Abstract
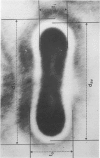
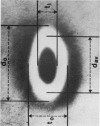
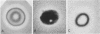
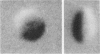
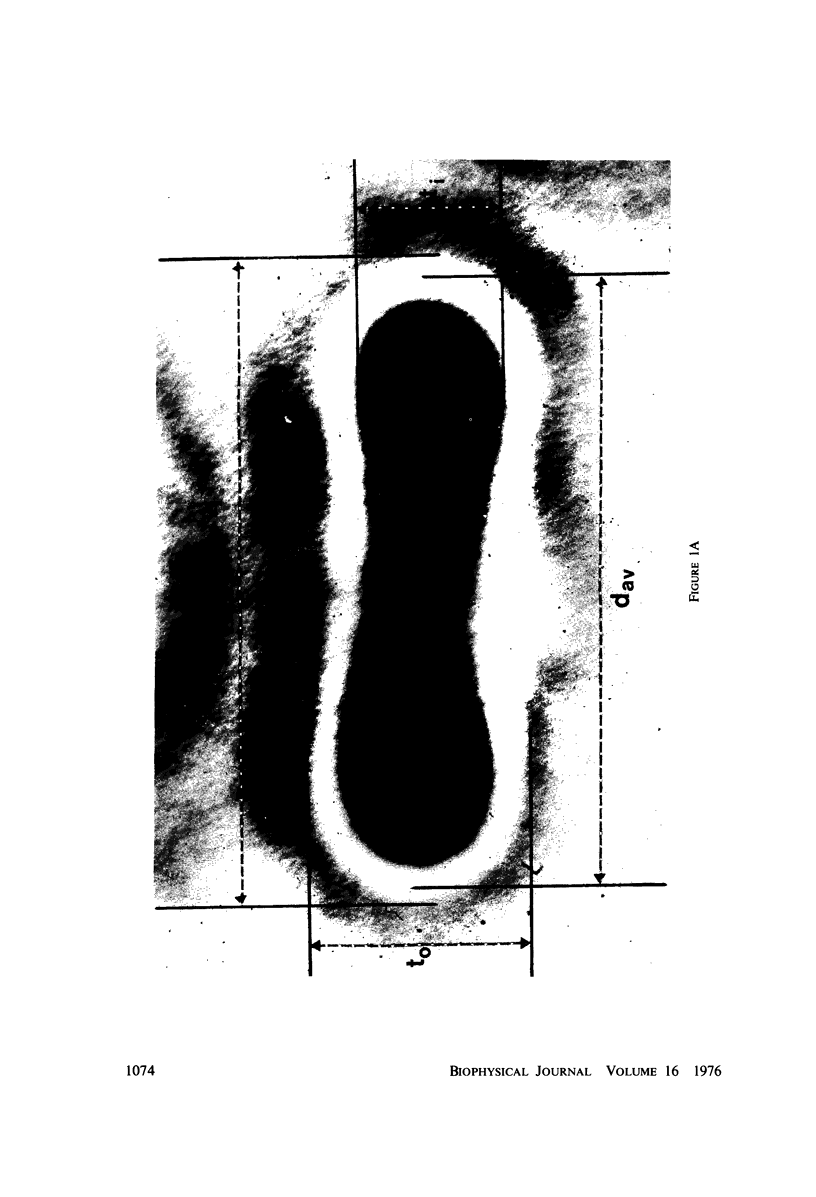
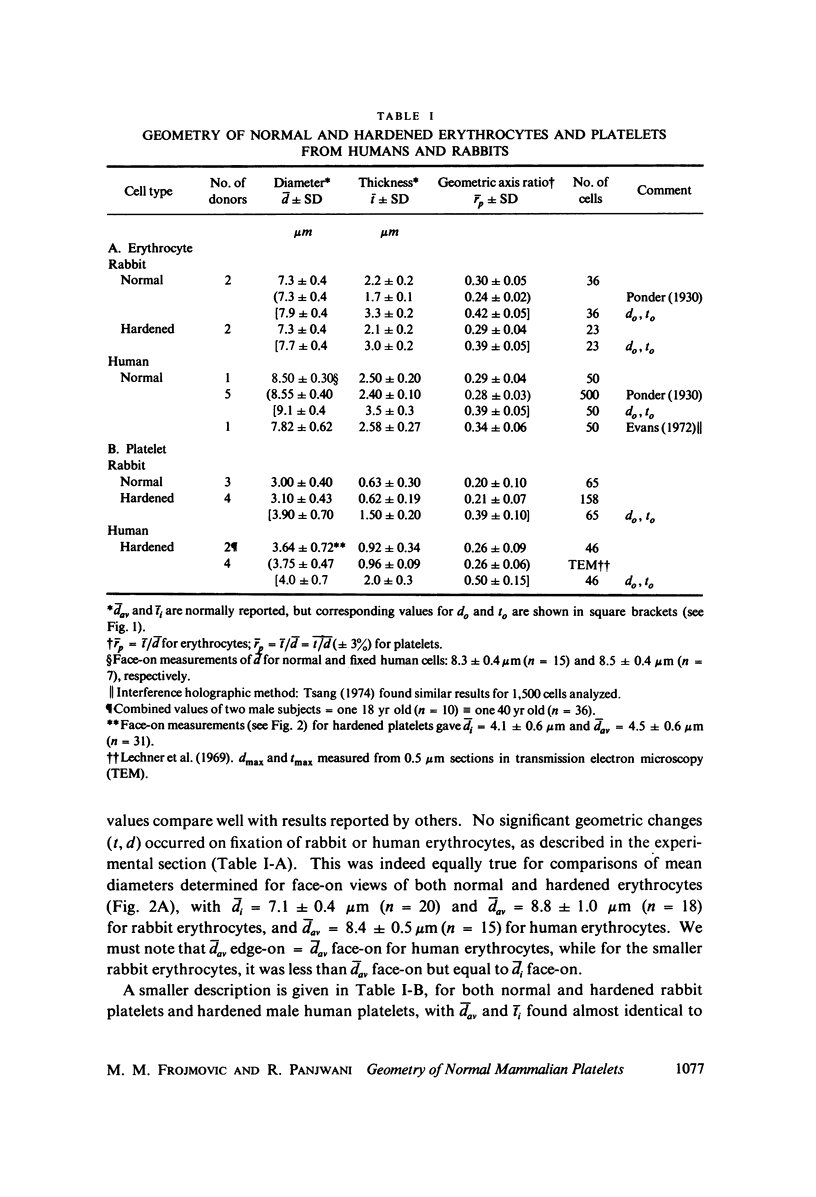
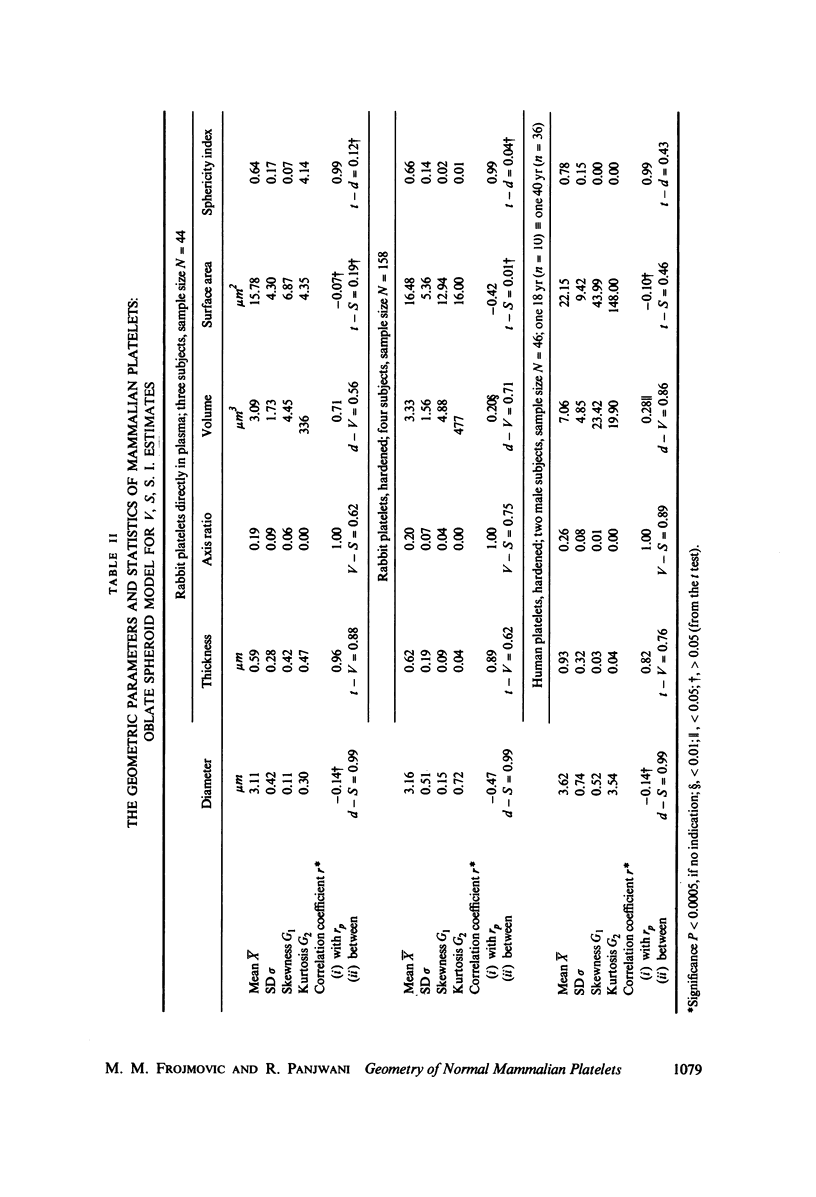
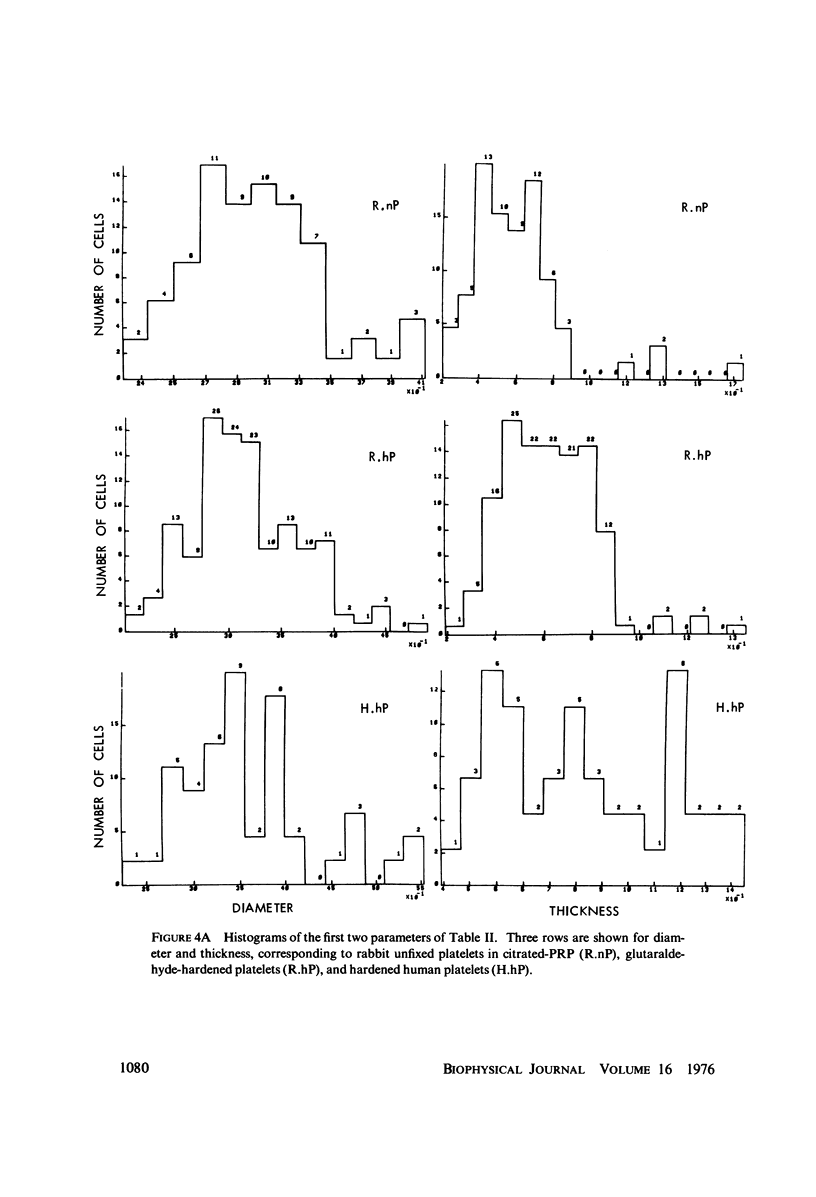
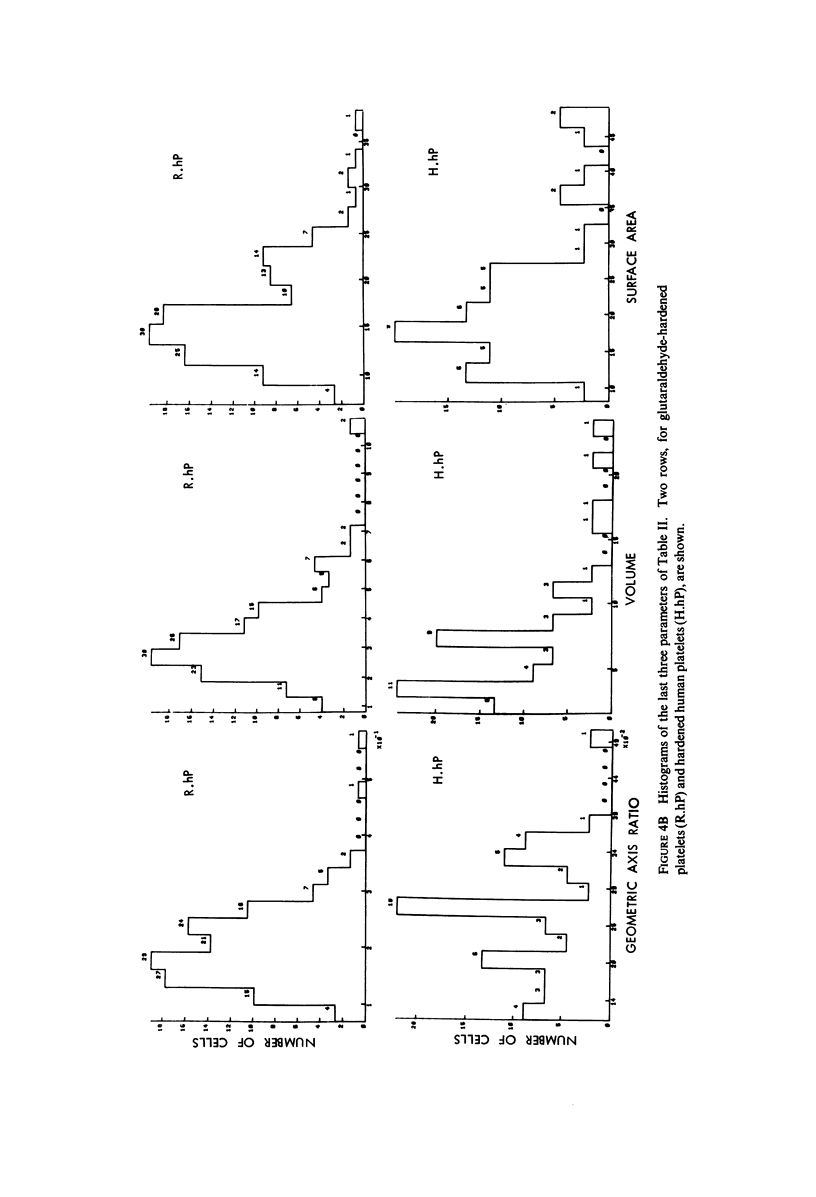
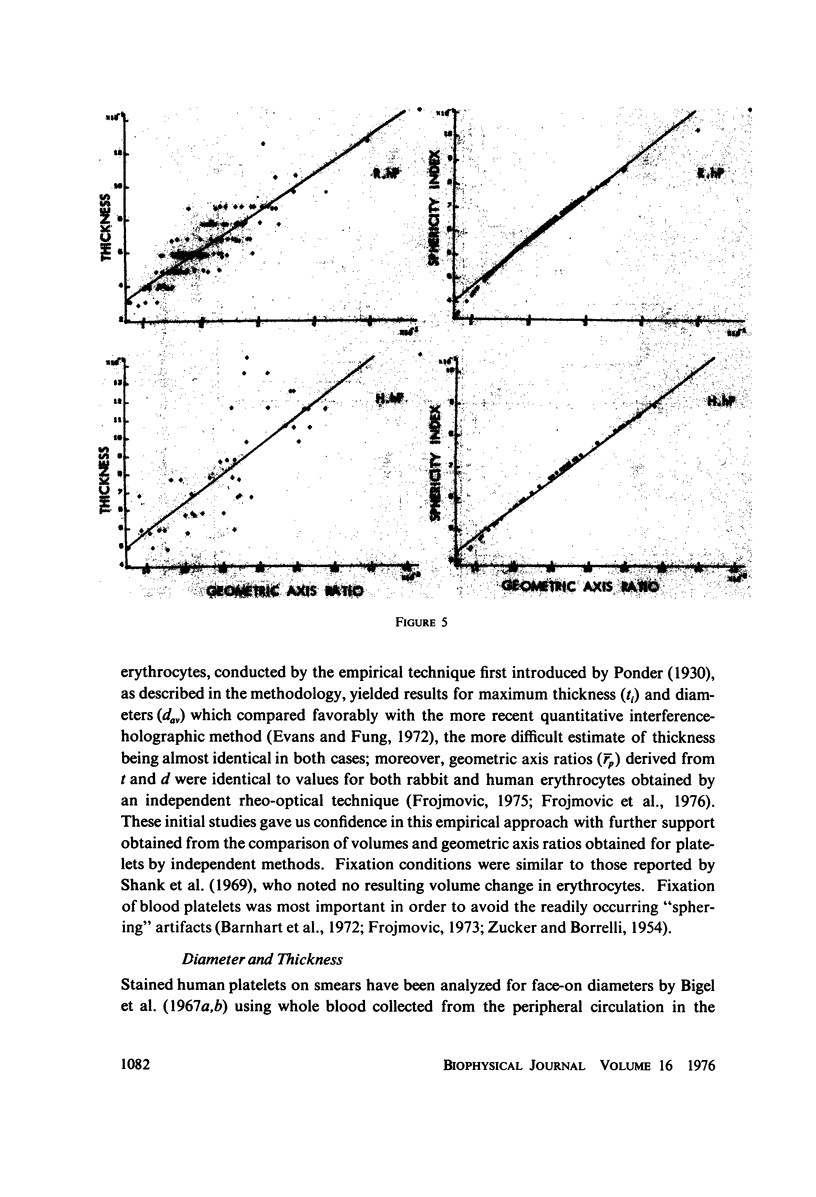
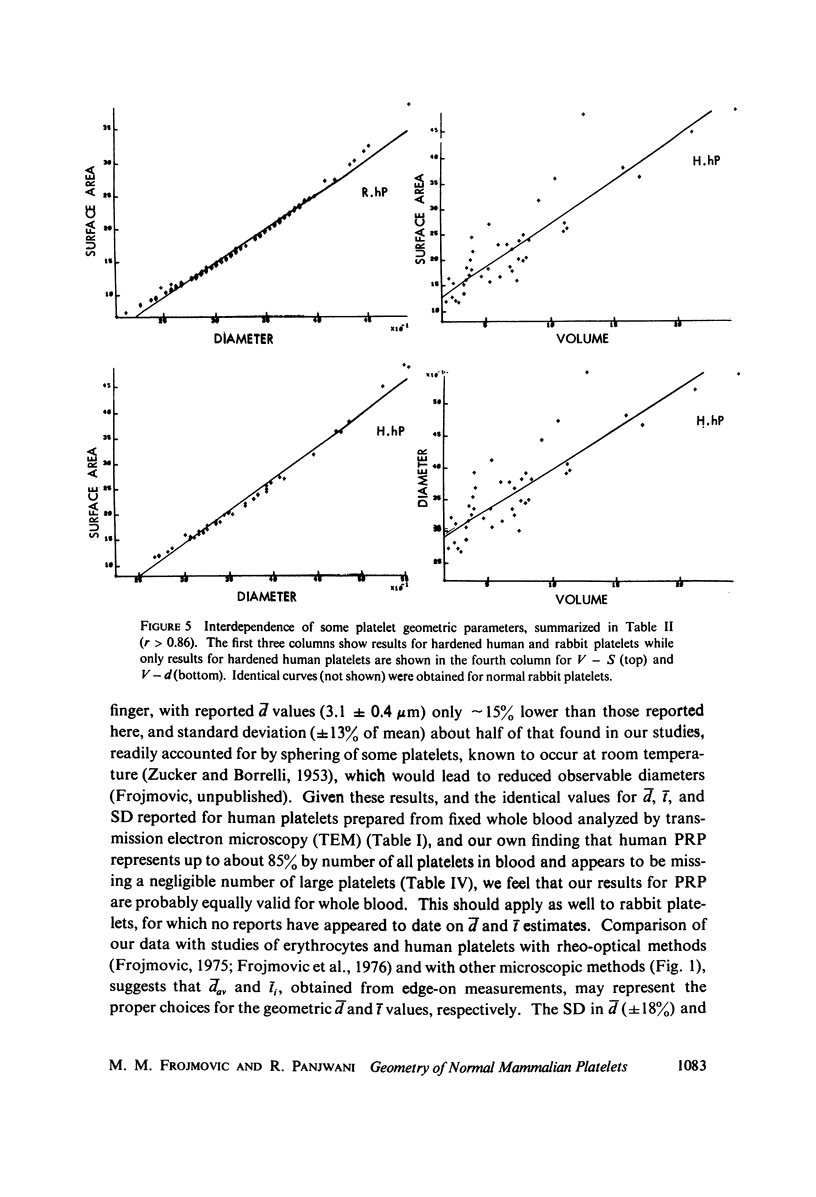
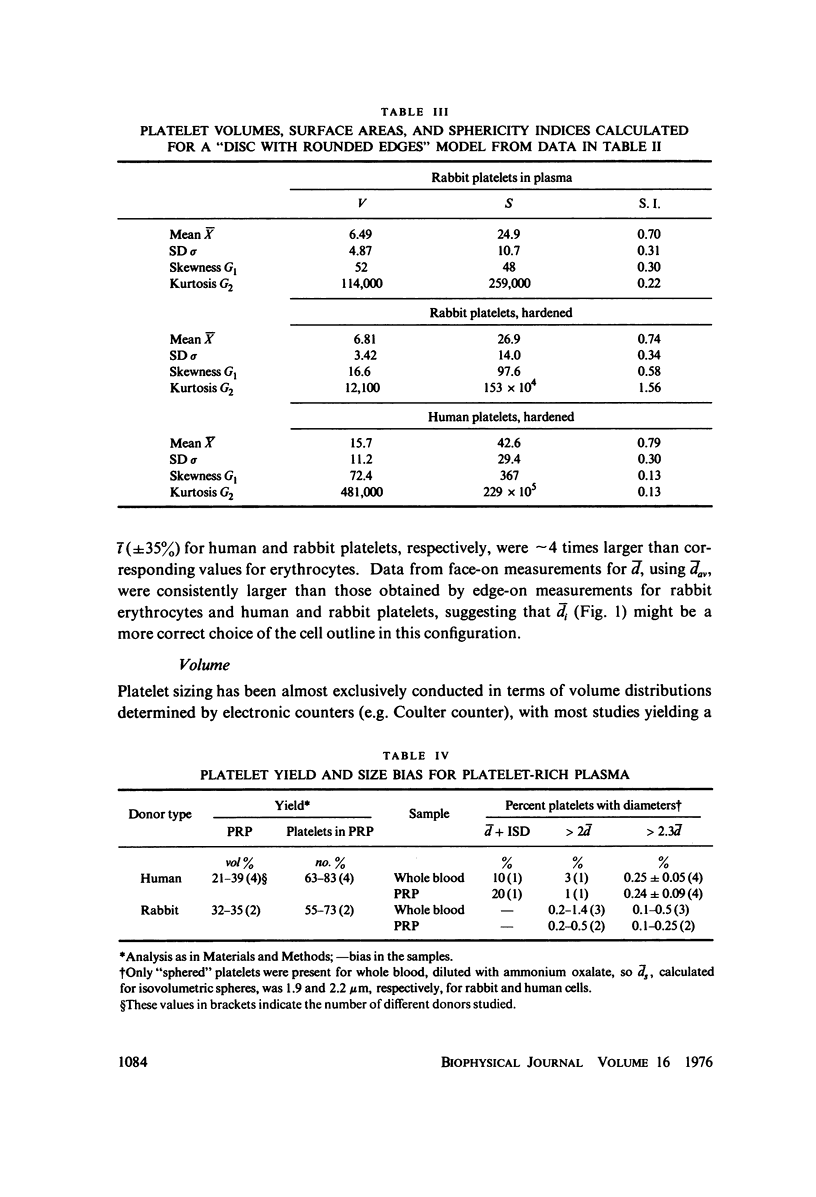
The shape distributions of normal and hardened human and rabbit erythrocytes and platelets were obtained for edge-on orientations of a few hundred freely rotating cells from analyses of microphotographs obtained similarly as by Ponder(1930, Q. J. Exp. Physiol. 20:29) by phase-contrast microscopy at 800 X magnification. Major average diameters (d) and thicknesses (t) were estimated for both normal and hardened cells, and were used to calculate an average geometric axis ratio, rp = t/d, which increases to unity as cells become more spherical. Our fixation procedure did not alter these shape parameters: rp was unchanged for erythrocytes, with d and t values similar to those reported by Ponder (1930); platelets had d X t = 3.6 +/- 0.7 mum X 0.9 +/- 0.3 mum and 3.1 +/- 0.4 mum X 0.6 +/- 0.3 mum, respectively, for human and rabbit cells, with rp = 0.26 and 0.20, respectively. Agreement in rp was found with data obtained by a novel rheo-optical method which allows for a direct statistical averaging for large populations (greater than 100 X 10(3) cells). Histograms and linear correlation studies were made of the above three parameters (d,t,rp), as well as volume (V), total surface area are (S), and sphericity index (S.I.) calculated for both "prolate ellipsoid" and "disc with rounded edges" models. Results indicate very high linear correlations between rp - t, rp - S. I., and d -S, with high correlations for t - V,d -V and S. Data are in agreement with the few reports in the literature determined by other methods, with the best model for platelets appearing to be an oblate spheroid.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnhart M. I., Walsh R. T., Robinson J. A. A three-dimensional view of platelet responses to chemical stimuli. Ann N Y Acad Sci. 1972 Oct 27;201:360–390. doi: 10.1111/j.1749-6632.1972.tb16311.x. [DOI] [PubMed] [Google Scholar]
- Bigel P., Lellouch J., Mayer S., Waitz R. Le diamètre thrombocytaire chez l'adulte normal. Nouv Rev Fr Hematol. 1967 Nov-Dec;7(6):900–903. [PubMed] [Google Scholar]
- Bigel P., Lellouch J., Mayer S., Waitz R. Les variations pathologiques des diamètres thrombocytaires. Nouv Rev Fr Hematol. 1967 Nov-Dec;7(6):903–907. [PubMed] [Google Scholar]
- Born G. V. Observations on the change in shape of blood platelets brought about by adenosine diphosphate. J Physiol. 1970 Aug;209(2):487–511. doi: 10.1113/jphysiol.1970.sp009176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull B. S., Zucker M. B. Changes in platelet volume produced by temperature, metabolic inhibitors, and aggregating agents. Proc Soc Exp Biol Med. 1965 Nov;120(2):296–301. doi: 10.3181/00379727-120-30516. [DOI] [PubMed] [Google Scholar]
- Canham P. B., Burton A. C. Distribution of size and shape in populations of normal human red cells. Circ Res. 1968 Mar;22(3):405–422. doi: 10.1161/01.res.22.3.405. [DOI] [PubMed] [Google Scholar]
- Child J. A., Bowry W. M., Knowles J. P. Abnormality of red-cell diameter-thickness ratio: findings in iron-deficiency anaemia. Br J Haematol. 1970 Aug;19(2):251–255. doi: 10.1111/j.1365-2141.1970.tb01621.x. [DOI] [PubMed] [Google Scholar]
- Eden M. Image processing techniques in relation to studies of red cell shape. Nouv Rev Fr Hematol. 1972 Nov-Dec;12(6):861–869. [PubMed] [Google Scholar]
- Enticknap J. B., Gooding P. G., Lansley T. S., Avis P. R. Platelet size and function in ischaemic heart disease. J Atheroscler Res. 1969 Jul-Aug;10(1):41–49. doi: 10.1016/s0368-1319(69)80080-3. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Leblond P. F. Geometric properties of individual red blood cell discocyte-spherocyte transformations. Biorheology. 1973 Sep;10(3):393–404. doi: 10.3233/bir-1973-10313. [DOI] [PubMed] [Google Scholar]
- Evans E., Fung Y. C. Improved measurements of the erythrocyte geometry. Microvasc Res. 1972 Oct;4(4):335–347. doi: 10.1016/0026-2862(72)90069-6. [DOI] [PubMed] [Google Scholar]
- Frojmovic M. M., Newton M., Goldsmith H. L. The microrheology of mammalian platelets: studies of rheo-optical transients and flow in tubes. Microvasc Res. 1976 Mar;11(2):203–215. doi: 10.1016/0026-2862(76)90052-2. [DOI] [PubMed] [Google Scholar]
- Frojmovic M. M., Okagawa A., Mason S. G. Rheo-optical transients in erythrocyte suspensions. Biochem Biophys Res Commun. 1975 Jan 6;62(1):17–24. doi: 10.1016/s0006-291x(75)80399-8. [DOI] [PubMed] [Google Scholar]
- Frojmovic M. M., Panjwani R. Blood cell structure-function studies: light transmission and attenuation coefficients of suspensions of blood cells and model particles at rest and with stirring. J Lab Clin Med. 1975 Aug;86(2):326–343. [PubMed] [Google Scholar]
- Frojmovic M. M. Quantitative parameterization of the light transmission properties of citrated, platelet-rich plasma as a function of platelet and adenosine diphosphate concentrations and temperature. J Lab Clin Med. 1973 Jul;82(1):137–153. [PubMed] [Google Scholar]
- Frojmovic M. M. Rheo-optical studies of blood cells. Biorheology. 1975 Jun;12(3-4):193–202. doi: 10.3233/bir-1975-123-409. [DOI] [PubMed] [Google Scholar]
- Garg S. K., Amorosi E. L., Karpatkin S. Use of the megathrombocyte as an index of megakaryocyte number. N Engl J Med. 1971 Jan 7;284(1):11–17. doi: 10.1056/NEJM197101072840103. [DOI] [PubMed] [Google Scholar]
- Grover N. B., Naaman J., Ben-Sasson S., Doljanski F. Electrical sizing of particles in suspensions. 3. Rigid spheroids and red blood cells. Biophys J. 1972 Sep;12(9):1099–1117. doi: 10.1016/s0006-3495(72)86147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover N. B., Naaman J., Ben-Sasson S., Doljanski F. Electrical sizing of particles in suspensions. I. Theory. Biophys J. 1969 Nov;9(11):1398–1414. doi: 10.1016/S0006-3495(69)86461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann R. G., Griggs V. G. Effect of counting solution and antihistaminics on platelet volume. Thromb Diath Haemorrh. 1967 Dec 31;18(3-4):705–712. [PubMed] [Google Scholar]
- Hirsh J. Platelet age: its relationship to platelet size, function and metabolism. Br J Haematol. 1972 Sep;23(Suppl):209–214. doi: 10.1111/j.1365-2141.1972.tb03520.x. [DOI] [PubMed] [Google Scholar]
- Jay A. W. Geometry of the human erythrocyte. I. Effect of albumin on cell geometry. Biophys J. 1975 Mar;15(3):205–222. doi: 10.1016/S0006-3495(75)85812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S. Heterogeneity of human platelets. I. Metabolic and kinetic evidence suggestive of young and old platelets. J Clin Invest. 1969 Jun;48(6):1073–1082. doi: 10.1172/JCI106063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S. Human platelet senescence. Annu Rev Med. 1972;23:101–128. doi: 10.1146/annurev.me.23.020172.000533. [DOI] [PubMed] [Google Scholar]
- Kraytman M. Platelet size in thrombocytopenias and thrombocytosis of various origin. Blood. 1973 Apr;41(4):587–598. [PubMed] [Google Scholar]
- Lechner K., Breddin K., Moser K., Stockinger L., Wenzel E. May-Hegglinsche Anomalie. Beschreibung einer neuen Familie und Untersuchungen zur Funktion, Biochemie und Ultrastruktur der Thrombozyten. Acta Haematol. 1969;42(5):303–320. doi: 10.1159/000208814. [DOI] [PubMed] [Google Scholar]
- Nakeff A., Ingram M. Platelet count: volume relationships in four mammalian species. J Appl Physiol. 1970 Apr;28(4):530–533. doi: 10.1152/jappl.1970.28.4.530. [DOI] [PubMed] [Google Scholar]
- O'Brien J. R. Platelet function: a guide to platelet membrane structure. Ser Haematol. 1970;3(4):68–82. [PubMed] [Google Scholar]
- RAND R. P., BURTON A. C. Area and volume changes in hemolysis of single erythrocytes. J Cell Comp Physiol. 1963 Jun;61:245–253. doi: 10.1002/jcp.1030610306. [DOI] [PubMed] [Google Scholar]
- Salzman E. W., Ashford T. P., Chambers D. A., Neri L. L., Dempster A. P. Platelet volume: effect of temperature and agents affecting platelet aggregation. Am J Physiol. 1969 Nov;217(5):1330–1338. doi: 10.1152/ajplegacy.1969.217.5.1330. [DOI] [PubMed] [Google Scholar]
- Shank B. B., Adams R. B., Steidley K. D., Murphy J. R. A physical explantation of the bimodal distribution obtained by electronic sizing of erythrocytes. J Lab Clin Med. 1969 Oct;74(4):630–641. [PubMed] [Google Scholar]
- Shimamoto T., Yamazaki H., Shimamoto T. Scanning electron microscopic observation of platelets in hemostasis. Thromb Diath Haemorrh. 1973 Feb 28;29(1):168–182. [PubMed] [Google Scholar]
- ZUCKER M. B., BORRELLI J. Reversible alterations in platelet morphology produced by anticoagulants and by cold. Blood. 1954 Jun;9(6):602–608. [PubMed] [Google Scholar]