time Effects. To test whether the sleep and time effects revealed here occurred in the same hippocampal region, we investigated the spatial overlap of activations in the left hippocampus between the day-to-day effects and the region where the correlation with the duration of slow-wave sleep was identified on day 1 [radius, 8 mm; centered at the local maximum for the negative sleep correlation (–22/0/–20)]. The cluster for the sleep effect overlapped with the cluster for the effect of days 1 versus 2 [cluster P = 0.01, (–22/–8/–20), P = 0.001, SVC]. Hence, the same anterior hippocampal region that showed reduced activity during confident remote memory retrieval with longer slow-wave sleep duration on day 1 that, in turn, was correlated with better recognition memory performance, also showed a consolidation effect over time.
Discussion
The present data reveal a progressively decreasing, retrieval-related activation of the hippocampus over the course of 3 months. Although standard consolidation theory is agnostic with regard to the necessity of such an effect, it is consistent with the findings of retrograde amnesia in patients with hippocampal damage (1) and experimental animal data (22–25, 27) suggesting that retrieval of remote memories can be executed without the hippocampus. It is also consistent with the findings of the temporal dynamics of experimentally induced long-term synaptic potentiation (30) and the notion that information stored in the hippocampus is cleared out during the consolidation process to make room for new memories (31). In animals, it is known that memory traces related to recently acquired information are replayed in hippocampal-neocortical circuits during postlearning rest/sleep (17, 18, 32), suggesting a consolidation process promoted during these off-line periods. Our findings showed that longer slow-wave sleep led to a greater hippocampal activation decrease during successful retrieval, specifically for those items encoded before sleep. This result suggests that the consolidation process itself may regulate hippocampal trace decay rather than simply following a passive time-dependent process associated with forgetting.
The question how memory consolidation affects overall performance in declarative memory tests is less straightforward than in procedural memory tests, where consolidation leads to performance improvements (e.g., ref. 33). Although declarative memory consolidation seems critical for efficient long-term memory storage, because it promotes stable memory traces in neocortical regions, it is also affected by forgetting (34). Furthermore, the process of stabilization and organization does not necessarily lead to faster access or more detailed information during retrieval. On the contrary, retrieval of consolidated memories might become slower and less vivid (35). For instance, Gilboa and coworkers (36) argued that the vividness of retrieved information is critical for the level of hippocampal activation. They studied brain activity related to the retrieval of autobiographical memories that were up to 53 years old and grouped into consecutive 5-year periods. In line with our findings, they found decreasing hippocampal activity with increasing remoteness, but this long-term decrease diminished if vividness of the retrieved information was equated. However, the results of that study and the present study are not directly comparable, because they differ substantially in the definition of remoteness, the kind of memory probed, and the memory tests used. Nevertheless, it seems unlikely that our results are related to a reduction of vividness, because the decrease in hippocampal activity on day 1 correlated with better memory performance and the largest hippocampal activity decrease occurred between days 1 and 2, an interval without a decline in recognition memory for remote items as assessed by performance and reaction times. In short, we observed a decrease in hippocampal activity associated with confident remote memory retrieval, independent of whether memory performance improved or remained stable.
The progressive increase found in the anterior ventral medial prefrontal activation associated with remote memory retrieval provides general support for the theory that consolidation involves a gradual strengthening of cortico-cortical connections (4–8, 27, 37). The role of this prelimbic region, which has reciprocal connections with the medial temporal lobe and wide-spread projections to the neocortex (38–40), in memory consolidation is unusual in the sense that lesions of this area do not impair acquisition of hippocampus-dependent memories. Even though the ventral medial prefrontal cortex is regarded as related to general-purpose, executive function and not to memory storage, it may play a crucial role in remote memory retrieval. Given its connectivity, this region is, like the hippocampus, ideally suited to integrate information from multiple neocortical regions during memory retrieval (41). Thus, it might take over the linking function from the hippocampus by indexing and binding information stored in distributed neocortical sites to retrieve coherent remote memories (23, 27). Such an idea may point toward a variant of the memory consolidation hypothesis by which the putative linking nodes that are created initially in the hippocampus in a rapid, sparse, and orthogonalized form might be gradually transferred to this prelimbic area in the form of more distributed codes that might also overlap with links related to other memory traces. This set of ideas is consistent with prospective autoradiographic studies in rodents (23), which show that this region is involved in remote memory retrieval. In addition, lesions to this region impair the retrieval of remote but not recent memories in rodents (24) and they are associated with temporally extensive retrograde amnesia in humans (42), suggesting a critical role in remote memory retrieval.
Nevertheless, there are alternative scenarios to these suggestions. For instance, the anterior ventral medial prefrontal region might inhibit the hippocampus when remote memories are successfully recalled, preventing the hippocampus from reen-coding existing memories (27) or it might be related to monitoring operations (43). Furthermore, medial prefrontal regions have been associated with processes related to error monitoring or task difficulty (44). However, these effects are observed in the anterior cingulate cortex further dorsally compared to our finding. Moreover, we observed a close linear relationship between the hippocampal activity decrease and the activity increase in the ventral medial prefrontal cortex, suggesting a functional interaction between these two brain regions. As discussed above, the hippocampal effect cannot be explained by reduced memory retrieval, and, thus, it seems unlikely that the progressive increase of ventral medial prefrontal activity associated with remote memory retrieval is related to task difficulty, decision making under conditions of uncertainty, or higher demands for error monitoring.
System level correlates of declarative memory consolidation were observed after a short period of slow-wave sleep and also over the time course of several weeks. This time course seems to contradict data derived from retrospective studies in humans (1, 21, 36), which suggest a time span of several years. This discrepancy might indicate either great uncertainties of retrospective studies in estimating the time course of memory consolidation, or a set of operations with greatly different time courses all defined as “consolidation.” Although we found evidence for rapid consolidation effects, these results are fully consistent with the idea that the process of declarative memory consolidation has not been completed within the time span of the current study and the idea that declarative memories may never become completely independent of the hippocampus (35, 43). Nevertheless, the time course observed in our data are in line with human and animal data obtained in prospective studies (9–14, 22–27), suggesting that at least one component of system-level consolidation is a relatively rapid process, which might be based on synaptic plasticity or structural neural changes occurring in the order of hours to a few days (45, 46).
These issues notwithstanding, we identified system level changes that are consistent with the theory that consolidation involves a gradual strengthening of neocortical connections as the initial hippocampal memory traces fades (6, 27, 47), and that these changes can occur within a rather short time frame of a few weeks and are promoted during slow-wave sleep. The sleep effect observed suggests that the hippocampal trace decay is not following a passive time-dependent process, but that the consolidation process itself may regulate it. In extension to the classical consolidation hypothesis (5) and in line with data in rodents (23, 24), evidence was found in humans that may suggest a transfer of linking nodes from the hippocampus to a ventral medial prefrontal region.
Methods
Subjects. Twenty-four subjects participated (12 females, 5 left-handed, mean age = 24.8 years). None of the subjects used any medication, had a history of drug abuse, head trauma, or neurological or psychiatric illness. Written informed consent was obtained according to the local medical ethics committee. Subjects reported to habitually sleep 6 to 9 h per night, and not to have had any sleep deprivation during the three days preceding the first day of the experiment (5.5 to 9 h on the previous night). Two subjects could not be investigated on day 2. Hence, all analyses that include day 2 data are based on 22 subjects.
Stimuli. We selected 960 color photographs showing large-scale spatial layouts of natural landscapes with or without buildings (480/480). Photographs were similar in terms of overall visual complexity, brightness, and contrast.
Behavioral Procedure. An overview of the study design is depicted in Fig. 5. On day 1 (0900 hours), subjects were instructed to memorize 320 photographs (160 with buildings; presentation time, 5.5 s; interstimulus interval, 125 ms). To encourage elaboration of the stimuli, subjects were given specific examples of learning strategies, such as “Where on the picture would you like to be most?”, “Where do you think the place is?”, and “Look for very special, distinct objects on the picture.” To monitor item processing, we introduced a building/no building decision task. After lunch at 1230 hours, subjects were instructed to lie on a bed in an electrically shielded, sound and light attenuated room for 2 to 3 h, during which polysomnographic recordings were obtained. This rest session was followed by another encoding session where subjects studied 80 new photographs with identical encoding instructions and presentation parameters as the initial encoding session, followed by the recognition test in the scanner 10 to 30 min later.
Fig. 5.
Experimental paradigm. On day 1, subjects were instructed to memorize 320 randomly selected photographs (i.e., “remote”). After the subsequent rest/nap, they studied 80 new photographs (i.e., “recent” of day 1) before scanning. During scanning, subjects performed a recognition memory test on randomly intermixed 80 remote, 80 recent, and 80 new photographs with three response options: (i) picture seen before with confidence, (ii) picture less certain to be seen before or not, and (iii) picture not seen before with confidence. This sequence of prescan study phase of new photographs and recognition memory test in the scanner was repeated on days 2, 30, and 90.
During scanning, subjects performed a recognition memory test on randomly intermixed 80 remote (initial study list), 80 recent (prescan list), and 80 new photographs (presentation time, 800 ms; mean interstimulus interval, 4,600 ms (range: 3,600–5,600 ms); 80 null events (presentation time, 2,000 ms identical to the fixation cross interstimulus interval). We offered the following response categories: (i) picture seen before with high confidence, (ii) picture less certain to be seen before or not, and (iii) picture not seen before with high confidence.
We repeated this sequence of prescan study and recognition test in the scanner on days 2, 30, and 90. Throughout the whole experiment, all remote and all recent pictures were seen only twice (once in the study session and once in the recognition test) and all new pictures once. The assignment of pictures to the three trial types was counterbalanced across subjects.
Polysomnographic Recordings. During the rest/nap session in the afternoon of day 1, polysomnographic recordings were obtained with 200 Hz sampling frequency, a 0.016 Hz high-pass filter, and a 30 Hz low-pass filter (Brain Products, Munich). Tin electrodes were placed on the scalp at C3, C4, O1, and O2 and referenced to the left mastoid. Additionally, horizontal and vertical eye movements, the electromyogram (cheek), and electrocardiogram were recorded. Each 30 s, epoch was scored offline manually according to standard criteria in ref. 48. The duration of the slow-wave sleep was determined as the sum of sleep stages 3 and 4.
MRI Data Acquisition. For fMRI, we acquired with ascending slice acquisition a T2*-weighted echo-planar imaging sequence (Sonata 1.5 T, Siemens, Munich; 33 axial slices; volume repetition time (TR), 2.29 s; echo time (TE), 30 ms; 90° flip angle; slice matrix, 64 × 64; slice thickness, 3.0 mm; slice gap, 0.5 mm; field of view, 224 mm). For structural MRI, we acquired a T1-weighted MP-RAGE sequence (176 sagittal slices; volume TR, 2,250 ms; TE, 3.93 ms; 15° flip angle; slice matrix, 256 × 256; slice thickness, 1.0 mm; no gap; field of view, 256 mm).
MRI Data Analysis. Image preprocessing and statistical analysis was performed by using the spm2 software (www.fil.ion.ucl.ac.uk/spm/software/spm2). The functional echo-planar imaging-blood oxygenation level-dependent contrast images were realigned, and the subject mean was coregistered with the corresponding structural MRI by using mutual information optimization. These images were subsequently slice-time corrected, spatially normalized and transformed into a common space, as defined by the spm2 Montreal Neurological Institute (MNI) T1 template, as well as spatially filtered by convolving the functional images with an isotropic 3D Gaussian kernel (10 mm full width at half maximum). The fMRI data were analyzed statistically by using the general linear model and statistical parametric mapping. The explanatory variables were temporally convolved with the canonical hemodynamic response function along with its temporal derivatives (49) provided by spm2. For the statistical analysis, relevant contrast parameter images were generated for each subject and subsequently subjected to a second-level random effects analysis with nonsphericity correction for correlated repeated measures. In the whole brain search, the results of the random effects analyses were thresholded at P = 0.001 (uncorrected) and the cluster-size statistics were used as the test statistic. Only clusters at P ≤ 0.05 (corrected for multiple comparisons; ref. 50) were considered significant. With respect to the medial temporal lobe, given its role as the primary focus of prior interest, region-specific investigations were performed by using spherical regions of interest, thresholded at P = 0.005 (uncorrected), and P values were SVC. All local maxima are reported as MNI coordinates (51) and were significant at P ≤ 0.05 corrected for multiple comparisons based on the false discovery rate (52).
Supplementary Material
Acknowledgments
We thank Paul Gaalman, Maarten van Hal, and Lucia Kerkhoffs for their technical support. This research was supported by The Netherlands Organization for Scientific Research Grant 051.04.100. B.L.M. is supported by Public Health Service Grant MH046823.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: fMRI, functional magnetic resonance imaging; n.s., not significant; SVC, small volume corrected.
Data deposition: The neuroimaging data have been deposited with the fMRI Data Center, www.fmridc.org (accession no. 2-2005-12ON7).
See Commentary on page 509.
References
- 1.Scoville, W. B. & Milner, B. (1957) J. Neurol. Neurosurg. Psychiatry 20, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teng, E. & Squire, L. R. (1999) Nature 400, 675–677. [DOI] [PubMed] [Google Scholar]
- 3.Eichenbaum, H. (2000) Nat. Rev. Neurosci. 1, 41–50. [DOI] [PubMed] [Google Scholar]
- 4.Marr, D. (1970) Proc. R. Soc. Lond. Ser. B 176, 161–234. [DOI] [PubMed] [Google Scholar]
- 5.McGaugh, J. L. (2000) Science 287, 248–251. [DOI] [PubMed] [Google Scholar]
- 6.Wiltgen, B. J., Brown, R. A. M., Talton, L. E. & Silva, A. J. (2004) Neuron 44, 101–108. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez, P. & Squire, L. R. (1994) Proc. Natl. Acad. Sci. USA 91, 7041–7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paller, K. A. (1997) Memory 5, 73–88. [DOI] [PubMed] [Google Scholar]
- 9.Plihal, W. & Born, J. (1997) J. Cogn. Neurosci. 9, 534–547. [DOI] [PubMed] [Google Scholar]
- 10.Maquet, P. (2001) Science 294, 1048–1052. [DOI] [PubMed] [Google Scholar]
- 11.Stickgold, R. & Walker, M. (2005) Trends Neurosci. 28, 408–415. [DOI] [PubMed] [Google Scholar]
- 12.Gais, S. & Born, J. (2004) Learn. Mem. 11, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peigneux, P., Laureys, S., Fuchs, S., Collette, F., Perrin, F., Reggers, J., Phillips, C., Degueldre, C., Del Fiore, G., Aerts, J., et al. (2004) Neuron 44, 535–545. [DOI] [PubMed] [Google Scholar]
- 14.Drosopoulos, S., Wagner, U. & Born, J. (2005) Learn. Mem. 12, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mölle, M., Marshall, L., Gais, S. & Born, J. (2004) Proc. Natl. Acad. Sci. USA 101, 13963–13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vertes, R. P. (2004) Neuron 44, 135–148. [DOI] [PubMed] [Google Scholar]
- 17.Wilson, M. A. & McNaughton, B. L. (1994) Science 265, 676–679. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman, K. L. & McNaughton, B.L. (2002) Science 297, 2070–2073. [DOI] [PubMed] [Google Scholar]
- 19.Nadasdy, Z., Hirase, H., Czurko, A., Csicsvari, J. & Buzsaki, G. (1999) J. Neurosci. 19, 9497–9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlides, C. & Winson, J. (1989) J. Neurosci. 9, 2907–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haist, F., Bowden Gore, J. & Mao, H. (2001) Nat. Neurosci. 4, 1139–1145. [DOI] [PubMed] [Google Scholar]
- 22.Zola-Morgan, S. M. & Squire, L. R. (1990) Science 250, 288–290. [DOI] [PubMed] [Google Scholar]
- 23.Bontempi, B., Laurent-Demir, C., Destrade, C. & Jaffard, R. (1999) Nature 400, 671–675. [DOI] [PubMed] [Google Scholar]
- 24.Takehara K., Kawahara, S. & Kirino, Y. (2003) J. Neurosci. 23, 9897–9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remondes, M. & Schuman, E. M. (2004) Nature 431, 699–703. [DOI] [PubMed] [Google Scholar]
- 26.Mednick, S., Nakayama, K. & Stickgold, R. (2003) Nat. Neurosci. 6, 697–698. [DOI] [PubMed] [Google Scholar]
- 27.Frankland, P. & Bontempi, B. (2005) Nat. Rev. Neurosci. 6, 119–130. [DOI] [PubMed] [Google Scholar]
- 28.Squire, L. R., Stark, C. E. & Clark, R. E. (2004) Annu. Rev. Neurosci. 27, 279–306. [DOI] [PubMed] [Google Scholar]
- 29.Rugg, M. D. & Yonelinas, A. P. (2003) Trends Cogn. Sci. 7, 313–319. [DOI] [PubMed] [Google Scholar]
- 30.Barnes, C. A. & McNaughton, B. L. (1980) in Psychobiology of Aging: Problems and Perspectives, ed. Stein, D. (Elsevier, New York), pp. 253–272.
- 31.Rosenzweig, E. S., Barnes, C. A. & McNaughton, B. L. (2002) Nat. Neurosci. 5, 6–8. [DOI] [PubMed] [Google Scholar]
- 32.McNaughton, B. L., Barnes, C. A., Battaglia, F. P., Bower, M. R., Cowen, S. L., Ekstrom, A. D., Gerrard, J. L., Hoffman, K. L., Houston, P. F., Karten, Y., et al. (2003) in Sleep and Brain Plasticity, eds. Maquet, P., Smith, C. & Stickgold, R. (Oxford Univ. Press, Oxford), pp. 225–246.
- 33.Walker, M. P., Brakefield, T., Morgan, A., Hobson, J. A. & Stickgold, R. (2002) Neuron 35, 205–211. [DOI] [PubMed] [Google Scholar]
- 34.Ebbinghaus, H. (1885) inÜber das Gedchtnis. Untersuchungen zur Experimentellen Psychologie (Duncker and Humblot, Leipzig, Germany).
- 35.Nadel, L. & Moscovitch, M. (2001) Trends Cogn. Sci. 5, 228–230. [DOI] [PubMed] [Google Scholar]
- 36.Gilboa, A., Winocur, G., Grady, C. L., Hevenor, S. J. & Moscovitch, M. (2004) Cereb. Cortex 14, 1214–1225. [DOI] [PubMed] [Google Scholar]
- 37.Murre, J. M. J. (1996) Hippocampus 6, 675–684. [DOI] [PubMed] [Google Scholar]
- 38.Ongur, D. & Price, J. L. (2000) Cereb. Cortex 10, 206–219. [DOI] [PubMed] [Google Scholar]
- 39.Pandya, D. N., Van Hoesen, G. W. & Mesulam, M. M. (1981) Exp. Brain Res. 42, 319–330. [DOI] [PubMed] [Google Scholar]
- 40.Uylings, H. B., Groenewegen, H. J. & Kolb, B. (2003) Behav. Brain Res. 146, 3–17. [DOI] [PubMed] [Google Scholar]
- 41.Miyashita, Y. (2004) Science 306, 435–440. [DOI] [PubMed] [Google Scholar]
- 42.Gilboa, A. & Moscovitch, M. (2002) in Handbook of Memory Disorders, eds. Baddeley A. D., Kopelman M. D. & Wilson, B. A. (Wiley, London), 2nd Ed., pp. 315–342.
- 43.Moscovitch, M., Rosenbaum, R. S., Gilboa, A., Addis, D. R., Westmacott, R., Grady, C., McAndrews, M. P., Levine, B., Black, S., Winocur, G., et al. (2005) J. Anat. 207, 35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ridderinkhof, R., Ullsperger, M., Crone, E. A. & Nieuwenhuis, S. (2004) Science 306, 443–447. [DOI] [PubMed] [Google Scholar]
- 45.Bliss, T. V. & Lomo, T. (1973) J. Physiol. 232, 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engert, F. & Bonhoeffer, T. (1999) Nature 399, 66–70. [DOI] [PubMed] [Google Scholar]
- 47.McClelland, J. L., McNaughton, B. L. & O'Reilly, R. C. (1995) Psychol. Rev. 102, 419–457. [DOI] [PubMed] [Google Scholar]
- 48.Rechtschaffen, A. & Kales, A. (1968) A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects (Brain Inf. Service, University of California, Los Angeles).
- 49.Friston, K. J., Fletcher P., Josephs, O., Holmes, A., Rugg, M. D. & Turner, R. (1998) NeuroImage 7, 30–40. [DOI] [PubMed] [Google Scholar]
- 50.Worsley, K. J., Marret, S., Neelin, P., Vandal, A. C., Friston, K. J. & Evans, A. C. (1996) Hum. Brain Mapp. 4, 58–73. [DOI] [PubMed] [Google Scholar]
- 51.Evans, A. C., Collins, D. L., Mills, S. R., Brown, E. D., Kelly, R. L. & Peters, T. M. (1993) in IEEE Conference Record, Nuclear Science Symposium, and Medical Imaging Conference (Inst. Electr. Electron. Eng., San Francisco), pp. 1813–1817.
- 52.Genovese, C. L., Lazar, N. A. & Nichols, T. (2002) NeuroImage 15, 870–878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.