Abstract
The effect of prior treatment by inducing agents on the radioresistance of cells of Escherichia coli has been studied. In order to separate the induction process from the radiation-damage process, cells were first treated with inducing agents such as ultraviolet light, ionizing radiation, or nalidixic acid, allowed to become induced by incubation for 50 min and then given rifampin to prevent further induction. They were then tested for radiation sensitivity. It was found that all strains tested except recA-, lex-, and recB showed very apparent protection. Induction by UV had the most effect and by nalidixic acid the least. The time course of development of protection was observed in one case: it is 50% established in 15 min. The absence of effect in recA- and lex- is explainable by the fact that these cells cannot be induced, for example, for prophage or the inducible inhibitor of post-irradiation DNA degradation. We suggest that the inducible inhibitor of postirradiation DNA degradation is one factor in a recovery system possessed by E. coli cells.
Full text
PDF


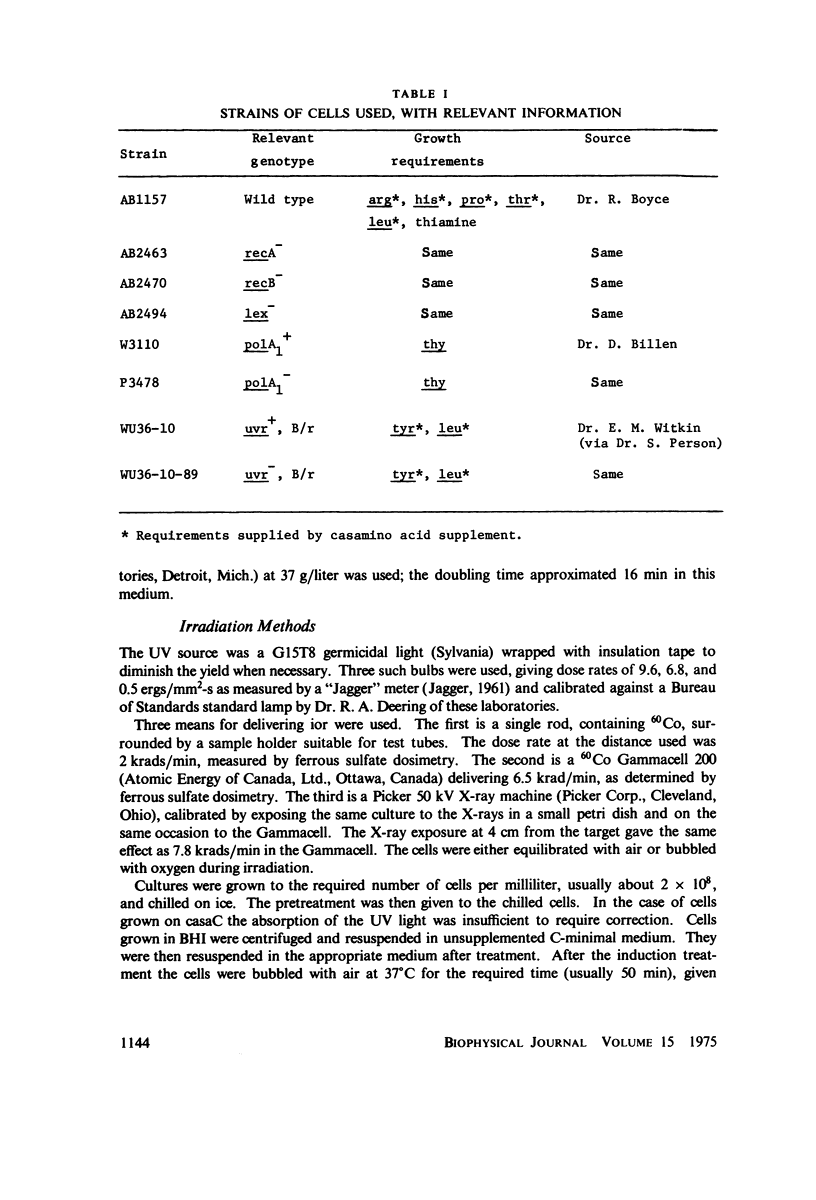

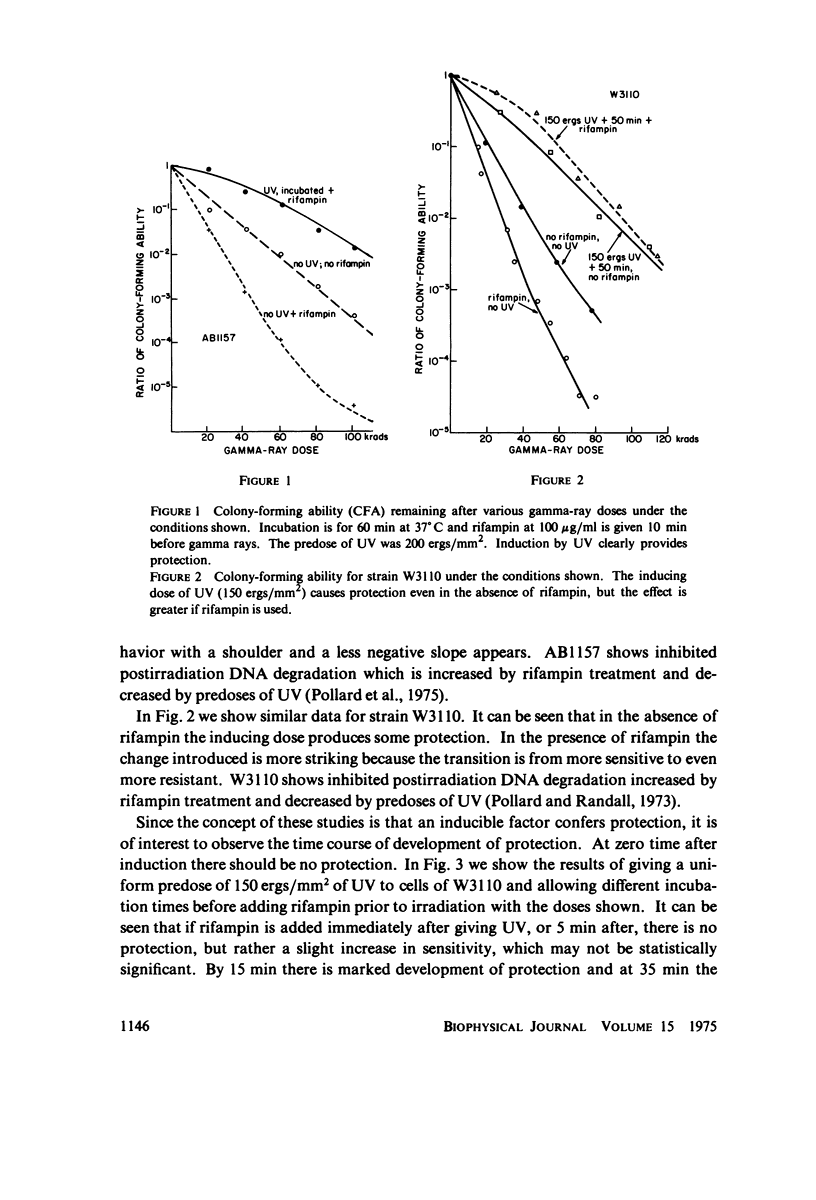
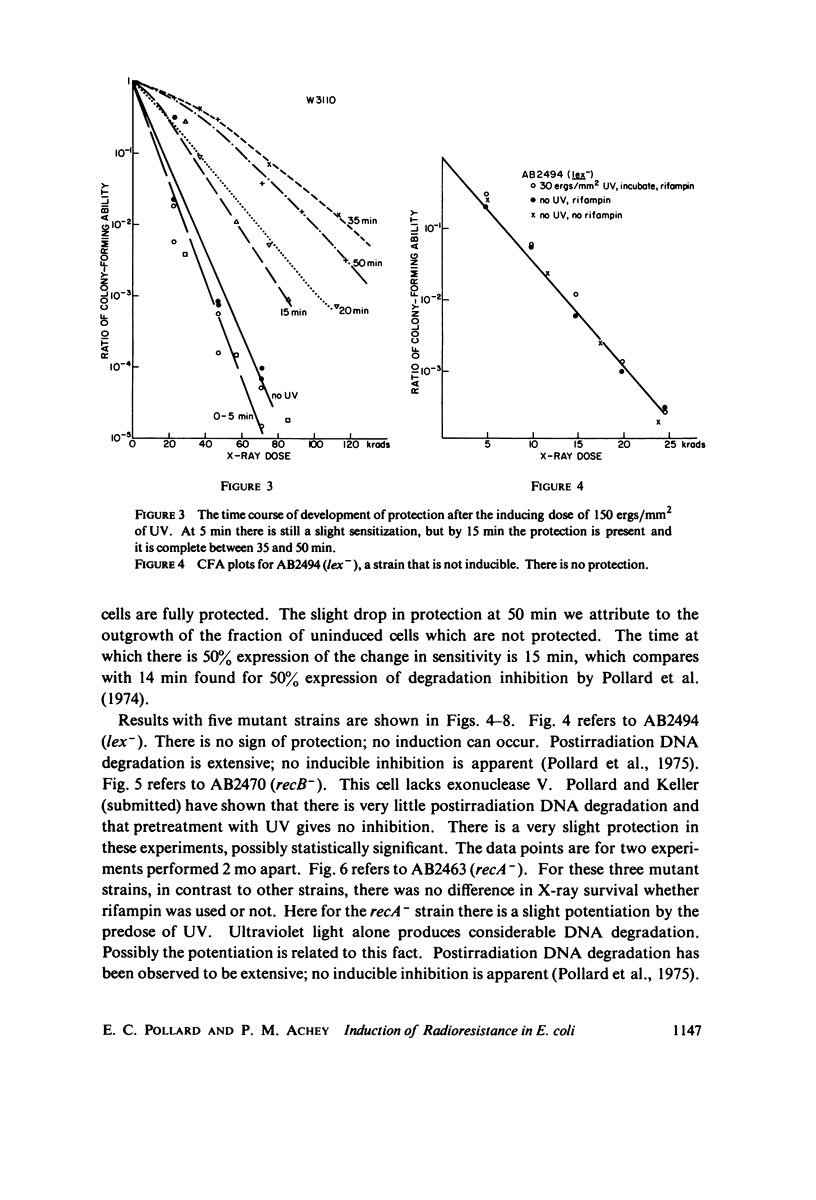
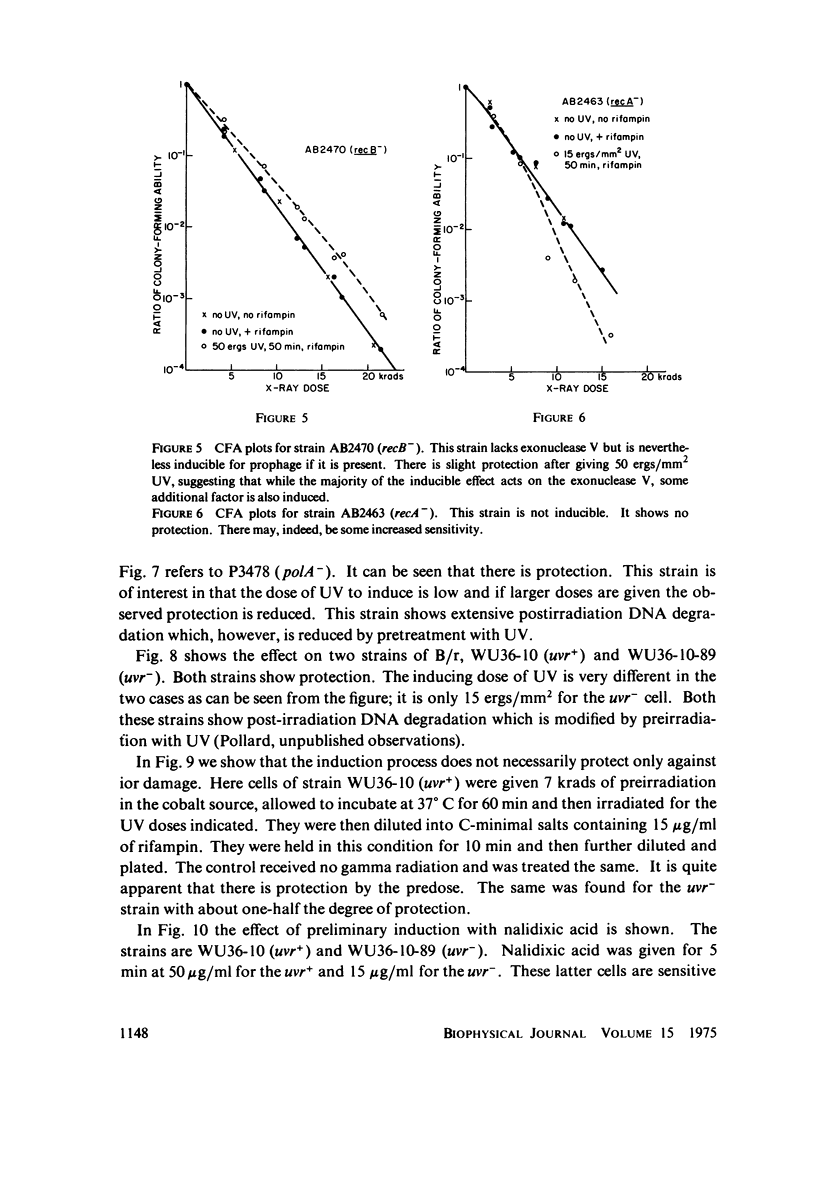



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J., Devoret R., Radman M. Indirect ultraviolet-reactivation of phage lambda. Proc Natl Acad Sci U S A. 1974 Jan;71(1):144–147. doi: 10.1073/pnas.71.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady L. J., Pollard E. C. Ionizing radiation-initiated degradation of deoxyribonucleic acid in bacteria. A possible role for defective prophage. Radiat Res. 1968 Oct;36(1):68–86. [PubMed] [Google Scholar]
- Green A. E., Burki J. A note on survival curves with shoulders. Radiat Res. 1974 Dec;60(3):536–540. [PubMed] [Google Scholar]
- JAGGER J. A small and inexpensive ultraviolet dose-rate meter useful in biological experiements. Radiat Res. 1961 Apr;14:394–403. [PubMed] [Google Scholar]
- MILETIC B., KUCAN Z., DRAKULIC M., ZAJEC L. Effect of chloramphenicol on the biosynthesis of DNA in x-irradiated Escherichia coli B. Biochem Biophys Res Commun. 1961 Apr 7;4:348–352. doi: 10.1016/0006-291x(61)90216-9. [DOI] [PubMed] [Google Scholar]
- MILETIC B., KUCAN Z., SASEL L. SYNTHESIS OF DEOXYRIBONUCLEIC ACID IN X-IRRADIATED BACTERIA TREATED WITH CHLORAMPHENICOL. Nature. 1964 Apr 18;202:311–312. doi: 10.1038/202311a0. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Pollard E. C., Ginoza W., Randall E. P. Involvement of recA and exr genes in the in vivo inhibition of the recBC nuclease. J Bacteriol. 1974 May;118(2):465–470. doi: 10.1128/jb.118.2.465-470.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard E. C., Billen D., Achey P. M. Kinetic studies of the effect of restoration of a required amino acid on post-irradiation DNA degradation and cell permeability. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Aug;26(2):107–119. doi: 10.1080/09553007414551041. [DOI] [PubMed] [Google Scholar]
- Pollard E. C., Randall E. P. Studies on the inducible inhibitor of radiation-induced DNA degradation of Escherichia coli. Radiat Res. 1973 Aug;55(2):265–279. [PubMed] [Google Scholar]
- Tolun A., Christensen R., Pollard E. C. Repair of radiation-induced strand breaks as related to the inducible inhibitor of postirradiation DNA degradation. Biophys J. 1974 Sep;14(9):691–696. doi: 10.1016/S0006-3495(74)85944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trgovcević Z., Rupp W. D. Interaction of bacterial and lambda phage recombination systems in the x-ray sensitivity of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1974 Feb;71(2):503–506. doi: 10.1073/pnas.71.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle J. J. Induction of Mutations in a Bacterial Virus. Proc Natl Acad Sci U S A. 1953 Jul;39(7):628–636. doi: 10.1073/pnas.39.7.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. The radiation sensitivity of Escherichia coli B: a hypothesis relating filament formation and prophage induction. Proc Natl Acad Sci U S A. 1967 May;57(5):1275–1279. doi: 10.1073/pnas.57.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs D. A., Bernstein I. A. Involvement of the recB-recC nuclease (exonuclease V) in the process of x-ray-induced deoxyribonucleic acid degradation in radiosensitive strains of Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):901–906. doi: 10.1128/jb.113.2.901-906.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


