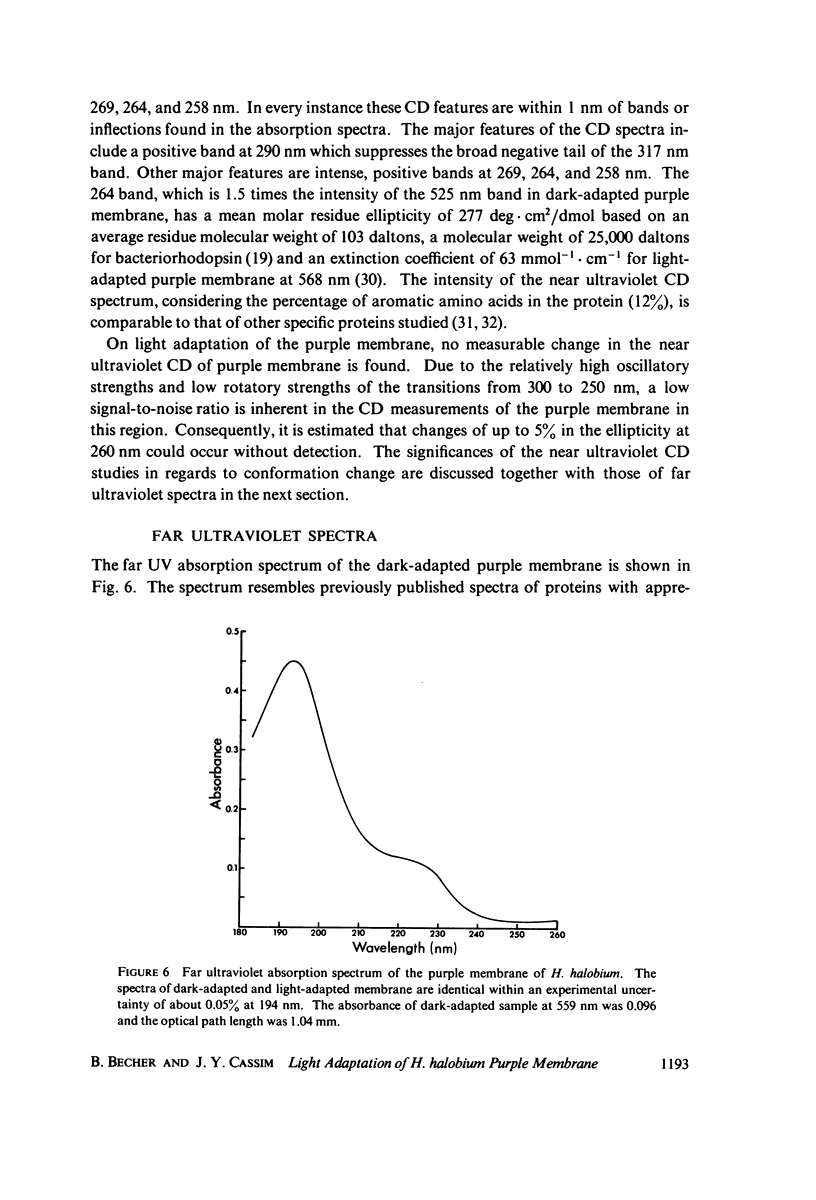
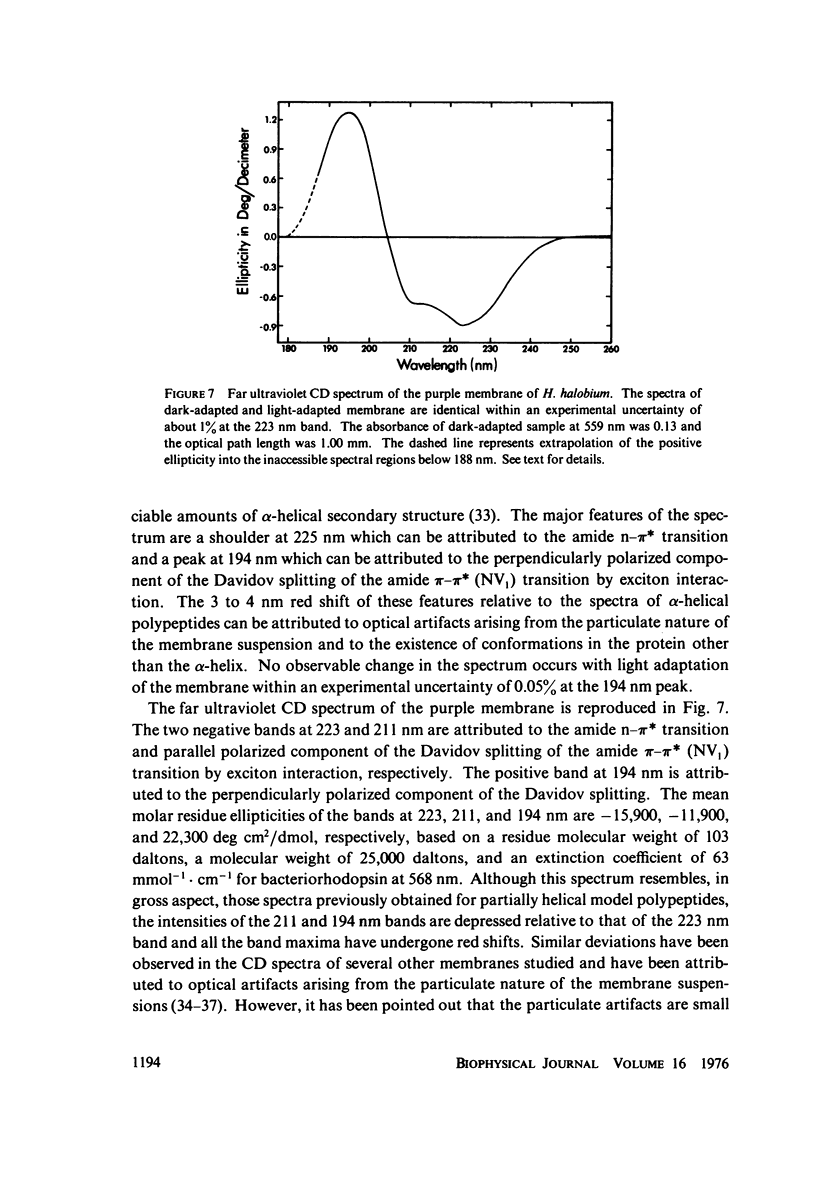
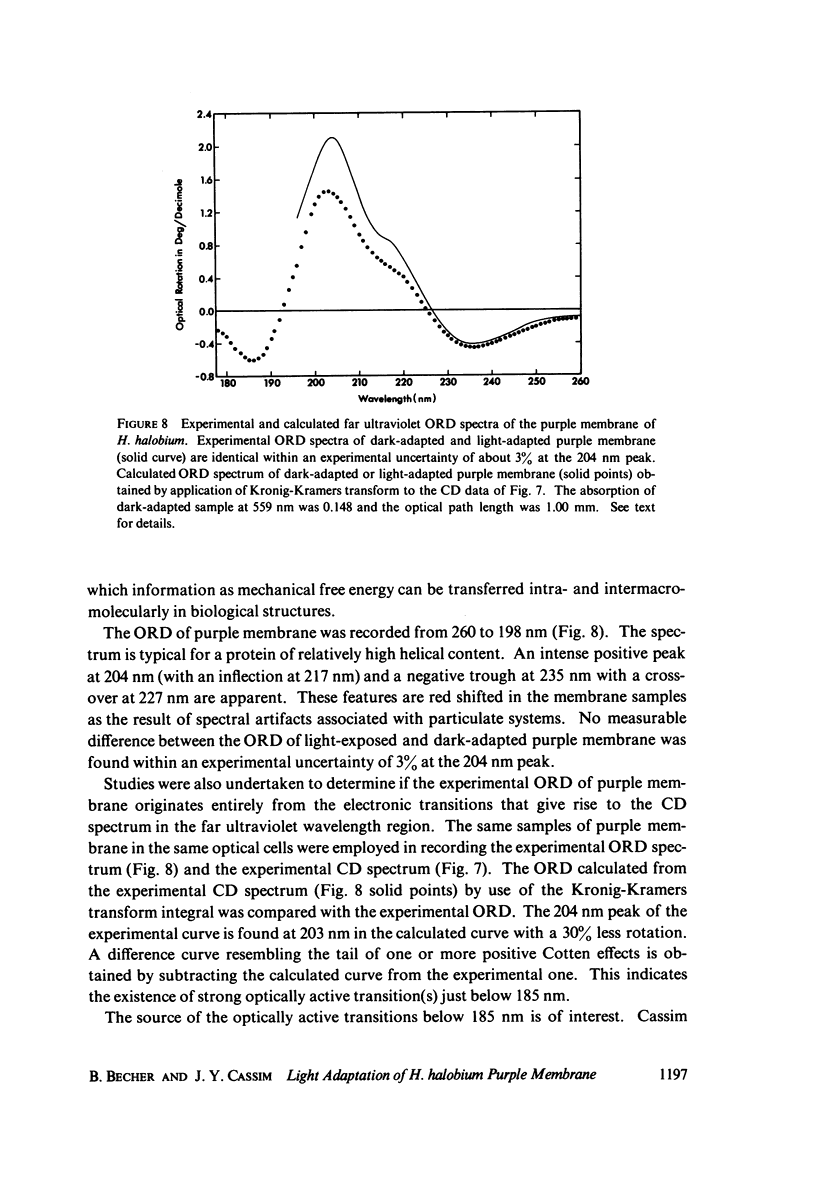
Abstract
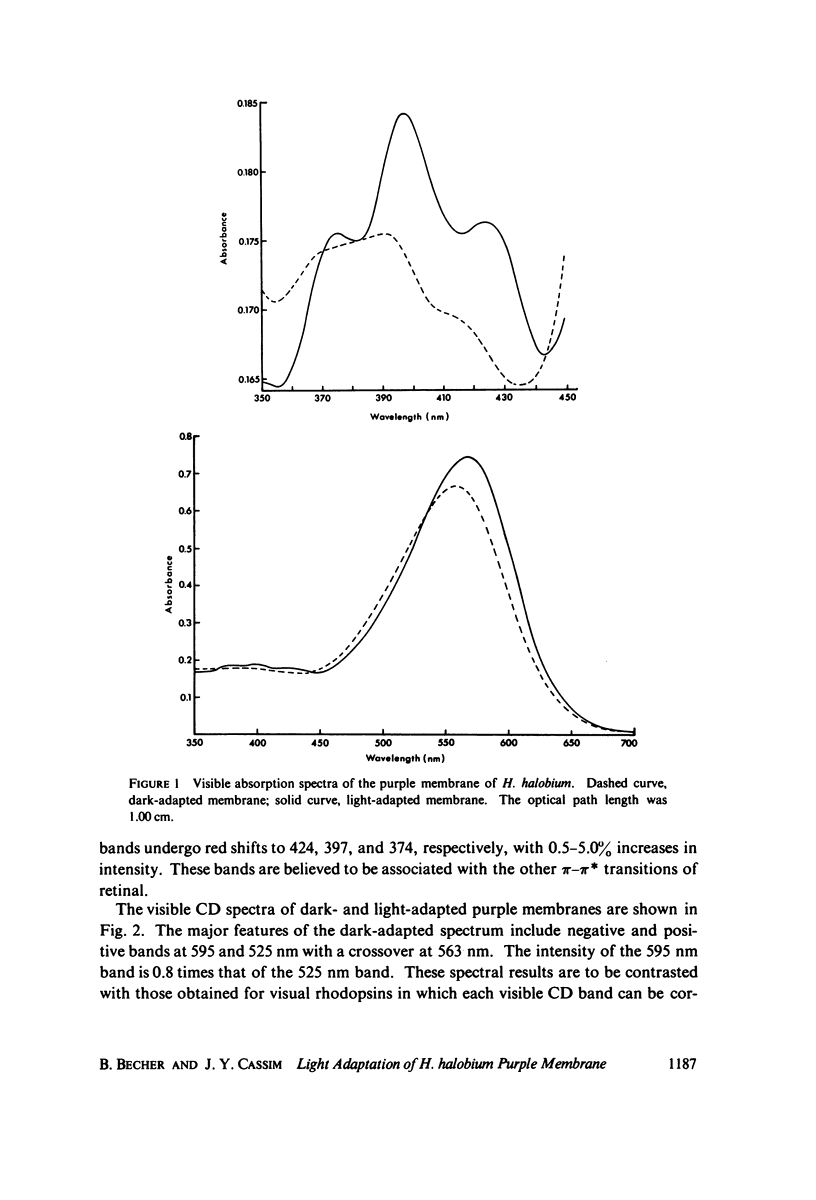
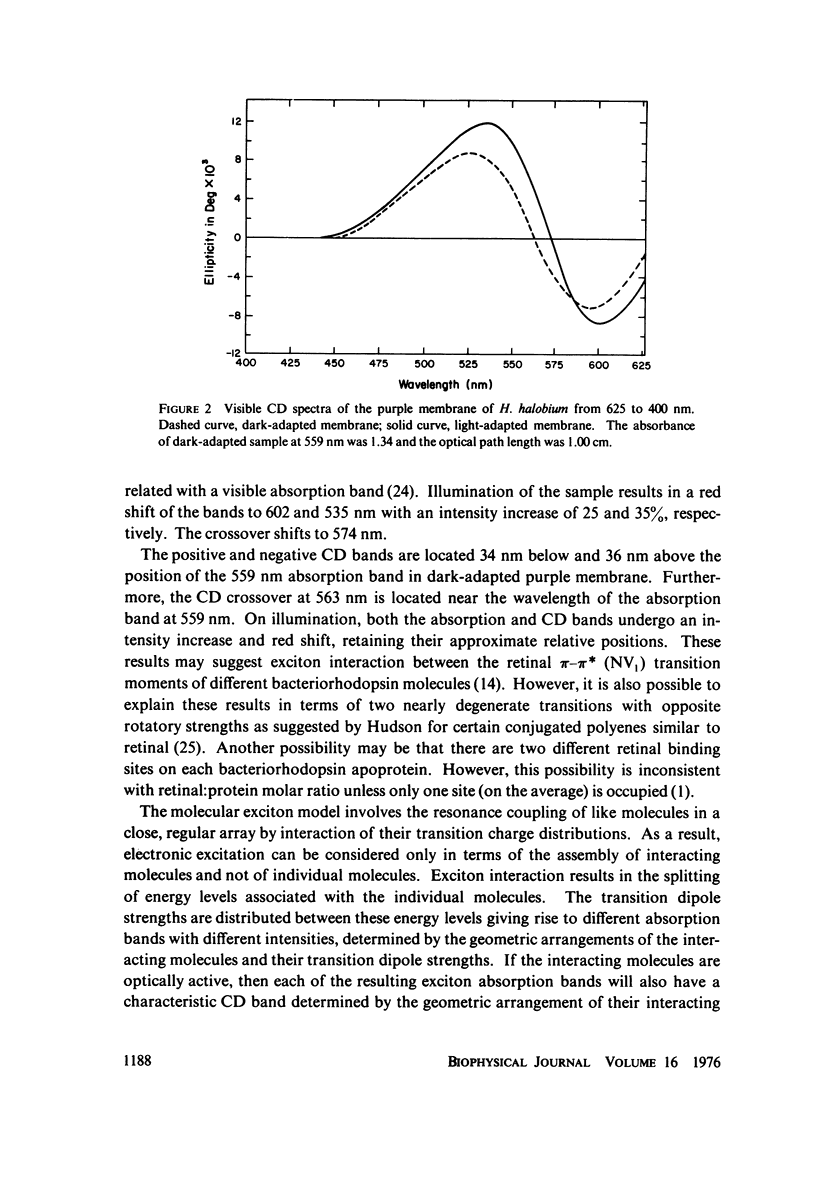
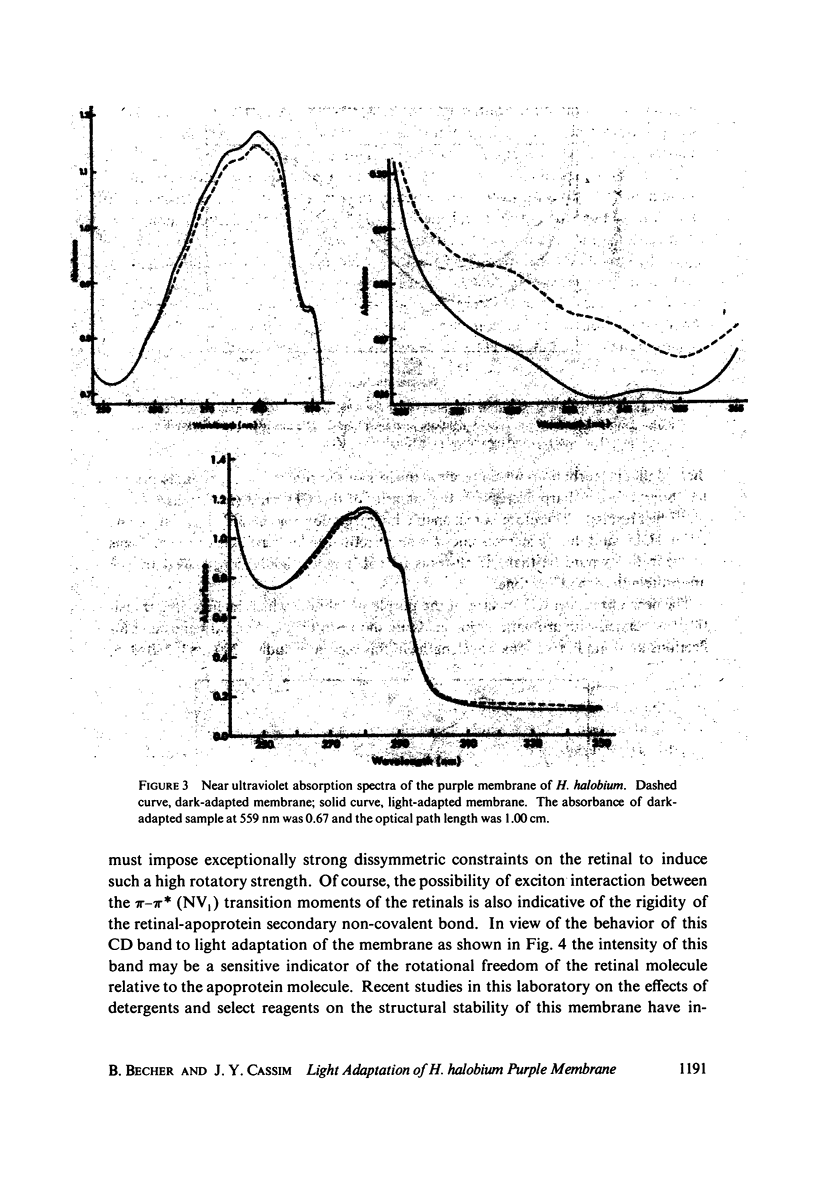
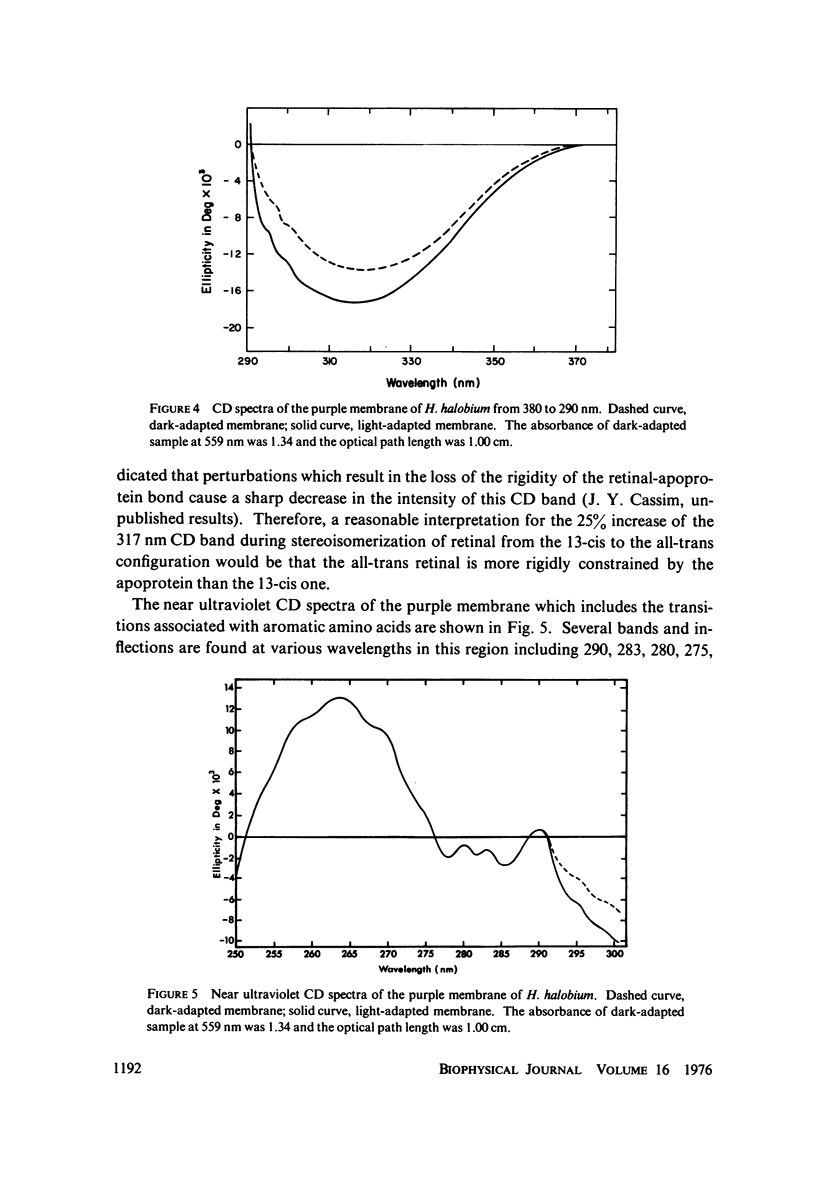
Absorption, circular dichroism and optical rotatory dispersion of the bacteriorhodopsin containing purple membrane form Halobacterium halobium were studied in regard to the structural stability of this membrane during the photoisomerization of the retinal of the bacteriorhodopsin from the 13-cis to the all-trans configuration. The following conclusions were reached: (a) the macromolecular structure (protein-protein interaction which may result in the possible exciton interaction of the retinal pi-pi* (NV1) transition moments and protein-lipid interaction) are not significantly altered, (b) possibilities of delocalized conformation changes of the apoprotein involving secondary and/or tertiary structure can be ruled out, (c) localized secondary structure conformation changes of the apoprotein must be limited to the involvement of no more than one or two amino acid residues and localized tertiary structure conformation changes of the apoprotein must be limited to a very short segment of the protein chain containing only a few aromatic amino acid residues, and (d) the interaction between the apoprotein and retinal seems to be relatively more pronounced when the retinal is in the all-trans form than the 13-cis from and also the apoprotein seems to impose a more pronounced dissymmetric constraint on the retinal in the all-trans form than in the 13-cis form.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barela T. D., Darnall D. W. Practical aspects of calculating protein secondary structure from circular dichroism spectra. Biochemistry. 1974 Apr 9;13(8):1694–1700. doi: 10.1021/bi00705a022. [DOI] [PubMed] [Google Scholar]
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Becher B., Ebrey T. G. Evidence for chromophore-chromophore (exciton) interaction in the purple membrane of Halobacterium halobium. Biochem Biophys Res Commun. 1976 Mar 8;69(1):1–6. doi: 10.1016/s0006-291x(76)80263-x. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E. Bacteriorhodospin: a trans-membrane pump containing alpha-helix. J Mol Biol. 1975 Apr 5;93(2):139–158. doi: 10.1016/0022-2836(75)90124-2. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- Bridgen J., Walker I. D. Photoreceptor protein from the purple membrane of Halobacterium halobium. Molecular weight and retinal binding site. Biochemistry. 1976 Feb 24;15(4):792–798. doi: 10.1021/bi00649a010. [DOI] [PubMed] [Google Scholar]
- Cassim J. Y., Yang J. T. A computerized calibration of the circular dichrometer. Biochemistry. 1969 May;8(5):1947–1951. doi: 10.1021/bi00833a026. [DOI] [PubMed] [Google Scholar]
- Cassim J. Y., Yang J. T. Critical comparison of the experimental optical activity of helical polypeptides and the predictions of the molecular exciton model. Biopolymers. 1970;9(12):1475–1502. doi: 10.1002/bip.1970.360091209. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T. A new approach to the calculation of secondary structures of globular proteins by optical rotatory dispersion and circular dichroism. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1285–1291. doi: 10.1016/s0006-291x(71)80225-5. [DOI] [PubMed] [Google Scholar]
- Chignell C. F., Chignell D. A. A spin label study of purple membranes from Halobacterium halobium. Biochem Biophys Res Commun. 1975 Jan 6;62(1):136–143. doi: 10.1016/s0006-291x(75)80415-3. [DOI] [PubMed] [Google Scholar]
- Danon A., Stoeckenius W. Photophosphorylation in Halobacterium halobium. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1234–1238. doi: 10.1073/pnas.71.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker R. V., Carraway K. L. Circular dichroism of erythrocyte membrane glycoproteins. Biochim Biophys Acta. 1975 Mar 28;386(1):52–61. doi: 10.1016/0005-2795(75)90245-7. [DOI] [PubMed] [Google Scholar]
- Dencher N., Wilms M. Flash photometric experiments on the photochemical cycle of bacteriorhodopsin. Biophys Struct Mech. 1975 May 30;1(3):259–271. doi: 10.1007/BF00535760. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Holzwarth G. Artifacts in the measured optic activity of membrane suspensions. Arch Biochem Biophys. 1971 Feb;142(2):481–488. doi: 10.1016/0003-9861(71)90511-x. [DOI] [PubMed] [Google Scholar]
- Gordon D. Discussion paper: classical scattering calculation of particulate artifacts in membrane optical activity. Ann N Y Acad Sci. 1972 Jun 20;195:147–149. [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Bauer P. J., Dencher N. A. A natural CD label to probe the structure of the purple membrane from Halobacterium halobium by means of exciton coupling effects. Biochem Biophys Res Commun. 1975 Dec 1;67(3):897–903. doi: 10.1016/0006-291x(75)90761-5. [DOI] [PubMed] [Google Scholar]
- Hildebrand E., Dencher N. Two photosystems controlling behavioural responses of Halobacterium halobium. Nature. 1975 Sep 4;257(5521):46–48. doi: 10.1038/257046a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y. The isomeric configuration of the bacteriorhodopsin chromophore. Vision Res. 1975 Oct;15:1081–1086. doi: 10.1016/0042-6989(75)90004-8. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr, Tinoco I., Jr Circular dichroism of polypeptide solutions in the vacuum ultraviolet. J Am Chem Soc. 1972 Jun 14;94(12):4389–4392. doi: 10.1021/ja00767a084. [DOI] [PubMed] [Google Scholar]
- Kemper D. L., Everse J. Active enzyme centrifugation. Methods Enzymol. 1973;27:67–82. doi: 10.1016/s0076-6879(73)27005-2. [DOI] [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi L. K. Light-induced leucine transport in Halobacterium halobium envelope vesicles: a chemiosmotic system. Biochemistry. 1975 Jul;14(13):2882–2889. doi: 10.1021/bi00684a014. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Hess B. Reversible photolysis of the purple complex in the purple membrane of Halobacterium halobium. Eur J Biochem. 1973 Aug 17;37(2):316–326. doi: 10.1111/j.1432-1033.1973.tb02990.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Meentzen M., Schuhmann L. Reversible dissociation of the purple complex in bacteriorhodopsin and identification of 13-cis and all-trans-retinal as its chromophores. Eur J Biochem. 1973 Dec 17;40(2):453–463. doi: 10.1111/j.1432-1033.1973.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Schneider A. S. Analysis of optical activity spectra of turbid biological suspensions. Methods Enzymol. 1973;27:751–767. doi: 10.1016/s0076-6879(73)27032-5. [DOI] [PubMed] [Google Scholar]
- Urry D. W. Conformation of protein in biological membranes and a model transmembrane channel. Ann N Y Acad Sci. 1972 Jun 20;195:108–125. [PubMed] [Google Scholar]
- Urry D. W., Masotti L., Krivacic J. Improved ellipticity data for several biological membranes. Biochem Biophys Res Commun. 1970 Nov 9;41(3):521–524. doi: 10.1016/0006-291x(70)90043-4. [DOI] [PubMed] [Google Scholar]
- Urry D. W. Protein conformation in biomembranes: optical rotation and absorption of membrane suspensions. Biochim Biophys Acta. 1972 Feb 14;265(1):115–168. doi: 10.1016/0304-4157(72)90021-4. [DOI] [PubMed] [Google Scholar]
- Wallach D. F., Gordon A. Lipid protein interactions in cellular membranes. Fed Proc. 1968 Nov-Dec;27(6):1263–1268. [PubMed] [Google Scholar]


