Abstract
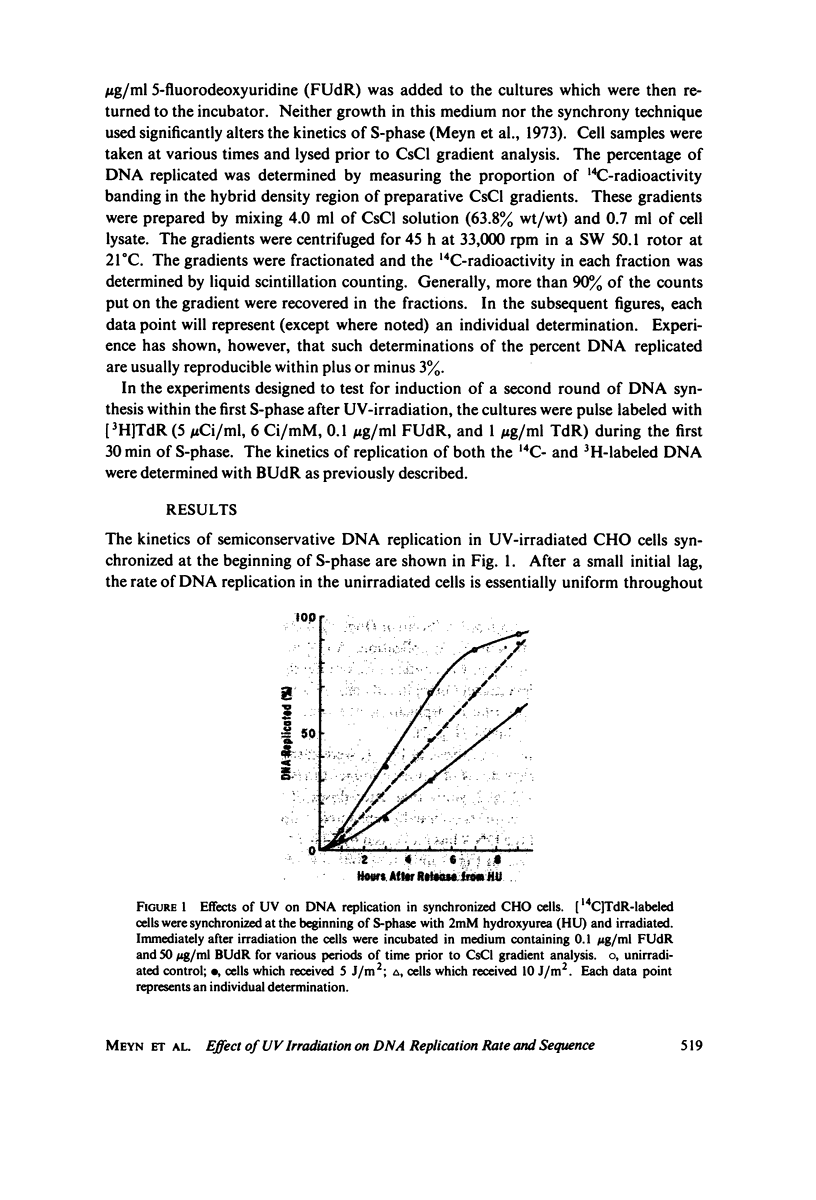
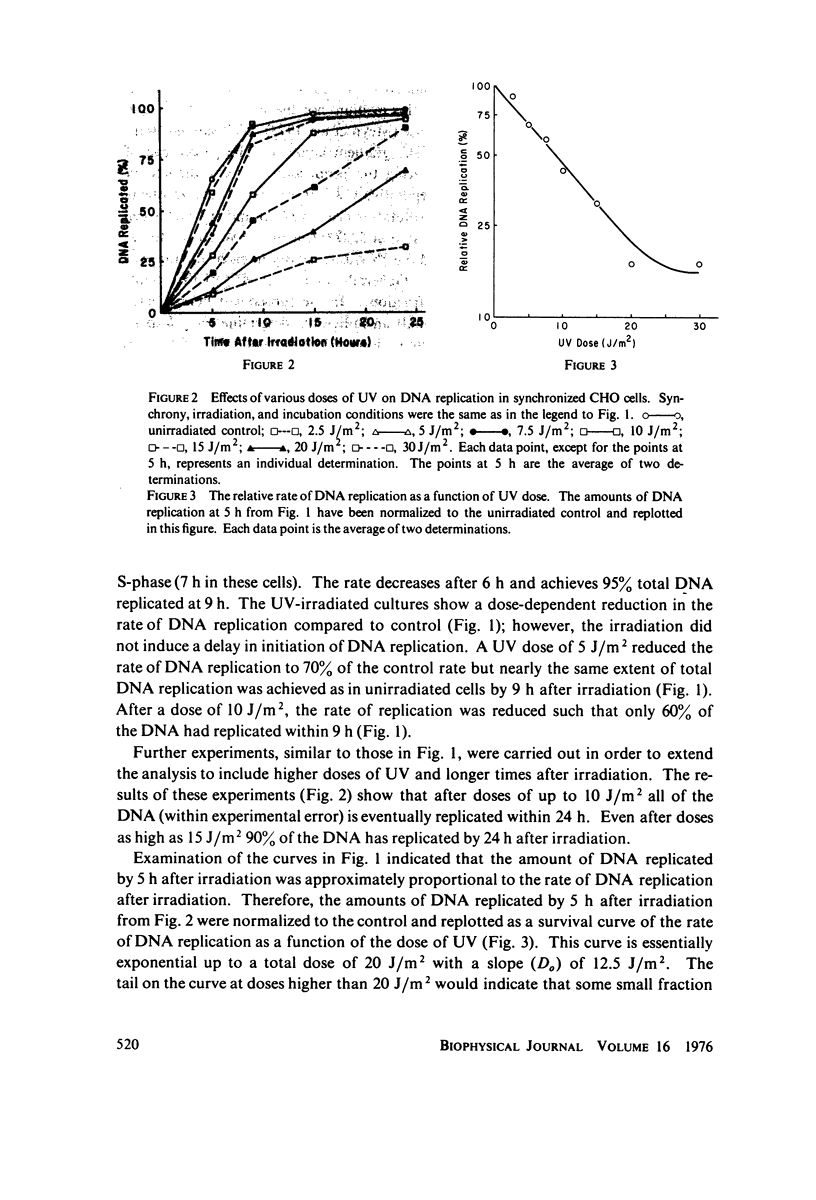
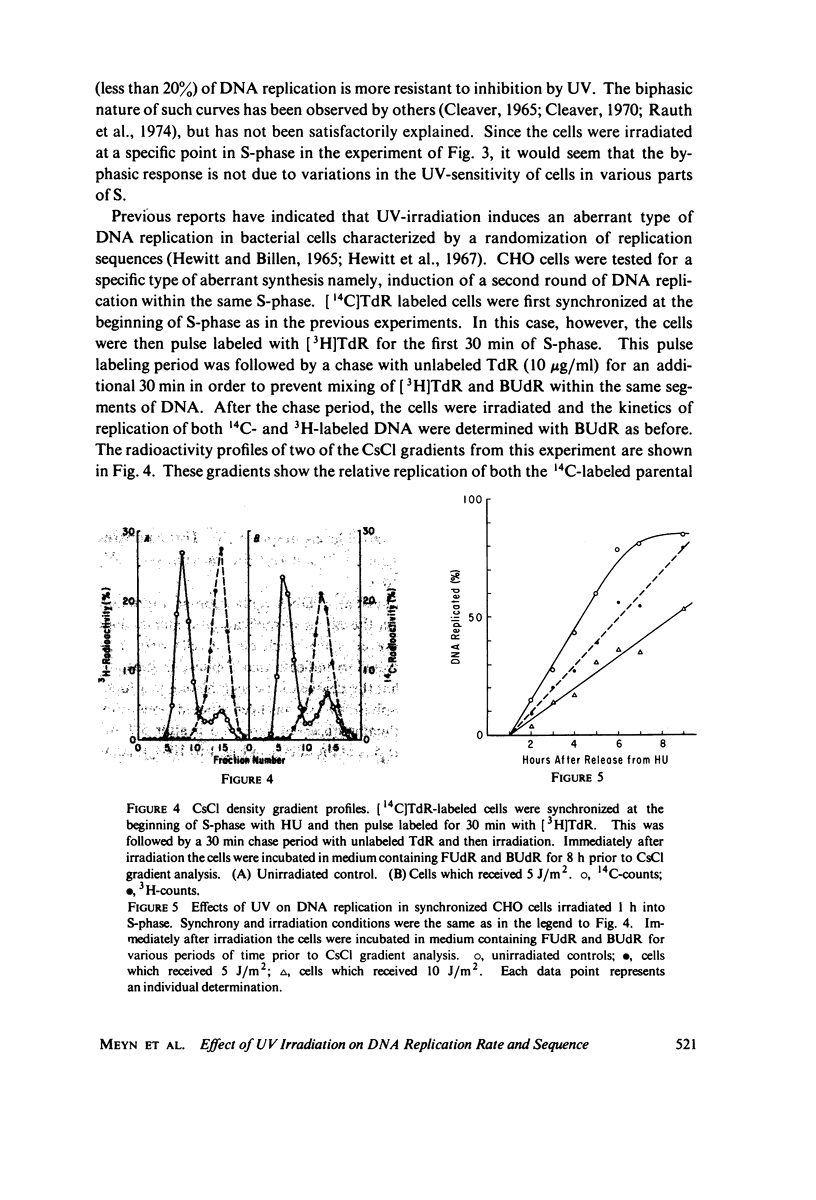
The effects of ultraviolet light (UV) irradiation on the rate of DNA replication in synchronized Chinese hamster ovary (CHO) cells were investigated. A technique for measuring semiconservative DNA replication was employed that involved growing the cells in medium containing 5-bromodeoxyuridine and subsequently determining the amount of DNA that acquired hybrid buoyant density in CsCl density gradients. One of the advantages of this technique was that it allowed a characterization of the extent of DNA replication as well as rate after irradiation. It was found that while there was a dose-dependent reduction in the rate of DNA replication following UV-irradiation, doses of up to 10 J/m2 (which produce many dimers per replication) did not prevent the ultimate replication of the entire genome. Hence, we conclude that dimers cannot be absolute blocks to DNA replication. In order to account for the total genome replication observed, a mechanism must exist that allows genome replication between dimers. The degree of reduction in the rate of replication by UV was the same whether the cells were irradiated at the G1-S boundary or 1 h into S-phase. Previous work had shown that cells in early S-phase are considerably more sensitive to UV than cells at the G1-S boundary. Experiments specifically designed to test for reiterative replication showed that UV does not induce a second round of DNA replication within the same S-phase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buhl S. N., Setlow R. B., Regan J. D. Recovery of the ability to synthesize DNA in segments of normal size at long times after ultraviolet irradiation of human cells. Biophys J. 1973 Dec;13(12):1265–1275. doi: 10.1016/S0006-3495(73)86061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. F., Rauth A. M. Nascent DNA synthesis in ultraviolet light-irradiated mouse L cells. Biochim Biophys Acta. 1972 Jan 31;259(2):164–174. doi: 10.1016/0005-2787(72)90056-1. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. DNA repair and radiation sensitivity in human (xeroderma pigmentosum) cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;18(6):557–565. doi: 10.1080/09553007014551491. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Investigations into the effects of ultraviolet light on the rate of deoxyribonucleic acid synthesis in mammalian cells. Biochim Biophys Acta. 1965 Sep 6;108(1):42–52. doi: 10.1016/0005-2787(65)90106-1. [DOI] [PubMed] [Google Scholar]
- Djordjevic B., Tolmach L. J. Responses of synchronous populations of HeLa cells to ultraviolet irradiation at selected stages of the generation cycle. Radiat Res. 1967 Oct;32(2):327–346. [PubMed] [Google Scholar]
- Domon M., Rauth A. M. Cell cycle specific recovery from fractionated exposures of ultraviolet light. Radiat Res. 1973 Jul;55(1):81–92. [PubMed] [Google Scholar]
- Domon M., Rauth A. M. Effects of caffeine on ultraviolet-irradiated mouse L cells. Radiat Res. 1969 Jul;39(1):207–221. [PubMed] [Google Scholar]
- Domon M., Rauth A. M. Ultraviolet irradiation of mouse L cells: effects on DNA synthesis and progression through the cell cycle. Radiat Res. 1968 Aug;35(2):350–368. [PubMed] [Google Scholar]
- Gautschi J. R., Kern R. M., Painter R. B. Modification of replicon operation in HeLa cells by 2,4-dinitrophenol. J Mol Biol. 1973 Nov 5;80(3):393–403. doi: 10.1016/0022-2836(73)90411-7. [DOI] [PubMed] [Google Scholar]
- HUMPHREY R. M., DEWEY W. C., CORK A. Relative ultraviolet sensitivity of different phases in the cell cycle of Chinese hamster cells grown in vitro. Radiat Res. 1963 Jun;19:247–260. [PubMed] [Google Scholar]
- Hewitt R., Billen D., Jorgensen G. Radiation-induced reorientation of chromosome replication sequence: generality in Escherichia coli; independence of prophage or 5-bromouracil toxicity. Radiat Res. 1967 Oct;32(2):214–226. [PubMed] [Google Scholar]
- Hewitt R., Billen D. Reorientation of chromosome replication after exposure to ultraviolet light of Escherichia coli. J Mol Biol. 1965 Aug;13(1):40–53. doi: 10.1016/s0022-2836(65)80078-x. [DOI] [PubMed] [Google Scholar]
- Hewitt R., Gaskins P. Influence of ultraviolet irradiation on chromosome replication in ultraviolet-sensitive bacteria. J Mol Biol. 1971 Nov 28;62(1):215–221. doi: 10.1016/0022-2836(71)90140-9. [DOI] [PubMed] [Google Scholar]
- Humphrey R. M., Sedita B. A., Meyn R. E. Recovery of Chinese hamster cells from ultra-violet irradiation damage. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;18(1):61–69. doi: 10.1080/09553007014550821. [DOI] [PubMed] [Google Scholar]
- Klímek M., Vlasínová M. Thymine and uracil-thymine dimers and deoxyribonucleic acid synthesis in mammalian cells irradiated with ultraviolet light. Int J Radiat Biol Relat Stud Phys Chem Med. 1966;11(4):329–337. doi: 10.1080/09553006714550031. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Post-replication repair of DNA in ultraviolet-irradiated mammalian cells. No gaps in DNA synthesized late after ultraviolet irradiation. Eur J Biochem. 1972 Dec 18;31(3):438–445. doi: 10.1111/j.1432-1033.1972.tb02550.x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Meyn R. E., Hewitt R. R., Humphrey R. M. Evaluation of S phase synchronization by analysis of DNA replication in 5-bromodeoxyuridine. Exp Cell Res. 1973 Nov;82(1):137–142. doi: 10.1016/0014-4827(73)90255-3. [DOI] [PubMed] [Google Scholar]
- Meyn R. E., Humphrey R. M. Deoxyribonucleic acid synthesis in ultraviolet-light-irradiated Chinese hamster cells. Biophys J. 1971 Mar;11(3):295–301. doi: 10.1016/S0006-3495(71)86215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. B. DNA damage and repair in eukaryotic cells. Genetics. 1974 Sep;78(1):139–148. doi: 10.1093/genetics/78.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauth A. M. Evidence for dark-reactivation of ultraviolet light damage in mouse L cells. Radiat Res. 1967 May;31(1):121–138. [PubMed] [Google Scholar]
- Rauth A. M., Tammemagi M., Hunter G. Nascent DNA synthesis in ultraviolet light-irradiated mouse, human and Chinese hamster cells. Biophys J. 1974 Mar;14(3):209–220. doi: 10.1016/S0006-3495(74)85908-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Regan J. D., German J., Carrier W. L. Evidence that xeroderma pigmentosum cells do not perform the first step in the repair of ultraviolet damage to their DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1035–1041. doi: 10.1073/pnas.64.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd P., Coohill T. P., Hellewell A. B., Mahoney J. A. Post-irradiation properties of cultured Chinese hamster cells exposed to ultraviolet light. Radiat Res. 1969 May;38(2):321–339. [PubMed] [Google Scholar]


