Abstract
Humans have three DNA polymerases, Polη, Polκ, and Polι, which are able to promote replication through DNA lesions. However, the mechanism by which these DNA polymerases are targeted to the replication machinery stalled at a lesion site has remained unknown. Here, we provide evidence for the physical interaction of human Polκ (hPolκ) with proliferating cell nuclear antigen (PCNA) and show that PCNA, replication factor C (RFC), and replication protein A (RPA) act cooperatively to stimulate the DNA synthesis activity of hPolκ. The processivity of hPolκ, however, is not significantly increased in the presence of these protein factors. The efficiency (Vmax/Km) of correct nucleotide incorporation by hPolκ is enhanced ∼50- to 200-fold in the presence of PCNA, RFC, and RPA, and this increase in efficiency is achieved by a reduction in the apparent Km for the nucleotide. Although in the presence of these protein factors, the efficiency of the insertion of an A nucleotide opposite an abasic site is increased ∼40-fold, this reaction still remains quite inefficient; thus, it is unlikely that hPolκ would bypass an abasic site by inserting a nucleotide opposite the site.
In both prokaryotes and eukaryotes, DNA polymerases belonging to the UmuC/Rad30/DinB family promote replication through DNA lesions (6, 16). In eukaryotes, DNA polymerase η (Polη) functions in the error-free replication of UV-damaged DNA, and both yeast Polη and human Polη (hPolη) replicate through a cis-syn thymine-thymine (T-T) dimer with the same efficiency and accuracy with which they replicate through undamaged T’s (13, 17, 31). Genetic studies of yeast have also implicated Polη in the error-free bypass of cyclobutane dimers formed at 5′-TC-3′ and 5′-CC-3′ sites (32), and Polη efficiently replicates through many other DNA lesions as well (9, 11). Mutations in Polη in humans cause a cancer-prone syndrome, the variant form of xeroderma pigmentosum (12, 22).
In addition to Polη, humans contain two other related polymerases, Polι and Polκ (14, 15, 25, 30). Unlike Polη, Polι does not bypass a cis-syn T-T dimer and it does not even insert a nucleotide opposite the 3′ T of the dimer (15). A role of Polι in lesion bypass, however, is indicated by its ability to incorporate nucleotides opposite the 3′ T of the (6-4) T-T photoproduct and opposite abasic sites (15). Subsequently, Polζ extends from the nucleotide inserted by Polι, thus completing the bypass process (15).
In contrast to Polη and Polι, which exist only in eukaryotes, the DinB protein and its homologs are found in both prokaryotes and eukaryotes (6, 16). In Escherichia coli, dinB-encoded Pol IV shows little ability to bypass a cis-syn T-T dimer, a (6-4) T-T photoproduct, or an abasic site (29). However, genetic studies have implicated Pol IV in the mutagenic replication of undamaged DNA, as deletion of the dinB gene in E. coli reduces the rates of frameshift and base substitution mutations. This effect of dinB in generating spontaneous mutations becomes more evident when the replicative polymerase has been partially disabled and the mismatch repair system has been inactivated (27). Also, overexpression of dinB in E. coli increases the rate of single base deletions in a run of guanines (20).
The human DINB1-encoded Polκ (previously called Polθ by us) is unable to bypass a cis-syn T-T dimer, a (6-4) T-T photoproduct, or an abasic site (14). However, Polκ can replicate through a guanine-AAF (N-2-acetyl-aminofluorine) adduct, and it may contribute to the bypass of other, as yet unidentified, DNA lesions as well (25). Transient expression of the mouse DINB1 gene in cultured mouse cells leads to an increase in the frequency of base substitution and frameshift mutations (23), which suggests a role for Polκ in the mutagenic replication of undamaged DNA. Thus, in both prokaryotes and eukaryotes, DinB contributes to spontaneous mutagenesis.
Although Polκ would need to be recruited to the stalled replication fork to perform its function in translesion synthesis, or to promote replication through certain sites in undamaged DNA, the manner by which this polymerase gains access to the replication machinery is not known. Here, we examine if interaction with proliferating cell nuclear antigen (PCNA) targets hPolκ to the replication machinery. PCNA, a ring-shaped homotrimeric protein, is loaded onto the primer-template junction by a multiprotein clamp loader, replication factor C (RFC), which couples the hydrolysis of ATP with the opening and closing of the PCNA ring around the DNA. After the loading of PCNA, RFC stays on the DNA via interaction with replication protein A (RPA) bound to single-stranded DNA (ssDNA) (1, 18, 33). The replicative DNA polymerase, Polδ, then assembles with PCNA and carries out processive synthesis of both the leading and lagging DNA strands (26, 28). Here, we provide evidence for the physical interaction of hPolκ with PCNA and show that PCNA, together with RFC and RPA, stimulates the DNA polymerizing activity of hPolκ.
MATERIALS AND METHODS
Proteins.
To obtain glutathione S-transferase (GST)-hPolκ and untagged hPolκ, the hDinB1 cDNA from pBJ733 (14) was cloned in frame with the GST gene (containing a PreScission Protease cleavage recognition sequence) in plasmid pBJ842 (7). hPolκ fused with GST was purified as described previously (14) and was used for the interaction studies described below (see Fig. 1). Untagged hPolκ was purified by treating GST-hPolκ (50 μg) bound to glutathione-Sepharose (Pharmacia) with 2 U of PreScission Protease (Amersham Pharmacia) overnight at 4°C and was used for DNA polymerase assays. Human PCNA, RFC, and RPA were purified as described previously (2, 4, 21). Six-His-tagged human PCNA (Six-His-hPCNA) used for the interaction studies shown in Fig. 1 was overexpressed in E. coli and purified as described previously (19).
FIG. 1.
Physical interaction of hPolκ with PCNA. Human six-His-hPCNA (2 μg) was mixed with GST-hPolκ (2 μg) (lanes 1 through 6). As controls, the same amounts of GST-hPolκ (lanes 7 through 9) or six-His-hPCNA (lanes 10 through 12) were used alone. After incubation, samples were bound to Ni-NTA (lanes 4 through 9) or glutathione-Sepharose (lanes 1 through 3 and 10 through 12) beads, followed by the washing and elution of the bound proteins by imidazole- or glutathione-containing buffer, respectively. Aliquots of each sample before additions of the beads (lanes L), the flowthrough-plus-washing fractions (lanes F), and the eluted proteins (lanes E) were precipitated by trichloroacetic acid and analyzed on a sodium dodecyl sulfate-12% polyacrylamide gel stained with Coomassie blue. The positions of GST-hPolκ and six-His-hPCNA are shown on the left.
Physical interaction of hPolκ with PCNA.
To constitute complexes, GST-hPolκ (2 μg) was mixed with Six-His-hPCNA (2 μg) in buffer I, which contained 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM dithiothreitol (DTT), 0.01% NP-40, and 10% glycerol. The 60-μl samples were incubated for 30 min at 4°C, followed by 10 min at 25°C. To 20 μl of these samples, 10 μl of glutathione-Sepharose or 10 μl of Ni-nitrilotriacetic acid (Ni-NTA) (Qiagen) beads was added to bind GST-hPolκ or six-His-hPCNA and their complexes, respectively, and the samples were further incubated with constant rocking for 30 min at 4°C. The glutathione-Sepharose and Ni-NTA beads were washed 5 times with buffer I, and then bound proteins were eluted with 40 mM glutathione or 500 mM imidazole containing buffer I, respectively. The samples containing the protein mixture before the additions of affinity beads, the flowthrough-plus-washing fractions, and the eluted proteins were precipitated with 5% trichloroacetic acid and separated on a sodium dodecyl sulfate-12% polyacrylamide gel, followed by Coomassie blue R-250 staining.
DNA polymerase assays.
The circular DNA substrate used for DNA synthesis studies (see Fig. 2A) was a 7.2-kb M13mp18 ssDNA primed with a nonlabeled 36-nucleotide (nt)-long oligomer spanning nt 6330 to 6294. For the DNA polymerase processivity studies (see Fig. 2B), the M13 derivative M13mp7L2 ssDNA was primed with the 35-nt-long, 5′ 32P-labeled oligomer primer LP-272 (5′-GGGTTTTCCCAGTCACGACGTTGTAAAACGACGGC-3′). Linear, running-start DNA substrates used for examining the DNA damage bypass ability of hPolκ (see Fig. 3) were generated by annealing a 75-nt oligomer template (5′-biotin-AGCAAGTCACCAATGTCTAAGAGTTCGTAXXATGCCTACACTGGAGTACCGGAGCATCGTCGTGACTGGGAAAAC-biotin-3′) or a 75-nt oligomer, 75AP-2biotin (5′-biotin-AAAAAAAAAAAAAAAAAAAAAAAAAAAGGG0ATGCCTACACTGGAGTACCGGAGCATCGTCGTGACTGGGAAAAC-biotin-3′), which contained one biotin molecule attached at each end and two undamaged T-residues, a cis-syn T-T dimer, or a (6-4) T-T photoproduct at positions 30 and 31 (XX) or an abasic site (a tetrahydrofuran moiety; Midland Company) at position 31 (0), respectively, attached to the 5′ 32P-labeled 26-nt primer LP283 (5′-CGACGATGCTCCGGTACTCCAGTGTA-3′). For steady-state kinetic analysis of nucleotide insertion opposite an undamaged C, G, T, or A or an abasic site template residue (see Fig. 4), the 75AP-2biotin template oligomer was annealed to the 5′ 32P-labeled oligomer primers LP-158 (5′-CGACGATGCTCCGGTACTCCAGTGTAG-3′), N4577 (5′-GTTTTCCCAGTCACGACGATGCTCCGGTA-3′), N4578 (5′-GTTTTCCCAGTCACGACGATGCTCCGGTACTCCAGTGT-3′), N4267 (5′-GTTTTCCCAGTCACGACGATGCTCCGGTACTCCAGTGTAGGCA-3′), or LP-159 (5′-CGACGATGCTCCGGTACTCCAGTGTAGGCAT-3′), respectively. To bind streptavidin to the biotin present at the ends of the linear DNA substrates, these primers-templates (2.5 pmol) were preincubated for 10 min at 30°C with streptavidin (5 μg) in 25 μl of DNA polymerase buffer which contained no MgCl2 before their addition to the DNA polymerase reactions. The standard DNA polymerase reaction buffer (10 μl) contained 40 mM Tris-HCl (pH 7.5), 8 mM MgCl2, 150 mM NaCl, 1 mM DTT, 10% glycerol, 100 μg of bovine serum albumin/ml, 500 μM ATP, and 100 μM (each) dGTP, dATP, dTTP, and dCTP. For the reactions in which circular DNA substrate was primed with nonlabeled oligonucleotide (Fig. 2A), [α-32P]dATP (∼200 to 500 cpm/pmol, final [dATP]) was added. As indicated in the figure legends, hPolκ (1 to 10 ng), PCNA (100 ng), RFC (50 ng), and/or RPA (50 to 250 ng) was incubated with 25 ng of M13 DNA substrate or with linear primer-template DNA (20 nM). Assay mixtures were assembled on ice, incubated at 37°C for 10 min, and stopped by the addition of loading buffer (40 μl) containing EDTA (20 mM), 95% formamide, 0.3% bromphenol blue, and 0.3% cyanole blue. The reaction products were resolved on 10% polyacrylamide gels containing 8 M urea. Quantitation of the results was done with Molecular Dynamics STORM PhosphorImager and ImageQuant software.
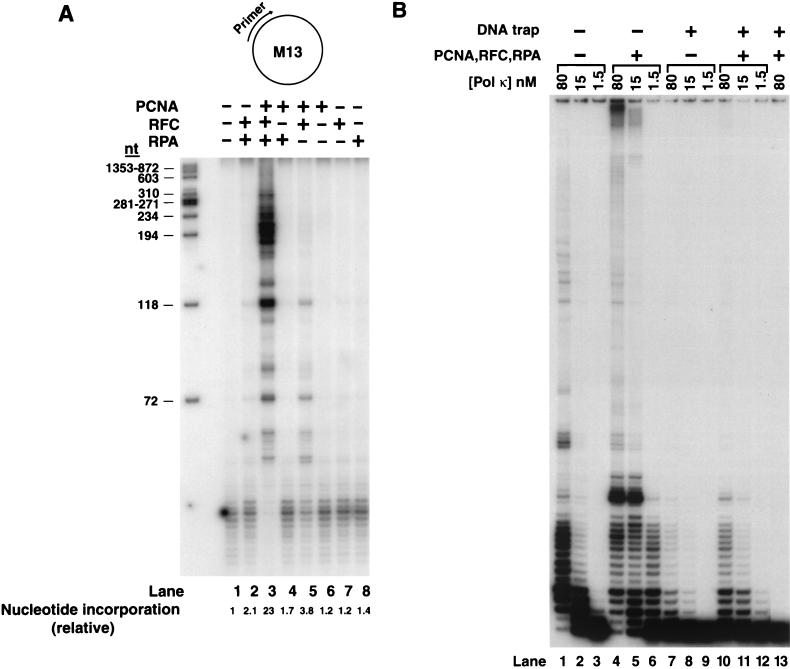
FIG. 2.
PCNA, RFC, and RPA stimulate the DNA polymerase activity of hPolκ. (A) Effect of PCNA, RFC, and RPA and various combinations of these proteins on DNA synthesis by hPolκ. The complete reaction mixture contained hPolκ (10 ng), along with singly primed M13 ssDNA (25 ng), PCNA (100 ng), RFC (50 ng), and RPA (250 ng), and all four deoxynucleotides (100 μM each) containing [α-32P]dATP (lane 3). PCNA, RFC, and RPA and combinations of these proteins were omitted from the reaction mixture as indicated at the tops of the lanes. The amount of DNA synthesis is indicated at the bottom of each lane as the relative nucleotide incorporation. The HaeIII-digested φX174 DNA labeled by polynucleotide kinase is shown on the left as a size standard. (B) Processivity of hPolκ in the presence of PCNA, RFC, and RPA. As indicated at the top, different concentrations of hPolκ (1.5 to 80 nM), alone (lanes 1 through 3 and 7 through 9) or in the presence of PCNA (100 ng), RFC (50 ng), and RPA (250 ng) (lanes 4 through 6 and 10 through 12), were preincubated with circular M13 template ssDNA (5 nM) singly primed with 5′ 32P-labeled oligonucleotide for 5 min at 37°C. Primer extension reactions were initiated by adding all four deoxynucleotides (500 μM each) (lanes 1 through 6) or all four deoxynucleotides and excess sonicated herring sperm DNA (0.5 mg/ml) as a trap (lanes 7 through 12). After 10 min of incubation at 37°C, the samples were quenched and run on a 10% polyacrylamide gel. To demonstrate the effectiveness of the trap, hPolκ plus PCNA, RFC, and RPA were preincubated with excess herring sperm DNA together with the primer-template substrate for 5 min at 37°C before the addition of dNTPs (lane 13).
FIG. 3.
Effects of PCNA, RFC, and RPA on synthesis by hPolκ on a lesion containing DNA substrates. A portion of the DNA substrate containing undamaged T-T residues, a cis-syn T-T dimer, or a (6-4) T-T photoproduct is shown in schematic I. The positions of the two undamaged or damaged T’s are indicated by asterisks. In the abasic site containing DNA substrate shown in schematic II, the abasic site in the template DNA is indicated by a “0” and is marked by an asterisk. The primer was 32P-labeled at its 5′ end, and the template contained biotin-streptavidin complex at both ends. Polκ (2 nM) was incubated with the DNA substrate (20 nM) in the presence of each of four dNTPs under standard reaction conditions. As indicated, the reactions were carried out in the presence or absence of PCNA (100 ng), RFC (50 ng), and RPA (50 ng).
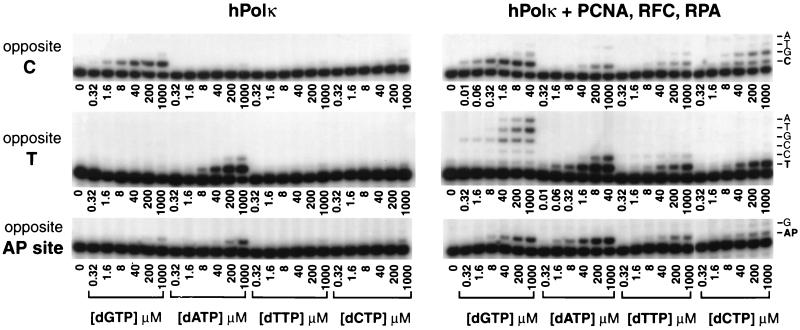
FIG. 4.
Deoxynucleotide incorporation opposite undamaged C and T residues, and opposite an abasic site, by hPolκ in the presence or absence of PCNA, RFC, and RPA. hPolκ (1 nM) was incubated with primer-template DNA substrate (20 nM) and increasing concentrations of a single deoxynucleotide in the absence or presence of PCNA (100 ng), RFC (50 ng), and RPA (50 ng) for 10 min opposite C and T and for 30 min opposite an abasic site at 37°C. The DNA substrates contained biotin-streptavidin complexes at both ends. The nucleotide incorporation rate was plotted against dNTP concentration, and the data were fit to the Michaelis-Menten equation describing a hyperbola. Apparent Km and Vmax values were obtained from the fit and used to calculate the efficiency of deoxynucleotide incorporation (Vmax/Km). AP, abasic.
Processivity assays.
Different concentrations of hPolκ (1.5 to 80 nM) in the presence or absence of PCNA, RFC, and RPA were preincubated for 5 min at 37°C with circular M13 ssDNA substrate primed with a 5′ 32P-labeled oligomer primer (5 nM) in the standard reaction buffer but without the deoxynucleotides. Reactions were initiated by the addition of all four deoxynucleotides (500 μM each) or all four deoxynucleotides plus excess sonicated herring sperm DNA (0.5 mg/ml) used as a trap, followed by 10 min of incubation at 37°C. To demonstrate the effectiveness of the trap, hPolκ was preincubated with the DNA trap and the primer-template substrate before the addition of deoxynucleotide triphosphates (dNTPs).
Determination of steady-state kinetic parameters.
Steady-state kinetic analyses for deoxynucleotide incorporation opposite a nondamaged G, A, T, or C residue or an abasic site were performed as described previously (3, 5). Briefly, hPolκ alone or in the presence of PCNA, RFC, and RPA was incubated with increasing concentrations of a single deoxynucleotide for 10 to 30 min under standard reaction conditions. Gel band intensities of the substrates and products were quantitated with a PhosphorImager, and the percentage of primer extension was plotted as a function of dNTP concentration. The data were fit by nonlinear regression by using SigmaPlot 5.0 to the Michaelis-Menten equation describing a hyperbola, v = (Vmax × [dNTP])/(Km + [dNTP]). Apparent Km and Vmax steady-state parameters were obtained from the fit and used to calculate the efficiency of deoxynucleotide incorporation (Vmax/Km).
RESULTS
Complex formation between hPolκ and PCNA.
To examine whether hPolκ physically interacts with PCNA, GST-hPolκ was incubated with six-His-hPCNA and a pull-down assay was performed using Ni-NTA and glutathione-Sepharose affinity beads (Fig. 1). The Ni-NTA beads have a high specificity for six-His-tagged proteins and bind six-His-hPCNA, whereas the glutathione-Sepharose beads bind GST-hPolκ. Our control experiments demonstrated that Ni-NTA beads do not bind GST-hPolκ (Fig. 1, lanes 7 through 9) and that glutathione-Sepharose beads do not bind six-His-hPCNA (Fig. 1, lanes 10 through 12). Hence, GST-hPolκ and six-His-hPCNA could have been pulled down together only if they had interacted with one another.
We preincubated GST-hPolκ and the six-His-hPCNA homotrimer at a 1:1 molar ratio. When GST-hPolκ was bound to glutathione-Sepharose beads, almost all of the input PCNA was retained on the beads (Fig. 1, lanes 1 through 3), and similarly, when six-His-hPCNA was bound to the Ni-NTA beads, nearly all of the input hPolκ remained bound to PCNA (Fig. 1, lanes 4 through 6). These observations provide evidence for the physical interaction of hPolκ with PCNA, and they indicate that the two proteins are able to form a complex in vitro.
PCNA, RFC, and RPA cooperatively stimulate DNA synthesis by hPolκ.
Next we examined the effect of replication accessory proteins PCNA, RFC, and RPA on the DNA synthesis activity of hPolκ. An unlabeled oligonucleotide primer annealed to a unique position of M13 circular ssDNA was elongated by hPolκ alone or in the presence of either PCNA, RFC, or RPA or different combinations of these proteins (Fig. 2A), and DNA synthesis was monitored by determining the incorporation of radioactive deoxynucleotides. Addition of either PCNA, RFC, or RPA did not significantly enhance the DNA synthesis activity of hPolκ (Fig. 2A, compare lane 1 to lanes 6 through 8). However, a robust (∼23-fold) stimulation of DNA synthesis by hPolκ was observed upon the addition of all three proteins, PCNA, RFC, and RPA (Fig. 2A, lane 3). This enhancement of the DNA synthesis activity of hPolκ requires all three accessory proteins, since in the absence of either PCNA, RFC, or RPA, only a weak stimulation was observed (Fig. 2A, compare lanes 1 through 5). From these results, we concluded that PCNA, RFC, and RPA cooperate to enhance the DNA synthesis activity of hPolκ.
Effect of PCNA on the processivity of hPolκ.
In a search for the mechanism by which PCNA, RFC, and RPA stimulate the DNA synthesis activity of hPolκ, we examined the effect of these accessory proteins on the processivity of hPolκ. Although a low processivity is desirable for a low-fidelity polymerase such as hPolκ (14, 24), as that would limit its activity to synthesizing only short stretches of DNA, thereby preventing high mutation rates, the possibility existed that an increase in processivity was in fact responsible for the stimulation of the DNA synthesis activity of hPolκ. To test whether PCNA, together with RFC and RPA, increased the processivity of hPolκ, we used a circular M13 template ssDNA primed with a 5′ 32P-labeled oligonucleotide primer (Fig. 2B) and we ensured in two different ways that we were observing deoxynucleotide incorporation resulting from a single DNA binding event of hPolκ. First, we decreased hPolκ concentration to a point where only a small fraction of the primer was extended, and second, we monitored DNA synthesis in the presence of an excess of nonradiolabeled, sonicated herring sperm DNA used as a trap. At a low enzyme concentration, hPolκ alone incorporated only 1 to 4 nt (Fig. 2B, lane 3). Although the addition of PCNA, RFC, and RPA greatly stimulated DNA synthesis by hPolκ (Fig. 2B, compare lanes 3 and 6), even in the presence of these accessory proteins, hPolκ incorporated only a few, ∼1 to 18 nt (Fig. 2B, lane 6). In the presence of excess nonlabeled herring sperm DNA, any hPolκ molecules that dissociated from the labeled DNA substrate would be trapped by the excess nonlabeled DNA, thereby preventing the rebinding of the enzyme to the primer-template junction. These reactions were performed by first preincubating hPolκ either alone (Fig. 2B, lanes 7 through 9) or in the presence of PCNA, RFC, and RPA (Fig. 2B, lanes 10 through 12) with the labeled DNA substrate. A mixture of excess herring sperm DNA and all four deoxynucleotides was then added to initiate the reaction. The effectiveness of the trap was verified by preincubating hPolκ with the DNA substrate and the excess herring sperm DNA before the addition of the nucleotides (Fig. 2B, lane 13). The lack of any DNA synthesis in this sample showed that excess herring sperm DNA (∼200-fold) was sufficient to trap all hPolκ molecules. In these reactions, wherein single-hit conditions were provided by excess herring sperm DNA, PCNA, RFC, and RPA did not significantly increase the processivity, since the overall pattern of nucleotide incorporation remained nearly the same in the presence and in the absence of these protein factors (Fig. 2B, compare lanes 7 through 9 and lanes 10 through 12).
Ability of hPolκ to bypass DNA lesions in the presence of PCNA, RFC, and RPA.
Although hPolκ belongs to the family of translesion synthesis DNA polymerases, it is unable to bypass a cis-syn T-T dimer, a (6-4) T-T photoproduct, or an abasic site (14). We next examined whether PCNA, RFC, and RPA promote translesion DNA synthesis by hPolκ through these DNA lesions. For this purpose, we were unable to use short, linear, damage-containing primer-template DNA substrates, probably because PCNA does not remain stably bound on such DNAs and may slide off at the ends. Therefore, we constructed DNA substrates wherein biotin was attached to both ends of the 75-nt oligonucleotide template, and following its annealing to the 5′ 32P-labeled oligonucleotide primer, both of these biotins were coupled to streptavidin, an ∼50-kDa protein (Fig. 3). The presence of streptavidin on both ends of the DNA substrate would inhibit PCNA from falling off after having been loaded onto the template-primer junction by RFC. On such undamaged linear DNA substrate, PCNA, RFC, and RPA stimulated the DNA synthesis activity of hPolκ (Fig. 3, compare lanes 1 and 2). However, even in the presence of these protein factors, hPolκ remained ineffective in bypassing a cis-syn T-T dimer, a (6-4) T-T photoproduct, or an abasic site (Fig. 3). In the presence of PCNA, RFC, and RPA, hPolκ exhibited some nucleotide insertion ability opposite an abasic site (Fig. 3, lane 8), but the presence of a strong stall site just before the lesion indicated an inhibition of insertion across from the abasic site, and the stall site opposite the lesion indicated an inhibition of extension of the 3′ primer end opposite the abasic site (Fig. 3, lane 8). The strong stall sites present 1 or 2 nt before the 3′ T of the cis-syn T-T dimer or the (6-4) T-T photoproduct (Fig. 3, lanes 4 and 6) indicated that PCNA, RFC, and RPA are unable to stimulate hPolκ’s ability to insert nucleotides opposite these DNA lesions.
Kinetic analyses of the efficiency of nucleotide incorporation by hPolκ in the presence of PCNA, RFC, and RPA.
To understand further the mechanism by which PCNA, RFC, and RPA stimulate hPolκ, we examined their effects on the steady-state kinetic parameters Km and Vmax for nucleotide insertion by hPolκ. DNA substrates bearing biotin-streptavidin complex on both ends were generated by annealing the 5′ 32P-labeled oligonucleotide primers to different positions of a linear 75-nt template oligonucleotide. The kinetics of insertion of a single deoxynucleotide opposite an undamaged G, A, T, or C or an abasic site, in a standing start reaction, were determined as a function of the deoxynucleotide concentration under steady-state conditions (Fig. 4). From the kinetics of deoxynucleotide incorporation, the steady-state apparent Km and Vmax values for each deoxynucleotide were obtained from the curve fit to the Michaelis-Menten equation. These Km and Vmax values and the efficiencies of single nucleotide incorporation (Vmax/Km) for hPolκ in the presence or absence of PCNA, RFC, and RPA are summarized in Table 1.
TABLE 1.
Kinetic parameters of nucleotide insertion reactions catalyzed by hPolκ opposite undamaged template residues and opposite an abasic site
| Template residue | Protein(s) | Incoming nucleotide | Km (μM) | Vmax (%/min) | Vmax/Km | fincb |
|---|---|---|---|---|---|---|
| C | Polκ | dGTP | 18.7 ± 1.5 | 3.7 ± 0.12 | 0.19 | ND |
| Polκ + PCNA, RFC, RPA | dGTP | 0.28 ± 0.02 | 3.4 ± 0.05 | 12.1 | 1 | |
| dATP | 344 ± 32 | 1.6 ± 0.2 | 0.0046 | 3.8 × 10−4 | ||
| dTTP | 114 ± 29 | 1.1 ± 0.05 | 0.0096 | 7.9 × 10−4 | ||
| dCTP | 58 ± 7 | 2.1 ± 0.09 | 0.036 | 2.9 × 10−3 | ||
| T | Polκ | dATP | 45 ± 3 | 6.4 ± 0.1 | 0.14 | ND |
| Polκ + PCNA, RFC, RPA | dGTP | 55 ± 14 | 2.3 ± 0.24 | 0.04 | 6.6 × 10−3 | |
| dATP | 0.87 ± 0.05 | 5.6 ± 0.17 | 6.43 | 1 | ||
| dTTP | 147 ± 39 | 2.2 ± 0.13 | 0.015 | 2.3 × 10−3 | ||
| dCTP | 216 ± 42 | 2.7 ± 0.3 | 0.0125 | 1.9 × 10−3 | ||
| A | Polκ | dTTP | 22 ± 12 | 1.9 ± 0.35 | 0.086 | ND |
| Polκ + PCNA, RFC, RPA | dGTP | 225 ± 46 | 2 ± 0.17 | 0.009 | 2.3 × 10−3 | |
| dATP | 342 ± 19 | 2.1 ± 0.23 | 0.006 | 1.5 × 10−3 | ||
| dTTP | 1.2 ± 0.27 | 4.8 ± 0.2 | 4 | 1 | ||
| dCTP | 431 ± 88 | 1.3 ± 0.08 | 0.003 | 7.5 × 10−4 | ||
| G | Polκ | dCTP | 38 ± 4.5 | 1.5 ± 0.1 | 0.039 | ND |
| Polκ + PCNA, RFC, RPA | dGTP | 57 ± 10 | 1.8 ± 0.08 | 0.03 | 3.2 × 10−3 | |
| dATP | 28 ± 9 | 1.7 ± 0.08 | 0.06 | 6.3 × 10−3 | ||
| dTTP | 140 ± 33 | 1.2 ± 0.2 | 0.0085 | 9 × 10−4 | ||
| dCTP | 0.41 ± 0.06 | 3.9 ± 0.08 | 9.5 | 1 | ||
| AP site | Polκ | dATP | 598 ± 249 | 0.35 ± 0.045 | 0.0006 | ND |
| Polκ + PCNA, RFC, RPA | dGTP | 64 ± 8.3 | 0.32 ± 0.08 | 0.005 | ND | |
| dATP | 39 ± 13 | 0.98 ± 0.1 | 0.025 | ND | ||
| dTTP | 248 ± 29 | 0.21 ± 0.05 | 0.0008 | ND | ||
| dCTP | 113 ± 20 | 0.26 ± 0.04 | 0.0023 | ND |
AP, abasic.
finc, fidelity of nucleotide incorporation. ND, not determined.
As indicated by the Vmax/Km values, PCNA, together with RFC and RPA, increased the efficiency of nucleotide incorporation by hPolκ opposite undamaged residues as well as opposite the abasic site. Opposite undamaged template residues and in the presence of PCNA, RFC, and RPA, hPolκ incorporates the correct nucleotide ∼50- to 200-fold more efficiently than it does in the absence of these proteins (Table 1). These increased efficiencies for nucleotide incorporation arise primarily from a marked reduction in the Km for the dNTP substrate, whereas only minor changes were observed in Vmax values. For example, in the presence of PCNA, RFC, and RPA, the ∼60-fold increase in the efficiency of G insertion opposite the C template nucleotide was accompanied by an ∼70-fold decrease in the Km for G, whereas the Vmax stayed about the same (Table 1). Opposite the abasic site, PCNA, RFC, and RPA increased the efficiency of a nucleotide A insertion ∼40-fold; nucleotide A is the nucleotide inserted most often by hPolκ, but the efficiency of this reaction still remained very low.
The incorporation of incorrect nucleotides is also enhanced by PCNA, RFC, and RPA; however, because of the low Vmax values for the insertion of incorrect nucleotides in the absence of these proteins, we did not quantitate this enhancement. Based on the comparison of the Vmax/Km values for the incorporation of correct and incorrect nucleotides, the fidelity of nucleotide insertion of hPolκ in the presence of PCNA, RFC, and RPA is low, ranging from 6.6 × 10−3 to 3.8 × 10−4.
DISCUSSION
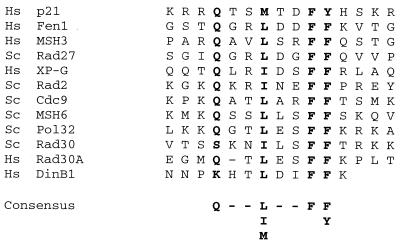
Here, we show that hPolκ physically interacts with PCNA and that PCNA, together with RFC and RPA, stimulates the DNA synthesis activity of hPolκ. Similar evidence for the physical and functional interaction of yeast Polη and hPolη with PCNA that has been reported recently has shown that this interaction is mediated via the conserved PCNA binding motif located at the extreme C terminus of these proteins (7, 8). A similar motif is also present at the C terminus of the human DinB1 protein (Fig. 5), and it may contribute to PCNA binding.
FIG. 5.
Putative PCNA binding motif of human DINB1-encoded Polκ. C-terminal amino acids 859 to 870 of hPolκ are aligned with the PCNA binding motifs in various PCNA binding proteins. The highly conserved residues are shown in bold. Hs, human; Sc, Saccharomyces cerevisiae.
PCNA, along with RFC and RPA, greatly stimulates the processivity of Polδ (26, 28). However, these protein factors do not significantly enhance the processivity of hPolκ. This result is similar to previous observations with yeast Polη and hPolη, where processivity was also not altered in the presence of these accessory proteins (7, 8). The absence of a significant increase in processivity is desirable for low-fidelity enzymes such as Polη and Polκ, for otherwise, there would be a large increase in mutation rates.
As judged from the Vmax/Km values, PCNA, RFC, and RPA stimulate the efficiency of correct nucleotide incorporation by ∼50- to 200-fold, and this increase in efficiency is achieved primarily by a reduction in the apparent Km for the nucleotide. Although the processivity of hPolκ is not increased in the presence of PCNA, RFC, and RPA, it remains possible that the binding of the enzyme to the 3′ primer end is stabilized in the presence of PCNA and that this accounts for the apparent increase in the affinity of the enzyme for the nucleotide. Alternatively, PCNA may contribute to the conformation of the active site, affording a higher affinity of hPolκ for the nucleotide.
Even with PCNA, RFC, and RPA, hPolκ was unable to insert a nucleotide opposite a cis-syn T-T dimer or a (6-4) T-T photoproduct and hence did not bypass these DNA lesions. Also, in the presence of these accessory factors, an abasic site remained a strong block to hPolκ and only a low level of nucleotide insertion occurred across from the lesion. Furthermore, although in the presence of PCNA, RFC, and RPA, steady-state kinetic analyses indicate an ∼40-fold increase in the efficiency of a nucleotide A insertion opposite the abasic site, this reaction still remained very inefficient. Compared to the insertion of an A opposite the template T, hPolκ inserted an A opposite an abasic site more than 250-fold less efficiently. Previously, hPolκ has been reported to bypass an abasic site via a looping-out mechanism, in which it first inserts an A opposite the lesion, and this was followed by the looping out of the lesion in the template strand and the pairing of the A with the T residue present 5′ to the abasic site in the template strand (25). However, even in the presence of PCNA, RFC, and RPA, the highly inefficient incorporation of an A, the nucleotide incorporated most frequently by hPolκ opposite the abasic site, makes this possibility untenable. Other considerations also provide no support for such a role of hPolκ. Genetic studies of yeast have indicated that although A is incorporated most frequently opposite an abasic site, the other nucleotides, C, G, and T, are also incorporated (10). Further, it has been shown previously that the bypass of an abasic site in eukaryotes requires the sequential action of two DNA polymerases, wherein the extension step depends solely upon Polζ but many different polymerases could contribute to the insertion step (10). Because of its proximity to the lesion site and its ability to insert an A opposite an abasic site, Polδ would play a major role in the bypass of this lesion. Rev1 and Polη, however, also could contribute to lesion bypass by promoting the insertion of a C or a G nucleotide, respectively (10). Although on its own, Polη is highly inefficient at inserting a nucleotide opposite an abasic site, the efficiency for nucleotide insertion rises dramatically in the presence of PCNA, RFC, and RPA (8). Thus, whereas yeast Polη incorporates a G opposite an abasic site more than 500-fold less efficiently than it inserts a G opposite an undamaged C template residue, in the presence of PCNA, RFC, and RPA, G is incorporated opposite an abasic site only 20-fold less efficiently than its incorporation opposite a C residue (8).
The interaction of hPolκ with PCNA implies that this polymerase gains access to the stalled replication fork via its binding to PCNA. This targeting mechanism is also used by another eukaryotic translesion synthesis DNA polymerase, Polη (7, 8). However, the mechanism by which Polδ dissociates and a translesion synthesis DNA polymerase gains access to the 3′ primer end remains to be identified.
Acknowledgments
This work was supported by National Institutes of Health grants GM19261 and GM38559.
REFERENCES
- 1.Bambara, R. A., R. S. Murante, and L. A. Henricksen. 1997. Enzymes and reactions at the eukaryotic DNA replication fork. J. Biol. Chem. 272:4647–4650. [DOI] [PubMed] [Google Scholar]
- 2.Cai, J., E. Gibbs, F. Uhlmann, B. Phillips, N. Yano, M. O’Donnell, and J. Hurwitz. 1997. A complex consisting of human replication factor C, p40, p37, and p36 subunits is a DNA-dependent ATPase and an intermediate in the assembly of the holoenzyme. J. Biol. Chem. 272:18974–18981. [DOI] [PubMed] [Google Scholar]
- 3.Creighton, S., L. B. Bloom, and M. F. Goodman. 1995. Gel fidelity assay measuring nucleotide misinsertion, exonucleolytic proofreading, and lesion bypass efficiencies. Methods Enzymol. 262:232–256. [DOI] [PubMed] [Google Scholar]
- 4.Gibbs, E., Z. Kelman, J. M. Gulbis, M. O’Donnell, J. Kuriyan, P. M. Burgers, and J. Hurwitz. 1997. The influence of the proliferating cell nuclear antigen-interacting domain of p21 (CIP1) on DNA synthesis catalyzed by the human and Saccharomyces cerevisiae polymerase delta holoenzymes. J. Biol. Chem. 272:2373–2381. [DOI] [PubMed] [Google Scholar]
- 5.Goodman, M. F., S. Creighton, L. B. Bloom, and J. Petruska. 1993. Biochemical basis of DNA replication fidelity. Crit. Rev. Biochem. Mol. Biol. 28:83–126. [DOI] [PubMed] [Google Scholar]
- 6.Goodman, M. F., and B. Tippin. 2000. Sloppier copier DNA polymerases involved in genome repair. Curr. Opin. Genet. Dev. 10:162–168. [DOI] [PubMed] [Google Scholar]
- 7.Haracska, L., R. E. Johnson, I. Unk, B. Phillips, J. Hurwitz, L. Prakash, and S. Prakash. 2001. Physical and functional interactions of human DNA polymerase η with PCNA. Mol. Cell. Biol. 21:7199–7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haracska, L., C. M. Kondratick, I. Unk, S. Prakash, and L. Prakash. 2001. Interaction with PCNA is essential for yeast DNA polymerase η function. Mol. Cell 8:407–415. [DOI] [PubMed] [Google Scholar]
- 9.Haracska, L., S. Prakash, and L. Prakash. 2000. Replication past O6-methylguanine by yeast and human DNA polymerase η. Mol. Cell. Biol. 20:8001–8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haracska, L., I. Unk, R. E. Johnson, E. Johansson, P. M. J. Burgers, S. Prakash, and L. Prakash. 2001. Roles of yeast DNA polymerases δ and ζ and of Rev1 in the bypass of abasic sites. Genes Dev. 15:945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haracska, L., S.-L. Yu, R. E. Johnson, L. Prakash, and S. Prakash. 2000. Efficient and accurate replication in the presence of 7,8-dihydro-8-oxoguanine by DNA polymerase η. Nat. Genet. 25:458–461. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, R. E., C. M. Kondratick, S. Prakash, and L. Prakash. 1999. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science 285:263–265. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, R. E., S. Prakash, and L. Prakash. 1999. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Polη. Science 283:1001–1004. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, R. E., S. Prakash, and L. Prakash. 2000. The human DINB1 gene encodes the DNA polymerase Polθ. Proc. Natl. Acad. Sci. USA 97:3838–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson, R. E., M. T. Washington, L. Haracska, S. Prakash, and L. Prakash. 2000. Eukaryotic polymerases ι and ζ act sequentially to bypass DNA lesions. Nature 406:1015–1019. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, R. E., M. T. Washington, S. Prakash, and L. Prakash. 1999. Bridging the gap: a family of novel DNA polymerases that replicate faulty DNA. Proc. Natl. Acad. Sci. USA 96:12224–12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, R. E., M. T. Washington, S. Prakash, and L. Prakash. 2000. Fidelity of human DNA polymerase η. J. Biol. Chem. 275:7447–7450. [DOI] [PubMed] [Google Scholar]
- 18.Kelman, Z., and J. Hurwitz. 1998. Protein-PCNA interactions: a DNA-scanning mechanism? Trends Biol. Sci. 23:236–238. [DOI] [PubMed] [Google Scholar]
- 19.Kelman, Z., N. Yao, and M. O’Donnell. 1995. Escherichia coli expression vectors containing a protein kinase recognition motif, His6-tag and hemagglutinin epitope. Gene 166:177–178. [DOI] [PubMed] [Google Scholar]
- 20.Kim, S.-R., G. Maenhaut-Michel, M. Yamada, Y. Yamamoto, K. Matsui, T. Sofuni, T. Nohmi, and H. Ohmori. 1997. Multiple pathways for SOS-induced mutagenesis in Escherichia coli: an overexpression of dinB/dinP results in strongly enhancing mutagenesis in the absence of any exogenous treatment to damage DNA. Proc. Natl. Acad. Sci. USA 94:13792–13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, S. H., T. Eki, and J. Hurwitz. 1989. Synthesis of DNA containing the simian virus 40 origin of replication by the combined action of DNA polymerases alpha and delta. Proc. Natl. Acad. Sci. USA 86:7361–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masutani, C., R. Kusumoto, A. Yamada, N. Dohmae, M. Yokoi, M. Yuasa, M. Araki, S. Iwai, K. Takio, and F. Hanaoka. 1999. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature 399:700–704. [DOI] [PubMed] [Google Scholar]
- 23.Ogi, T., T. Kato, Jr., T. Kato, and H. Ohmori. 1999. Mutation enhancement by DINB1, a mammalian homologue of the Escherichia coli mutagenesis protein DinB. Genes Cells 4:607–618. [DOI] [PubMed] [Google Scholar]
- 24.Ohashi, E., K. Bebenek, T. Matsuda, W. J. Feaver, V. L. Gerlach, E. C. Friedberg, H. Ohmori, and T. A. Kunkel. 2000. Fidelity and processivity of DNA synthesis by DNA polymerase κ, the product of the human DINB1 gene. J. Biol. Chem. 275:39678–39684. [DOI] [PubMed] [Google Scholar]
- 25.Ohashi, E., T. Ogi, R. Kusumoto, S. Iwai, C. Masutani, F. Hanaoka, and H. Ohmori. 2000. Error-prone bypass of certain DNA lesions by the human DNA polymerase κ. Genes Dev. 14:1589–1594. [PMC free article] [PubMed] [Google Scholar]
- 26.Prelich, G., M. Kostura, D. R. Marshak, M. B. Matthews, and B. Stillman. 1987. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature 326:471–475. [DOI] [PubMed] [Google Scholar]
- 27.Strauss, B. S., R. Roberts, L. Francis, and P. Pouryazdanparast. 2000. Role of the dinB gene product in spontaneous mutation in Escherichia coli with an impaired replicative polymerase. J. Bacteriol. 182:6742–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan, C. K., C. Castillo, A. G. So, and K. M. Downey. 1986. An auxiliary protein for DNA polymerase δ from fetal calf thymus. J. Biol. Chem. 261:12310–12316. [PubMed] [Google Scholar]
- 29.Tang, M., P. Pham, X. Shen, J.-S. Taylor, M. O’Donnell, R. Woodgate, and M. F. Goodman. 2000. Roles of E. coli DNA polymerase IV and V in lesion-targeted and untargeted SOS mutagenesis. Nature 404:1014–1018. [DOI] [PubMed] [Google Scholar]
- 30.Tissier, A., J. P. McDonald, E. G. Frank, and R. Woodgate. 2000. Polι, a remarkably error-prone human DNA polymerase. Genes Dev. 14:1642–1650. [PMC free article] [PubMed] [Google Scholar]
- 31.Washington, M. T., R. E. Johnson, S. Prakash, and L. Prakash. 2000. Accuracy of thymine-thymine dimer bypass by Saccharomyces cerevisiae DNA polymerase η. Proc. Natl. Acad. Sci. USA 97:3094–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu, S.-L., R. E. Johnson, S. Prakash, and L. Prakash. 2001. Requirement of DNA polymerase η for error-free bypass of UV-induced CC and TC photoproducts. Mol. Cell. Biol. 21:185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuzhakov, A., Z. Kelman, J. Hurwitz, and M. O’Donnell. 1999. Multiple competition reactions for RPA order the assembly of the DNA polymerase δ holoenzyme. EMBO J. 18:6189–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]