Abstract
Raffinose family oligosaccharides (RFOs) are synthesized by a set of galactosyltransferases, which sequentially add galactose units from galactinol to sucrose. The accumulation of RFOs was studied in maturing seeds of two pea (Pisum sativum) lines with contrasting RFO composition. Seeds of the line SD1 accumulated stachyose as the predominant RFO, whereas verbascose, the next higher homolog of stachyose, was almost absent. In seeds of the line RRRbRb, a high level of verbascose was accumulated alongside with stachyose. The increase in verbascose in developing RRRbRb seeds was associated with galactinol-dependent verbascose synthase activity. In addition, a galactinol-independent enzyme activity was detected, which catalyzed transfer of a galactose residue from one stachyose molecule to another. The two enzyme activities synthesizing verbascose showed an optimum at pH 7.0. Both activities were almost undetectable in SD1. Maximum activity of stachyose synthase was about 4-fold higher in RRRbRb compared with SD1, whereas the activities of galactinol synthase and raffinose synthase were only about 1.5-fold higher in RRRbRb. The levels of galactinol synthase and stachyose synthase activity were reflected by steady-state levels of corresponding mRNAs. We suggest that the accumulation of verbascose in RRRbRb was controlled by a coordinated up-regulation of the last steps of verbascose biosynthesis.
Many higher plants accumulate raffinose family oligosaccharides (RFOs) during seed maturation. These carbohydrates consist of Gal units linked to Suc via α-(1→6) glycosidic linkages. RFOs have been proposed to act as protective agents during desiccation and storage of seeds in the dry state (for review, see Horbowicz and Obendorf, 1994; Obendorf, 1997), although there is no evidence for a causal relationship between their accumulation and the acquisition of desiccation tolerance (Black et al., 1999; Bentsink et al., 2000; Buitink et al., 2000). Although RFOs have long been regarded as antinutritional factors in human nutrition, recent data support a beneficial role of RFOs as so-called prebiotics, by specifically stimulating growth of remedial gut bacteria (Voragen, 1998; Aranda et al., 2000).
A set of galactosyltransferases is involved in the biosynthesis of RFOs (for review, see Peterbauer and Richter, 2001). Galactinol synthase (EC 2.4.1.123) catalyzes the synthesis of galactinol (O-α-d-gal-actopyranosyl-[1→1]-l-myo-inositol) from UDP-d-Gal and myo-inositol (Liu et al., 1998; Sprenger and Keller, 2000). Raffinose and stachyose are then synthesized by addition of Gal units from galactinol to Suc and raffinose, respectively. These reversible reactions are mediated by raffinose synthase (EC 2.4.1.82; Lehle and Tanner, 1973) and stachyose synthase (EC 2.4.1.67; Peterbauer and Richter, 1998; Peterbauer et al., 1999). Transfer of a further Gal residue from galactinol to stachyose gives verbascose. This reaction is probably catalyzed by a bifunctional stachyose synthase or by a very similar verbascose synthase (Tanner et al., 1967; Kandler and Hopf, 1982). In addition to the galactinol-dependent pathway, a galactinol-independent enzyme has been identified in leaves of Ajuga reptans, which catalyzes the transfer of the terminal Gal unit from one RFO molecule to another (Bachmann et al., 1994). For example, it is able to form verbascose and raffinose when incubated with stachyose. By analogy with fructosyltransferases of the fructan biosynthetic pathway, the enzyme has been termed galactan:galactan galactosyltransferase (GGT). Unlike the other enzymes of RFO biosynthesis, GGT shows an acidic pH optimum and is located exclusively in the vacuole (Bachmann and Keller, 1995; Braun and Keller, 2000). GGT activity has also been detected in leaves of Coleus blumei (Gilbert et al., 1997), but has not yet been found in seed tissues.
The metabolic regulation of RFO accumulation during seed development is poorly understood. RFOs appear to be synthesized within the seeds, even in plant species that translocate RFOs in the phloem (Kandler and Hopf, 1982; Handley et al., 1983a). Considerable genetic variation for the content of RFOs of mature seeds has been reported, in particular for that of stachyose and verbascose (Frias et al., 1994, 1999; Pattee et al., 2000). In seeds of various pea (Pisum sativum) lines, the content of stachyose ranged from 0.7% to 4.1% of dry matter, whereas verbascose ranged from undetectable levels to 3.1% (Jones et al., 1999). Recently, a major quantitative trait locus affecting the content of raffinose and stachyose in seeds of Arabidopsis has been identified (Bentsink et al., 2000). The corresponding genomic region contained, among others, genes putatively encoding for a galactinol synthase and for a raffinose synthase, but the locus has not been fine-mapped.
In a number of previous studies, differences in the total amount of RFOs deposited during seed development have been related to variation in the level of galactinol synthase activity (Handley et al., 1983b; Saravitz et al., 1987; Lowell and Kuo, 1989). Based on these and other correlative data, it has been proposed that galactinol synthase is the key enzyme that regulates partitioning of carbon into the RFO pool (Keller and Pharr, 1996). In this study, we extended the characterization of the pathway to include all known enzymes involved in RFO biosynthesis. Expression of corresponding genes and changes in the carbohydrate composition of developing seeds were studied in two pea lines with contrasting RFO content and composition. The two lines, RRRbRb and SD1, were selected based on previously published data on their seed carbohydrate composition (Jones et al., 1999; Górecki et al., 2000). We demonstrate that seeds of the high-verbascose line RRRbRb line contain the complete set of galactinol-dependent enzymes, including verbascose synthase activity, but are devoid of acidic GGT activity. However, we describe a galactinol-independent verbascose synthase activity operating at neutral pH values. In the low-verbascose pea line SD1, both pathways for verbascose synthesis were blocked.
RESULTS
Seed Growth and Changes in Soluble Carbohydrates
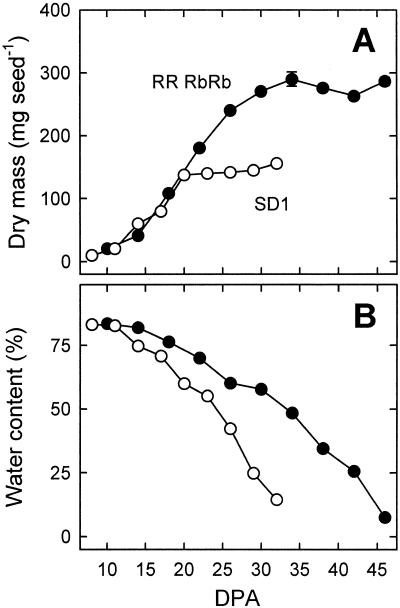
Under the conditions used in this study, the initial growth rates of RRRbRb and SD1 seeds were similar (Fig. 1A). Maximum dry mass was observed at 20 d post-anthesis (DPA) in SD1 and at 34 DPA in RRRbRb, respectively. The longer seed fill duration of RRRbRb seeds resulted in about 2-fold higher dry mass at maturity compared with seeds of the SD1 line. Water content declined faster in SD1 than in RRRbRb (Fig. 1B).
Figure 1.
Changes in dry mass (A) and water content (B) during maturation of pea seeds of RRRbRb (black symbols) and SD1 (white symbols) lines. Symbols represent the means ± se of three to six replications. Error bars are contained within the symbol when not shown.
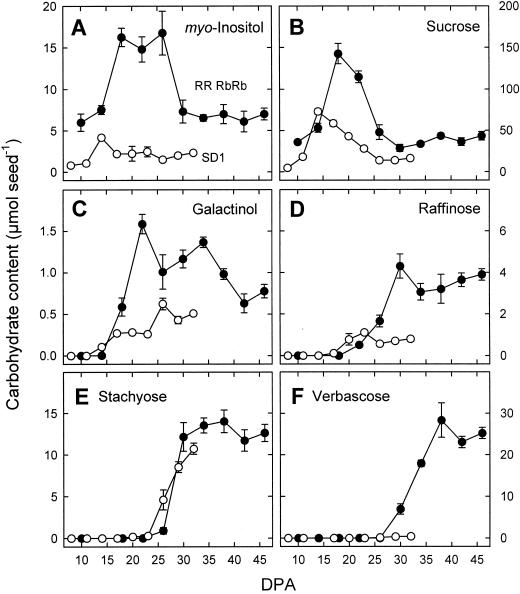
myo-inositol and Suc were present throughout seed development (Fig. 2, A and B). At almost all developmental stages, both compounds showed higher levels in RRRbRb seeds as compared with SD1 seeds. Changes in the level of myo-inositol were small in SD1, whereas a transient, 2-fold increase was observed in RRRbRb (14–30 DPA). Galactinol and RFOs were absent during early stages of seed development (Fig. 2, C–F). Galactinol appeared at 14 DPA in SD1 and at 18 DPA in RRRbRb (Fig. 2C). Raffinose was detected 3 d later in SD1 seeds and 5 d later in RRRbRb seeds (Fig. 2D). The levels of galactinol and raffinose were two to five times higher in RRRbRb compared with SD1. Starting at 23 DPA in SD1 and at approximately 26 DPA in RRRbRb, stachyose accumulated rapidly (Fig. 2E). The final stachyose content was very similar in the two lines. From 26 to 38 DPA, verbascose accumulated almost linearily in RRRbRb, but not in SD1 (Fig. 2F). At maturity, RRRbRb seeds contained 25.2 μmol verbascose seed−1, whereas only 0.4 μmol seed−1 was detected in SD1. The majority of RFOs (mainly stachyose in SD1 and verbascose in RRRbRb) were accumulated after maximum dry mass had been reached.
Figure 2.
Contents of soluble carbohydrates in maturing pea seeds of RRRbRb (black symbols) and SD1 (white symbols) lines as determined by capillary gas chromatography. Values are means ± se of three independent replications.
Activity and Expression of Enzymes of the RFO Pathway
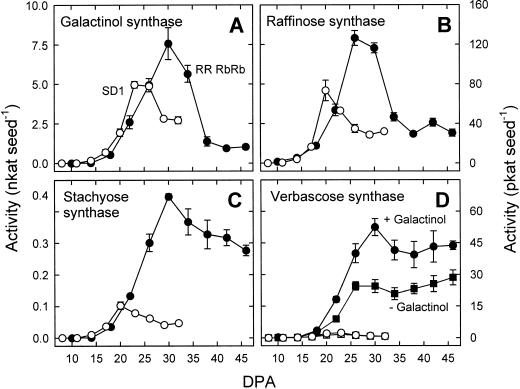
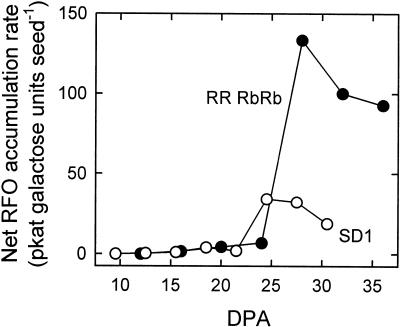
Activity of all enzymes of the RFO biosynthetic pathway was near zero in SD1 seeds younger than 14 DPA and in RRRbRb seeds younger than 18 DPA (Fig. 3). Galactinol synthase activity increased rapidly in both lines, reaching 5.0 nkat seed−1 at 23 DPA in SD1 and 7.6 nkat seed−1 at 30 DPA in RRRbRb. Activity subsequently fell to a plateau in both lines. To compare the levels of galactinol synthase activity with changes in RFO levels, rates of RFO accumulation were calculated by dividing the increase in the total amount of Gal units in galactinol and RFOs between two consecutive harvests by the interval of time between the harvests (Fig. 4). The highest value obtained was 34.2 pkat seed−1 (23–26 DPA) in SD1 and 133.4 pkat seed−1 (26–30 DPA) in RRRbRb. It is important to note that the data in Figure 4 can only be used to compare the rate of RFO accumulation with galactinol synthase activity. Maximum increase in raffinose units, for example, was considerably lower (16.4 pkat seed−1 in SD1 and 60.3 pkat seed−1 in RRRbRb) because stachyose and verbascose contain two and three Gal units, respectively, but only one raffinose skeleton (data not shown).
Figure 3.
In vitro enzyme activities in maturing pea seeds of RRRbRb (black symbols) and SD1 (white symbols) lines. All assays were performed at pH 7.0. Verbascose synthase activity was assayed in the presence (circles) or in the absence (squares) of galactinol. Details regarding assay conditions are given in “Materials and Methods.” Values are means ± se of three independent replications.
Figure 4.
Estimation of the net rate of RFO accumulation during development of pea seeds of RRRbRb (black symbols) and SD1 (white symbols) lines. Rates represent the increase in total Gal units in galactinol and RFOs (in picomoles) between two consecutive harvests (Fig. 2) divided by the time (in seconds) between the harvests. Data represent the means of three independent replications.
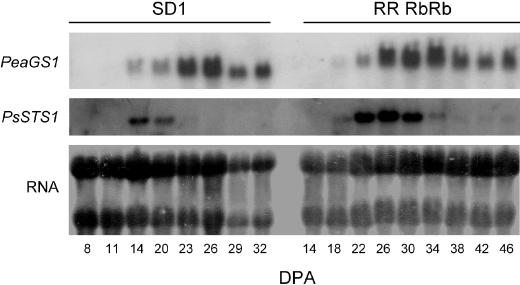
Steady-state transcript levels for galactinol synthase were analyzed by probing an RNA gel blot with a fragment of PeaGS1 encoding for pea galactinol synthase (Fig. 5). A hybridization signal of the expected size (about 1.5 kb) appeared at 14 DPA in SD1 and at 18 DPA in RRRbRb. Subsequent changes in mRNA abundance coincided with changes in galactinol synthase activity, with a maximum about one-half of the way through seed development and reduced levels in mature seeds. The signal intensity was almost identical in the two lines.
Figure 5.
Northern-blot analysis of steady-state levels of transcripts encoding galactinol synthase (PeaGS1, GenBank accession no. AJ243815) and stachyose synthase (PsSTS1, GenBank accession no. AJ311087) in maturing pea seeds of SD1 and RRRbRb lines. Each lane contained total RNA (10 μg) from seeds harvested at the indicated DPA. For a loading control, total RNA was stained with methylene blue.
Raffinose synthase activity showed a very similar pattern, but peaked slightly earlier than galactinol synthase activity in RRRbRb and SD1 (Fig. 3B). Maximum raffinose synthase activity was 73.4 pkat seed−1 in SD1 (20 DPA) and 126.5 pkat seed−1 in RRRbRb (26 DPA), respectively. The corresponding gene has not yet been cloned.
In contrast to raffinose synthase, maximum stachyose synthase activity coincided with galactinol synthase activity (Fig. 3C). The highest values of stachyose synthase activity were 102.5 pkat seed−1 in SD1 (20 DPA) in SD1 and 446.3 pkat seed−1 in RRRbRb (30 DPA). It is surprising that stachyose synthase activity peaked in SD1 before substantial amounts of stachyose had been accumulated. The increased levels of activity in RRRbRb compared with SD1 were associated with higher levels of mRNA for stachyose synthase (Fig. 5). However, in both lines, the highest mRNA levels were detected a few days prior to maximum activity. Although stachyose synthase activity decreased moderately toward maturity in both lines, almost no transcripts were detected in RNA from seeds harvested during late maturation stages.
Enzyme Activities Synthesizing Verbascose and Hydrolytic Activities
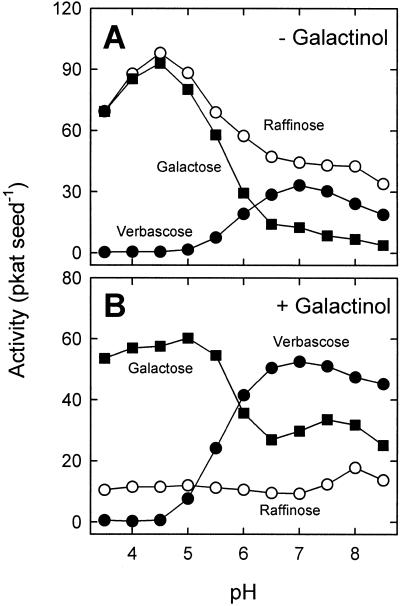
In an initial attempt to characterize the biosynthesis of verbascose in pea seeds, extracts from mature RRRbRb seeds were tested for verbascose synthase and for GGT activity. Only traces of GGT activity were detected when assayed at pH 5.0 with stachyose, whereas substantial activity of verbascose synthase was found at pH 7.0 (data not shown). These observations initially suggested that only the galactinol-dependent pathway is operative in pea seeds. However, we recognized that verbascose was not only produced in the presence of galactinol. Considerable amounts of verbascose were also formed in control assays without galactinol. The activity was specific for stachyose as substrate. No chain elongation was detected when extracts were incubated with raffinose. The novel activity, as well as the galactinol-dependent verbascose synthase activity, showed an optimum at pH 7.0 (Fig. 6). In the absence of galactinol, raffinose, the coproduct arising by transfer between two stachyose molecules, was formed at a higher rate than verbascose (Fig. 6A). The additional amount of raffinose produced (corresponding to 12.6 pkat seed−1 at pH 7.0) can be explained by the presence of hydrolytic α-galactosidase activity in the desalted extracts. The highest hydrolytic activity (98.1 pkat seed−1) was detected at pH 4.5. In the presence of galactinol, the rate of verbascose formation from stachyose was increased by 58.2%, whereas the rate of the raffinose formation was reduced by 88.3% at pH 4.5 and by 79.0% at pH 7.0 (Fig. 6B). These results indicate that the hydrolytic activity toward stachyose, as well as the galactinol-independent activity synthesizing verbascose, was inhibited by galactinol. At pH 4.5, the rate of Gal formation from stachyose and galactinol was reduced by 62.0% compared with that from stachyose alone, suggesting that galactinol itself was a poor substrate for α-galactosidase. A second, lower maximum (33.5 pkat seed−1) was observed at pH 7.5. This maximum was probably caused by raffinose synthase and stachyose synthase, which display some hydrolytic activity toward galactinol in the absence of their acceptors (Lehle and Tanner, 1973; Holthaus and Schmitz, 1991).
Figure 6.
Effect of pH on the rate of formation of verbascose (●), raffinose (○) and Gal (▪) in reaction mixtures containing 20 mm stachyose and desalted protein extracted from mature RRRbRb seeds. Reactions were performed in the absence (A) and in the presence (B) of 10 mm galactinol.
During seed development, galactinol-dependent verbascose synthase activity was initially detected at 18 DPA in RRRbRb (Fig. 3D). Activity increased to 59.7 pkat seed−1 at 30 DPA and remained fairly high during later stages. The galactinol-independent activity showed a very similar pattern. In contrast, both activities were hardly detectable in seeds of the SD1 line, with maximum values of 2.4 (galactinol-dependent activity) and 1.6 pkat seed−1 (galactinol-independent activity).
DISCUSSION
In this study, we attempted to analyze the mechanisms that control RFO accumulation in pea seeds. Throughout seed development, changes in galactinol synthase activity followed changes in the level of transcripts (Figs. 3A and 5). Likewise, higher stachyose synthase activity in RRRbRb as compared with SD1 was associated with higher mRNA abundance (Figs. 3C and 5). Thus, the biosynthesis of RFOs appears to be mainly regulated at the level of transcription. Nonetheless, additional post-transcriptional regulatory processes were apparent. Toward maturation, mRNA levels for stachyose synthase were not correlated with enzymatic activity. The latter observations are similar to changes in mRNA and protein levels of stachyose synthase in adzuki bean (Vigna angularis) seeds, which suggested that degradation of newly synthesized protein is at least in part delayed until germination (Peterbauer et al., 1999).
As expected, accumulation of verbascose was associated with higher activities synthesizing verbascose in RRRbRb as compared with the low-verbascose line SD1 (Fig. 3D), whereas the differences in galactinol synthase activity were rather small. Moreover, galactinol synthase activity as determined under the conditions used was more than 50-fold higher than the minimum activity required to explain the observed accumulation of RFOs in RRRbRb and almost 150-fold higher in SD1. These observations do not support the proposed key role of galactinol synthase in channeling flux into the pathway (Handley et al., 1983b; Saravitz et al., 1987). To explain these findings, it should be considered that the accumulation of RFOs is not only governed by the kinetics of the involved reactions, but also by thermodynamic constraints. There is little doubt that galactinol synthase and the galactinol-dependent galactosyltransferases colocalize with their metabolites in the cytosol (Keller and Matile, 1985; Keller, 1992; Bachmann and Keller, 1995). Because the entire RFO pathway represents a sequence of reversible reactions (Haritatos et al., 1996), all enzymes will more or less approach equilibrium once products accumulate, depending on individual mass action ratios and equilibrium constants. Since all Gal units in RFOs are provided by galactinol, each chain-elongating reaction ultimately consumes galactinol and produces myo-inositol. Hence, the operation of additional galactosyltransferases in RRRbRb could positively affect the mass-action ratio for galactinol synthesis. Thereby, the net rate of RFO accumulation could be increased and a higher final concentration of RFOs could be reached without a corresponding up-regulation of galactinol synthase activity. In addition, the synthesis of galactinol in RRRbRb was probably supported by a higher myo-inositol concentration, in particular during the initial phase of galactinol accumulation (Fig. 2A). The latter observation is in agreement with dramatic effects of lowered myo-inositol concentration on RFO metabolism in transgenic potato (Solanum tuberosum) tubers (Keller et al., 1998). Taken together, these considerations clearly indicate that the mere fact that galactinol synthase catalyzes the first step in the pathway does not justify the assumption that it exerts a higher degree of control than other enzymes.
It might be argued that thermodynamically unfavorable conditions for RFO synthesis are prevented by transport of end products such as stachyose and verbascose into vacuolar compartments. Although high gradients in RFO concentration are maintained across the tonoplast in vacuoles of leaves and tubers (Keller and Matile, 1985; Bachmann and Keller, 1995), there is evidence that the concentration of RFOs in the specialized protein storage vacuoles of seeds is not higher than in the cytoplasm (Muller and Jacks, 1983; Plant and Moore, 1983). Therefore, transport of RFOs into protein storage vacuoles may be important (Tanner et al., 1968), but is certainly not able to eliminate the inhibitory interactions in the cytoplasm outlined above. However, hydrolytic α-galactosidases colocalize with RFOs in protein storage vacuoles (Dey, 1984; Herman and Shannon, 1985). As a consequence, it has been speculated that RFO accumulation is regulated by synthesis and simultaneous hydrolysis (Keller and Pharr, 1996; Kuo et al., 1997). Although we cannot exclude that futile cycling of RFOs occurs, α-galactosidase activity as determined with stachyose and galactinol as substrates (Fig. 6) was considerably lower than previously reported values, which were acquired with artificial substrates (Lowell and Kuo, 1989; Hendrix, 1990; Kuo et al., 1997). Moreover, maximum α-galactosidase activity was found at pH 4.5, whereas the pH in the lumen of protein storage vacuoles may be as high as pH 6.7 (Swanson et al., 1998). At pH 7.0, α-galactosidase activity toward stachyose was very low compared with stachyose synthase activity. Hydrolytic activity toward galactinol was considerably higher at neutral pH values, but this amount of activity represented only 1.1% of galactinol synthase activity. Taken together, these results indicate that hydrolytic activities may not necessitate the levels of the anabolic enzymes observed in pea seeds.
Consistent with the assumption that the pH in protein storage vacuoles is high, no acidic GGT activity, which would be inactive under these conditions, was detected in RRRbRb seeds. Instead, we found a similar activity operating at neutral pH values (Fig. 6A). Previously described procedures to assay the synthesis of verbascose in vitro relied on the use of [Gal-14C]galactinol (Tanner et al., 1967; Kandler and Hopf, 1982) and, hence, failed to detect such a galactinol-independent activity. Unlike the acidic leaf enzyme, the galactinol-independent activity from pea seeds was inactive on raffinose. Thus, in a strict sense, it is not a GGT that acts on all RFOs (Bachmann et al., 1994). Expression of the activity appeared to be regulated in a coordinated manner with the galactinol-dependent verbascose synthase, which is also active in pea seeds (Fig. 3D). The latter activity, in turn, has been suggested to be catalyzed by a bifunctional stachyose synthase in broad bean (Vicia faba), since both activities copurified in the course of a 55-fold enrichment (Tanner et al., 1967). In support of this view, it has also been demonstrated that verbascose effectively inhibits stachyose synthase activity. However, this inhibitory effect also suggests that feedback regulation of verbascose accumulation by inhibition of stachyose synthase could occur. It is interesting to note that an increased level of stachyose synthase activity was observed in RRRbRb (Fig. 3C). If verbascose is synthesized by stachyose synthase in pea seeds, up-regulation of stachyose synthase could probably counterbalance such an inhibitory effect of verbascose.
In summary, our results demonstrate that the biosynthesis of verbascose in pea seeds is more complex than previously anticipated. We were able to show that a galactinol-dependent as well as a galactinol-independent pathway is operative. The latter is catalyzed by a galactosyltransferase activity acting on stachyose. In contrast to the corresponding acidic activity from leaves of A. reptans (Bachmann et al., 1994), activity was highest at pH 7.0. The increased demand for Gal units for the accumulation of verbascose in RRRbRb was not associated with a corresponding up-regulation of galactinol synthase. Therefore, we suggest that the additional Gal units for verbascose accumulation in RRRbRb were pulled into the pathway by the enzyme systems catalyzing the synthesis of higher RFOs rather than being pushed into it by galactinol synthase. Our model may explain the high degree of variation for the content and composition of RFOs better than the previous model emphasizing the role of galactinol synthase as a key enzyme. However, it is evident that the concentration of the initial substrates such as myo-inositol or Suc and feedback loops within the pathway can affect the final content of RFOs deposited in mature seeds. A more detailed analysis of flux control of the pathway will require sets of transgenic plants with suppressed or overexpressed activity of individual enzymes.
MATERIALS AND METHODS
Plant Material and Chemicals
Pea (Pisum sativum) plants (BC3 near-isoline RRRbRb and line SD1; Jones et al., 1999) were grown in 15-cm pots (two plants per pot) containing a mixture of peat:soil:perlite (3:2:1, v/v) in a greenhouse with a 14-h/10-h light/dark cycle at approximately 21°C during the light and at approximately 17°C in the dark. Plants were illuminated by fluorescent lamps producing 190 μmol m−2 s−1 photosynthetic photon flux density. The plants were watered daily and fertilized weekly with NPK fertilizer (3:1:1; 1 g pot−1). Flowers at the first node were tagged when fully opened. Seeds were exclusively collected from pods at the first node at intervals of 3 (SD1) or 4 d (RRRbRb). Water content was determined after drying at 100°C to constant weight. For the extraction of soluble protein and RNA, respectively, seeds were frozen in liquid nitrogen and stored at −70°C.
Galactinol was purified from a leaf sample of sage (Salvia officinalis) and Melissa officinalis as previously described (Peterbauer, 1998). myo-inositol and raffinose were from Sigma (Vienna). Suc, stachyose, and UDP-d-Gal were from Fluka (Vienna). Verbascose was from Megazyme (Wicklow, Ireland).
Analysis of Soluble Carbohydrates
Freshly harvested seeds were homogenized with mortar and pestle in ethanol:water (1:1, v/v) containing 300 μg of phenyl α-d-glucopyranoside as internal standard. The homogenate was heated at 90°C for 30 min and was centrifuged at 20,000g for 20 min. Aliquots (0.4 mL) of clear supernatant were passed through a 10,000-Mr cut-off filter and were evaporated to dryness under a stream of nitrogen. To remove traces of water, residues were stored overnight over phosphorus pentoxide in a desiccator, and were derivatized with a mixture of trimethylsilyl imidazole:pyridine (1:1, v/v). Trimethylsilyl-derivatives of soluble carbohydrates were analyzed by capillary gas chromatography (Górecki et al., 1997). The gas chromatograph (GC-14A, Shimadzu, Kyoto) was equipped with a DB-1 capillary column (15 m × 0.25 mm × 0.1 μm, J & W Scientific, Folsom, CA) and a flame-ionization detector. Helium was used as a carrier gas with a linear velocity of 35 cm s−1. The column was operated with an initial temperature of 150°C, adjusted to 160°C at 3°C min−1, held at 160°C for 3 min, and adjusted to 175°C, 225°C, 245°C, and 325°C at 3°C, 7°C, 4°C, and 7°C min−1, respectively. The final temperature was held for 15 min. The injector port was operated in the split mode (1:50) at 335°C, and the detector was maintained at 350°C.
Enzyme and Protein Assay
Frozen seeds were finely ground in liquid nitrogen using mortar and pestle. Approximately 200 mg of the powder was suspended in 1 mL of ice-cold extraction buffer containing 50 mm HEPES (N-[2-hydroxyethyl]piperazine-N′[2-ethansulfonic acid])-NaOH, pH 7.0, 1 mm dithiothreitol (DTT), and 1% (w/v) polyvinyl polypyrrolidone, and was homogenized with a Polytron tissue homogenizer. The suspension was centrifuged at 26,000g for 20 min at 4°C. Aliquots of the supernatant (0.3 mL) were desalted by centrifugal gel filtration (Helmerhorst and Stokes, 1980) through Sephadex G-25 superfine columns (3.5-mL bed volume) preequilibrated in gel filtration buffer consisting of 50 mm HEPES-NaOH, pH 7.0, and 1 mm DTT. More than 95% of the soluble carbohydrates present in the crude extracts could be removed by this technique. To further reduce the concentration of soluble carbohydrates, the desalted extracts were diluted 5-fold and reconcentrated at 4°C by centrifugal ultrafiltration (Centricon-10; Millipore, Vienna).
Enzyme activities were determined as previously described (Peterbauer et al., 1998) with minor modifications. The enzyme extracts were incubated at 30°C with appropriate substrates in gel filtration buffer in a final volume of 30 μL. Reaction mixtures for the determination of galactinol synthase activity contained 10 μL of desalted enzyme extract, 5 mm MnCl2, 5 mm UDP-d-Gal, and 20 mm myo-inositol. The reaction mixtures were incubated for 15 min. The activities of raffinose synthase, stachyose synthase, and verbascose synthase were assayed in reaction mixtures containing 20 μL of desalted enzyme extract, 10 mm galactinol, and 40 mm Suc (raffinose synthase), 20 mm raffinose (stachyose synthase), or 20 mm stachyose (galactinol-dependent verbascose synthase), respectively. For estimation of galactinol-independent verbascose synthase activity, reactions mixtures contained only stachyose (20 mm). The reaction mixtures were incubated for 2 (stachyose synthase activity) to 4 h (raffinose synthase and verbascose synthase activities). All reactions were terminated by boiling for 5 min. The reaction mixtures were diluted to 1 mL, and 100 mg of a 2:3 (w/w) mixture of ion-exchange resins (Dowex 50W × 8, H+ and 1 × 8, formate) was added. The samples were shaken at 700 rpm for 60 min and were centrifuged. Aliquots of the supernatants (10 μL) were analyzed by HPLC with pulsed amperometric detection employing a Carbopac PA10 column (Dionex, Vienna) as previously described (Peterbauer et al., 1999). Control reactions contained gel filtration buffer instead of substrates. In addition, each batch of substrate was tested for the presence of traces of reactions products. Reaction rates were corrected for the amount of products introduced by the ultrafiltrated extracts and by the substrate solutions. All reactions were linear with time under these assay conditions.
For determination of pH-dependent activity profiles, desalted protein extracts were repeatedly ultrafiltrated in 10 mm Na-phosphate (pH 7.0) and 1 mm DTT (Centricon Plus-20, Millipore), diluted 10-fold with McIlvaine buffer (0.2 m Na2 HPO4 adjusted to various pH values with 0.1 m citric acid), and assayed for enzyme activity. Soluble protein was estimated with bovine serum albumin as a standard using the Bradford dye-binding procedure (Bio-Rad protein assay; Bio-Rad, Vienna).
Preparation of Probes and Northern-Blot Analysis
A cDNA-fragment of PeaGS1 encoding for pea galactinol synthase (GenBank accession no. AJ243815) was isolated by reverse transcriptase-PCR. Total RNA from developing RRRbRb seeds (about 20 d after flowering) was reverse transcribed with an Omniscript reverse transcriptase kit and an oligo-dT primer (Qiagen, Hilden, Germany). PCR was performed with the primers 5′-ATG GCA CCG GAG ATC GTT CAG-3′ and 5′-GGT TGC ACC TCC TCA TTC TTA TTC C-3′. The resulting 1.0-kb fragment (bp 153–1196) was isolated from an agarose gel and was cloned into the pCR4-TOPO vector (Invitrogen, Groningen, The Netherlands). The identity of the fragment was verified by sequencing using a BigDye Primer Cycle Sequencing Ready Reaction kit and an ABI Prism 310 sequencer (Applied Biosystems, Vienna). A digoxigenin-labeled probe was prepared by amplification of the cDNA fragment in the presence of digoxigenin-11-dUTP (Roche Diagnostics, Vienna) as suggested by the manufacturer. For a digoxigenin-labeled probe specific for pea stachyose synthase, a 1.5-kb fragment of PsSTS1 (GenBank accession no. AJ311087, bp 221–1694) was amplified with the primers 5′-AAA TGC ACC ACC TTC ACT TCT TCA-3′ and 5′-ATC ATC CCC AAC TCT TCC CAT AG-3′. A pCR2.1 vector containing the coding region of PsSTS1 was used as a template. Before use, labeled probes were purified from agarose gels with a Prep-A-Gene Master kit (Bio-Rad) and were denatured by boiling for 10 min.
Total RNA was extracted with an RNeasy Plant Mini kit (Qiagen). Equal amounts of RNA (10 μg) were size fractionated on a 1.2% (w/v) agarose gel containing formaldehyde and were capillary blotted with 20× standard saline sodium citrate (SSC) onto a positively charged nylon membrane (Roche Diagnostics). The blot was hybridized overnight at 50°C with the PeaGS1 probe in DIG Easy Hyb buffer (Roche Diagnostics), washed twice in 2× SSC and 0.1% (w/v) SDS at room temperature, and twice in 0.5× SSC and 0.1% (w/v) SDS at 68°C. Bound probe was visualized with an anti-digoxigenin-alkaline phosphatase conjugate and chemiluminescence detection. The membrane was subsequently stripped as suggested by the manufacturer and was probed with the labeled PsSTS1 fragment. Digoxigenin-labeled RNA fragments (Roche Diagnostics) were used as size markers. For a loading control, the RNA was visualized with methylene blue (Hermsmeier et al., 2001).
Footnotes
This work was supported by the Austrian Science Foundation (grant no. P13955–BIO).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010534.
LITERATURE CITED
- Aranda P, Dostalova J, Frias J, Lopez-Jurado M, Kozlowska H, Pokorny J, Urbano G, Vidal-Valverde C, Zdyunczyk Z. Nutrition. In: Hedley CL, editor. Carbohydrates in Grain Legume Seeds: Improving Nutritional Quality and Agronomic Characteristics. Wallingford, UK: CAB International; 2000. pp. 61–87. [Google Scholar]
- Bachmann M, Keller F. Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans L.: inter- and intracellular compartmentation. Plant Physiol. 1995;109:991–998. doi: 10.1104/pp.109.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M, Matile P, Keller F. Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans L. Cold acclimation, translocation, and sink to source transition: discovery of a chain elongation enzyme. Plant Physiol. 1994;105:1335–1345. doi: 10.1104/pp.105.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentsink L, Alonso-Blanco C, Vreugdenhil D, Tesnier K, Groot SPC, Koornneef M. Genetic analysis of seed-soluble oligosaccharides in relation to seed storability of Arabidopsis. Plant Physiol. 2000;124:1595–1604. doi: 10.1104/pp.124.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M, Corbineau F, Gee H, Come D. Water content, raffinose, and dehydrins in the induction of desiccation tolerance in immature wheat embryos. Plant Physiol. 1999;120:463–471. doi: 10.1104/pp.120.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R, Keller F. Vacuolar chain elongation of raffinose oligosaccharides in Ajuga reptans. Aust J Plant Physiol. 2000;27:743–746. [Google Scholar]
- Buitink J, Hemminga MA, Hoekstra FA. Is there a role for oligosaccharides in seed longevity? An assessment of intracellular glass stability. Plant Physiol. 2000;122:1217–1224. doi: 10.1104/pp.122.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey PM. Occurrence of glycoprotein glycosidases in mature seeds of mung bean (Vigna radiata) Phytochemistry. 1984;23:257–260. [Google Scholar]
- Frias J, Bakhsh A, Jones DA, Arthur AE, Vidal-Valverde C, Rhodes MJC, Hedley CL. Genetic analysis of the raffinose oligosaccharide pathway in lentil seeds. J Exp Bot. 1999;50:469–476. [Google Scholar]
- Frias J, Vidal-Valverde C, Bakhsh A, Arthur AE, Hedley C. An assessment of variation for nutritional and non-nutritional carbohydrates in lentil seeds (Lens culinaris) Plant Breed. 1994;113:170–173. [Google Scholar]
- Gilbert GA, Wilson C, Madore MA. Root-zone salinity alters raffinose oligosaccharide metabolism and transport in Coleus. Plant Physiol. 1997;115:1267–1276. doi: 10.1104/pp.115.3.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górecki RJ, Lahuta LB, Jones AD, Hedley CL. Soluble sugars in maturing pea seeds of different lines in relation to desiccation tolerance. In: Black M, Bradford KJ, Vásquez-Ramos J, editors. Seed Biology: Advances and Applications. Wallingford, UK: CAB International; 2000. pp. 67–74. [Google Scholar]
- Górecki RJ, Piotrowicz-Cieslak AI, Lahuta LB, Obendorf RL. Soluble carbohydrates in desiccation tolerance of yellow lupin seeds during maturation and germination. Seed Sci Res. 1997;7:107–115. [Google Scholar]
- Handley LW, Pharr DM, McFeeters RF. Carbohydrate changes during maturation of cucumber fruit: implications for sugar metabolism and transport. Plant Physiol. 1983a;72:498–502. doi: 10.1104/pp.72.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley LW, Pharr DM, McFeeters RF. Relationship between galactinol synthase activity and sugar composition of leaves and seeds of several crop species. J Am Soc Hort Sci. 1983b;108:600–605. [Google Scholar]
- Haritatos E, Keller F, Turgeon R. Raffinose oligosaccharide concentrations measured in individual cell and tissue types in Cucumis melo L. leaves: implications for phloem loading. Planta. 1996;198:614–622. doi: 10.1007/BF00262649. [DOI] [PubMed] [Google Scholar]
- Helmerhorst E, Stokes GB. Microcentrifuge desalting: a rapid, quantitative method for desalting small amounts of protein. Anal Biochem. 1980;104:130–135. doi: 10.1016/0003-2697(80)90287-0. [DOI] [PubMed] [Google Scholar]
- Hendrix DL. Carbohydrates and carbohydrate enzymes in developing cotton ovules. Physiol Plant. 1990;78:85–92. [Google Scholar]
- Herman EM, Shannon LM. Accumulation and subcellular localization of α-galactosidase-hemagglutinin in developing soybean cotyledons. Plant Physiol. 1985;77:886–890. doi: 10.1104/pp.77.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsmeier D, Schittko U, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata: I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiol. 2001;125:683–700. doi: 10.1104/pp.125.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthaus U, Schmitz K. Stachyose synthesis in mature leaves of Cucumis melo: purification and characterization of stachyose synthase (EC 2.4.1.67) Planta. 1991;184:525–531. doi: 10.1007/BF00197902. [DOI] [PubMed] [Google Scholar]
- Horbowicz M, Obendorf RL. Seed desiccation tolerance and storability: dependence on flatulence-producing oligosaccharides and cyclitols: review and survey. Seed Sci Res. 1994;4:385–405. [Google Scholar]
- Jones DA, DuPont MS, Ambrose MJ, Frias J, Hedley CL. The discovery of compositional variation for the raffinose family of oligosaccharides in pea seeds. Seed Sci Res. 1999;9:305–310. [Google Scholar]
- Kandler O, Hopf H. Oligosaccharides based on sucrose (sucrosyl oligosaccharides) Encyclopedia of Plant Physiology (New Series) 1982;13A:348–383. [Google Scholar]
- Keller F. Galactinol synthase is an extravacuolar enzyme in tubers of Japanese artichoke (Stachys sieboldii) Plant Physiol. 1992;99:1251–1253. doi: 10.1104/pp.99.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller F, Matile P. The role of the vacuole in storage and mobilization of stachyose in tubers of Stachys sieboldii. J Plant Physiol. 1985;119:369–380. [Google Scholar]
- Keller F, Pharr DM. Metabolism of carbohydrates in sinks and sources: galactosyl-sucrose oligosaccharides. In: Zamski E, Schaffer AA, editors. Photoassimilate Distribution in Plants and Crops. New York: Marcel Dekker; 1996. pp. 115–184. [Google Scholar]
- Keller R, Brearley CA, Trethewey RN, Muller-Rober B. Reduced inositol content and altered morphology in transgenic potato plants inhibited for 1D-myo-inositol 3-phosphate synthase. Plant J. 1998;16:403–410. [Google Scholar]
- Kuo TM, Lowell CA, Smith PT. Changes in soluble carbohydrates and enzymic activities in maturing soybean seed tissues. Plant Sci. 1997;125:1–11. [Google Scholar]
- Lehle L, Tanner W. The function of myo-inositol in the biosynthesis of raffinose: purification and characterization of galactinol:sucrose 6-galactosyltransferase from Vicia faba seeds. Eur J Biochem. 1973;38:103–110. doi: 10.1111/j.1432-1033.1973.tb03039.x. [DOI] [PubMed] [Google Scholar]
- Liu JJJ, Krenz DC, Galvez AF, de Lumen BO. Galactinol synthase (GS): increased enzyme activity and levels of mRNA due to cold and desiccation. Plant Sci. 1998;134:11–20. [Google Scholar]
- Lowell CA, Kuo TM. Oligosaccharide metabolism and accumulation in developing soybean seeds. Crop Sci. 1989;29:459–465. [Google Scholar]
- Muller LL, Jacks TJ. Intracellular distribution of free sugars in quiescent cottonseed. Plant Physiol. 1983;71:703–704. doi: 10.1104/pp.71.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obendorf RL. Oligosaccharides and galactosyl cyclitols in seed desiccation tolerance. Seed Sci Res. 1997;7:63–74. [Google Scholar]
- Pattee HE, Isleib TG, Giesbrecht FG, McFeeters RF. Investigations into genotypic variations of peanut carbohydrates. J Agric Food Chem. 2000;48:750–756. doi: 10.1021/jf9910739. [DOI] [PubMed] [Google Scholar]
- Peterbauer T. Structure, synthesis and function of galactosylcyclitols in Vigna seeds. PhD thesis. Vienna, Austria: University of Vienna; 1998. [Google Scholar]
- Peterbauer T, Mucha J, Mayer U, Popp M, Glössl J, Richter A. Stachyose synthesis in seeds of adzuki bean (Vigna angularis): molecular cloning and functional expression of stachyose synthase. Plant J. 1999;20:509–518. doi: 10.1046/j.1365-313x.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- Peterbauer T, Puschenreiter M, Richter A. Metabolism of galactosylononitol in seeds of Vigna umbellata. Plant Cell Physiol. 1998;39:334–341. [Google Scholar]
- Peterbauer T, Richter A. Galactosylononitol and stachyose synthesis in seeds of adzuki bean: purification and characterization of stachyose synthase. Plant Physiol. 1998;117:165–172. doi: 10.1104/pp.117.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterbauer T, Richter A. Biochemistry and physiology of raffinose family oligosaccharides and galactosyl cyclitols in seeds. Seed Sci Res. 2001;11:185–187. [Google Scholar]
- Plant AR, Moore KG. The protein, lipid and carbohydrate composition of protein bodies from Lupinus angustifolius seeds. Phytochemistry. 1983;22:2359–2363. [Google Scholar]
- Saravitz DM, Pharr DM, Carter TE. Galactinol synthase activity and soluble sugars in developing seeds of four soybean genotypes. Plant Physiol. 1987;83:185–189. doi: 10.1104/pp.83.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger N, Keller F. Allocation of raffinose family oligosaccharides to transport and storage pools in Ajuga reptans: the roles of two distinct galactinol synthases. Plant J. 2000;21:249–258. doi: 10.1046/j.1365-313x.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- Swanson SJ, Bethke PC, Jones RL. Barley aleurone cells contain two types of vacuoles: characterization of lytic organelles by use of fluorescent probes. Plant Cell. 1998;10:685–698. doi: 10.1105/tpc.10.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner W, Lehle L, Kandler O. myo-Inositol, a cofactor in the biosynthesis of verbascose. Biochem Biophys Res Commun. 1967;29:166–171. doi: 10.1016/0006-291x(67)90581-5. [DOI] [PubMed] [Google Scholar]
- Tanner W, Seifarth H, Kandler O. Der Umsatz der Oligosaccharide in reifenden und keimenden Samen von Phaseolus vulgaris. Z Pflanzenphysiol. 1968;58:369–377. [Google Scholar]
- Voragen AGJ. Technological aspects of functional food-related carbohydrates. Trends Food Sci Technol. 1998;9:328–335. [Google Scholar]