Abstract
Protein kinases of the microtubule affinity-regulating kinase (MARK) family were originally discovered because of their ability to phosphorylate certain sites in tau protein (KXGS motifs in the repeat domain). This type of phosphorylation is enhanced in abnormal tau from Alzheimer brain tissue and causes the detachment of tau from microtubules. MARK-related kinases (PAR-1 and KIN1) occur in various organisms and are involved in establishing and maintaining cell polarity. Herein, we report the ability of MARK2 to affect the differentiation and outgrowth of cell processes from neuroblastoma and other cell models. MARK2 phosphorylates tau protein at the KXGS motifs; this results in the detachment of tau from microtubules and their destabilization. The formation of neurites in N2a cells is blocked if MARK2 is inactivated, either by transfecting a dominant negative mutant, or by MARK2 inhibitors such as hymenialdisine. Alternatively, neurites are blocked if the target KXGS motifs on tau are rendered nonphosphorylatable by point mutations. The results suggest that MARK2 contributes to the plasticity of microtubules needed for neuronal polarity and the growth of neurites.
INTRODUCTION
The establishment of neuronal polarity and the generation of cell processes require the interplay between signaling mechanisms (from extracellular cues to the cytoplasm and to the nucleus), which enable the cell to decide when and where to grow a neurite, and mechanochemical elements (cytoskeleton, motors, and membranes) that allow the neurite to push outward. The actin network in the cell cortex tends to resist gross shape changes, and consequently actin-disassembly drugs facilitate neurite outgrowth (Edson et al., 1993; Knowles et al., 1994; Bradke and Dotti, 1999), whereas microtubules provide the core for a growing cell process, and therefore microtubule-disassembly poisons prevent the outgrowth (Baas and Ahmad, 1993; Rochlin et al., 1996). In addition microtubules must be dynamically unstable to allow growth cone formation, and therefore both microtubule-stabilizing and -destabilizing drugs can inhibit neurite outgrowth (Liao et al., 1995; Tanaka et al., 1995; Jordan and Wilson, 1998; Kaverina et al., 1998; Waterman-Storer and Salmon, 1999; Goode et al., 2000; Kabir et al., 2001). In this study, we focus on the neuronal microtubule-associated protein tau, its role in neurite outgrowth, and its regulation by phosphorylation. Tau is a mixture of six splicing isoforms (Figure 1; Lee et al., 1988; Goedert et al., 1989) that become largely axonal during development (Binder et al., 1985; Hirokawa et al., 1996). The accepted role of tau is that of a microtubule stabilizer (Cleveland et al., 1977; Drubin and Kirschner, 1986; Butner and Kirschner, 1991; Gustke et al., 1994; Panda et al., 1999), although other roles such as a regulator of axonal traffic (Ebneth et al., 1998; Stamer et al., 2002), anchor for kinases and phosphatases (Lee et al., 1998; Liao et al., 1998; Sontag et al., 1999), or membrane linker (Brandt et al., 1995) have recently emerged. Tau strongly promotes neurite outgrowth during differentiation (Caceres and Kosik, 1990; Esmaeli-Azad et al., 1994; Leger et al., 1994; Hirokawa et al., 1996), and even in nonneuronal cells tau induces cell processes with a cytoskeletal organization reminiscent of neurites (Kanai et al., 1989; Knops et al., 1991; Barlow et al., 1994; Biernat and Mandelkow, 1999). The interaction between tau and microtubules is regulated by phosphorylation. This aspect has been studied intensely because hyperphosphorylation of tau, detachment from microtubules, and abnormal aggregation are hallmarks of Alzheimer's disease (reviewed in Delacourte and Buee, 2000). One would therefore expect that phosphorylation of tau at sites that cause its detachment from microtubules would counteract neurite formation. However, other observations complicate this view of tau's role. One is that the concentration of tau along an axon seems to be greatest near the tip where the microtubules are few and of lower stability in spite of the presence of tau (Black et al., 1996). This pool of tau is also thought to be in a state of low phosphorylation that would be expected to favor association with microtubules (Mandell and Banker, 1996). Thus, there is no simple correlation between the concentration of tau, its phosphorylation, and the local stability of microtubules. Other counterintuitive results come from nonneuronal cell models such as Sf9 cells transfected with tau, which induces them to develop neurite-like cell processes (Baas et al., 1991; Knops et al., 1991). In this case, the type of phosphorylation that most potently detaches tau from microtubules in vitro (at KXGS motifs in the repeat domain) is necessary for cell process outgrowth, rather than inhibitory (Biernat and Mandelkow, 1999).
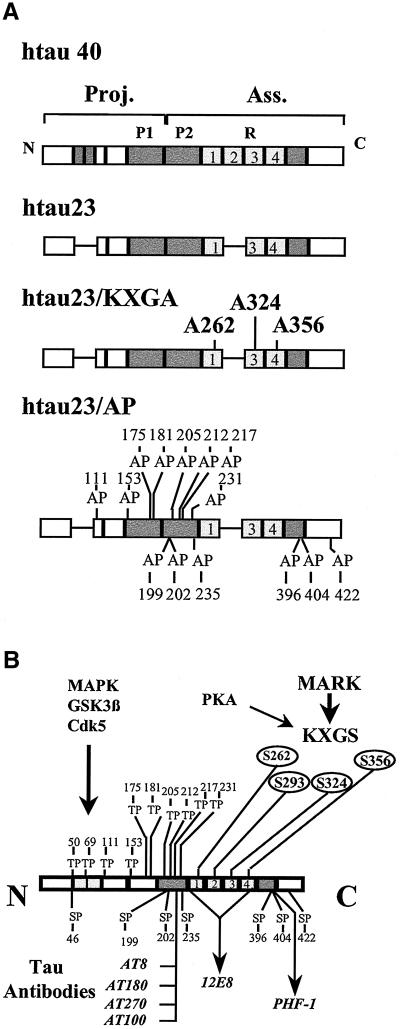
Figure 1.
Bar diagram of human tau, phosphorylation sites, and antibody epitopes. (a) N-terminal half is the “projection domain” because it projects from the microtubule surface but does not bind itself. The C-terminal half is the “assembly domain” because it binds to microtubules and promotes their assembly. The binding is mediated by the three to four pseudorepeats (∼31 residues each) and the flanking domains (shaded). Compared with the longest isoform in the CNS (htau40, 441 residues), the fetal isoform htau23 (352 residues) lacks the second repeat (exon 10) and two inserts near the N terminus (58 residues). The SP or TP motifs (outside the repeat domain, 14 in htau23, 17 in htau40) are marked; in the AP-htau23 mutants the S or T residues are replaced by A so that they cannot be phosphorylated by proline-directed kinases. The KXGS motifs (one per repeat) are the targets of MARK; in the KXGA-htau23 mutant the S residues are replaced by A, making them nonposphorylatable. (b) Antibody epitopes in htau40 sensitive to phosphorylation are indicated. The main targets of cdk5 or cdc2 on htau40 are the double motifs T231/S235 (epitope of antibody AT-180), S202/T205 (antibody AT-8), and S404 (only weak reaction with PHF-1); the main targets of GSK-3β are S396/S404 (strong reaction with antibody PHF-1), and S202/T205 (AT-8 epitope) (Illenberger et al., 1998); the main target of MARK is S262 (epitope of antibody 12E8).
These observations require a critical evaluation of the kinases of tau and their role in neurite outgrowth. Tau contains many phosphorylation sites targeted by several kinases. One can broadly distinguish two classes of sites: 1) Tau contains many Ser-Pro or Thr-Pro motifs phosphorylated by proline-directed kinases such as GSK-3β, cdc2, cdk5, or mitogen-activated protein (MAP) kinase. These sites account for the major fraction of tau phosphorylation (Figure 1; Illenberger et al., 1998; Biernat and Mandelkow, 1999) and have received attention because they are prominent in tau from Alzheimer brain (Morishima-Kawashima et al., 1995). 2) Other sites can be phosphorylated by nonproline-directed kinases such as protein kinase A (PKA), protein kinase C, calcium/calmodulin-dependent protein kinase II, or microtubule affinity-regulating kinase (MARK). Some sites have pronounced effects on tau's binding to microtubules, notably, the KXGS motifs in the repeats that can be phosphorylated by MARK (Biernat et al., 1993; Drewes et al., 1997). Similar KXGS motifs occur in other MAPs (MAP2 and MAP4), suggesting a generalized mechanism of regulation (Illenberger et al., 1996; Ozer and Halpain, 2000). There is a family of MARK kinases in mammals with homology to kinases in other organisms (par-1, kin1+; reviewed in Drewes et al., 1998; Nelson and Grindstaff, 1997; Kemphues, 2000), members of the SNF1/AMPK subfamily of kinases (Hanks and Hunter, 1995). They are important for the establishment of cell polarity in different contexts, e.g., asymmetric distribution of P-granules in the Caenorhabditis elegans zygote (par-1; Guo and Kemphues, 1995), polar growth of Schizosaccharomyces pombe (kin1+; Levin and Bishop, 1990), axis formation in the Drosophila embryo (Shulman et al., 2000; Tomancak et al., 2000), or the polar structure of epithelial cell layers (Böhm et al., 1997). Kinases of the MARK/PAR-1 family seem to be elevated in fetal tissue (Lopez and Sheetz, 1995; Drewes et al., 1997; Jenkins and Johnson, 1997; Brown et al., 1999). We therefore studied whether MARK plays a role in neurite development and whether it operates through its target tau. The results shown herein suggest that this is indeed the case.
MATERIALS AND METHODS
Cells and Viruses
N2a neuroblastoma cells were grown in minimal essential medium Earle's medium supplemented with 10% fetal calf serum, 2 mM glutamine (Biochrom, Berlin, Germany), and 0.1% nonessential amino acids (Sigma Chemie, Deisenhofen, Germany), at 37°C and 5% CO2 in a humidified chamber. Cells were seeded onto coverslips at a density 2 × 104 cells/cm2 in 24-well culture dishes, transfected with 1 μg of plasmid DNA by Effectene (QIAGEN, Hilden, Germany) or N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate (Roche Applied Science, Mannheim, Germany) according to the manufacturer's protocol. N2a cells were differentiated and transfected (before or afterward) with plasmids encoding tau, MARK2, or their mutants. Differentiation of N2a cells was induced by serum deprivation and 1 μM retinoic acid (Sigma Chemie) for 24 h. The N2a/htau40 cell line stably transfected with htau40 was described previously (Ebneth et al., 1998). Sf9 cells were obtained from Invitrogen (San Diego, CA) and were grown at 27°C in monolayer culture Grace's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 50 μg/ml gentamicin, and 2.5 μg/ml amphotericin. BaculoGold was obtained from BD PharMingen (San Diego, CA) and pVL1392 was from Invitrogen. Concentrations of tau were determined by an enzyme-linked immunosorbent assay described previously (Ackmann et al., 2000).
Plasmids and Recombinant Baculoviruses Containing Mutated Tau Genes
The cDNAs of tau and tau mutants were inserted into a derivative of pRc/CMV vector (Invitrogen, Leek, The Netherlands) by using NdeI and BamHI restriction sites to yield vectors for tau (htau23) or tau mutants AP (all SP or TP motifs changed to AP) or KXGA (all KXGS motifs changed to KXGA). The same procedure was used for the kinase MARK2 or its inactive mutant MARK2/T208A/S212A (numbering as in Drewes et al., 1997). The construction of recombinant baculoviruses containing mutated tau genes was described previously (Biernat and Mandelkow, 1999)
Quantitation of Tau-induced Process Formation in Sf9 Cells
The frequency of cell processes was determined in monolayer culture. Cells (3 × 106) were plated on 60-mm Petri dishes, infected with recombinant baculoviruses at a multiplicity of infection (MOI) of 1–5, and incubated at 27°C. The morphological analysis was performed on unfixed cells because the cells have the tendency to detach and processes are lost during the fixation procedure. Process morphology was quantitated in three independent experiments, scoring 200 cells each. To determine the effects of the kinase inhibitors hymenialdisine (HD), flavopiridol (FL), H89, or LiCl on cell processes, Sf9 cells were allowed to express tau for 48 h (at this time a robust growth of processes is observed in transfected cells), and then treated with 50 μM HD or FL inhibitors or 50 mM LiCl, or 10 μM H89, for 3 h and scored subsequently.
Quantitation of Neurite Outgrowth in N2a Cells Transfected with tau23 Constructs
N2a cells were allowed to differentiate for 24 h by treatment with retinoic acid in the presence of 0.1% fetal calf serum and subsequently transfected with plasmids encoding tau constructs. Twenty-four hours after transfection the coverslips were fixed with methanol and incubated with the rabbit polyclonal pan-tau antibody K9JA (Dako Diagnostika, Hamburg, Germany) and the mouse monoclonal anti-tubulin antibody DM1A (Sigma Chemie). Tau-containing cells were scored for cell processes only when they reached a length of more than two cell diameters (>40 μm; three independent experiments, 100 cells each).
Quantitation of Neurite Outgrowth in N2a Cells Treated with Kinase Inhibitors
Control N2a/htau40 cells were differentiated by serum deprivation and treatment with 1 μM retinoic acid, fixed with 4% paraformaldehyde, and stained for immunofluorescence with tau antibody K9JA. N2a/htau40 cells differentiated for 12 h by serum deprivation and treatment with retinoic acid were incubated for 2–5 h in the presence of 50 μM FL, an inhibitor of cdk5 and GSK-3β (Leclerc et al., 2001) or of 50 μM HD, also an inhibitor of GSK-3β and cdk5 (Meijer et al., 2000), fixed, and stained for immunofluorescence with tau antibody K9JA. Cells with extended neurites were counted and scored as the percentage of total cells.
Transfection of N2a Cells with MARK2, Mutant MARK2, and Cotransfection with tau23-KXGA
N2a cells were transiently transfected with plasmids encoding MARK2 or dominant negative mutant of MARK2. Twenty-four hours after transfection the coverslips were fixed with methanol and incubated with monoclonal antibody 12CA5 (staining for MARK2) and with the mouse monoclonal anti-tubulin antibody DM1A. MARK2- or dnMARK2-containing cells were counted for extended neurites and scored as the percentage of total transfected cells. In cotransfection experiments, the N2a cells were cotransfected transiently with plasmids encoding green fluorescent protein (GFP)-MARK2 and KXGA/htau23 and after 16 h differentiated for 24 h, fixed with 4% paraformaldehyd for 5 min and incubated with the rabbit polyclonal pan-tau antibody K9JA and the mouse monoclonal anti-tubulin antibody (DM1A). Double transfected cells with extended neurites were counted and scored as the percentage of total transfected cells.
Western Blot Analysis
Sf9 cells were infected with recombinant virus at an MOI of 1–5. Cell lysates were prepared in hypotonic lysis buffer (50 mM Tris-HCl pH 7.4, 120 mM NaCl, 10% glycerol, 1% Nonidet-P40, 5 mM dithiothreitol, 1 mM EGTA, 20 mM NaF, 1 mM orthovanadate, 5 μM microcystin, 100 μg/ml each of protease inhibitors leupeptin, aprotinin, and pepstatin). The lysed cells were centrifuged at 16,000 × g for 15 min, and the supernatant and pellet were separated. The supernatant was adjusted to 500 mM NaCl, boiled for 10 min, and centrifuged at 16,000 × g for 15 min. Then 1- to 3-μg aliquots of proteins in the supernatant were electrophoresed by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and blotted with the following monoclonal antibodies: 12E8 (1:5000; a gift of P. Seubert, Elan Pharma, South San Francisco, CA), AT-8 (stock 1 mg/ml, diluted 1:2000), AT-180 (1 mg/ml, 1:2000), AT-100 (1 mg/ml, 1:500 (Innogenetics, SA, Ghent, Belgium), PHF-1 (1:600; a gift of P. Davies, Albert Einstein College, Bronx, NY), polyclonal rabbit anti-tau antibody K9JA (1:2000, Dako Diagnostika), and polyclonal rabbit antibody SA6941, raised against the regulatory loop peptide of MARK2 phosphorylated at T208 and S212. The antibody was affinity purified. The immunostaining was visualized using enhanced chemiluminescence (Amersham Biosciences, Braunschweig, Germany).
Immunofluorescence
Cells were washed in MTSB buffer (80 mM HEPES, pH 6.9, 1 mM MgCl2, 1 mM EGTA, 4% polyethylene glycol) and subsequently fixed with methanol at −20°C for 5 min, washed with phosphate-buffered saline, and treated with 5% bovine serum albumin in phosphate-buffered saline and 0.1% Triton X-100 for 1 h. Fixed cells were incubated with rabbit polyclonal pan-tau antibody K9JA (1:500; Dako Diagnostika), rat monoclonal anti-tubulin antibody YL1/2 (1:200; Serotec, Oxford, United Kingdom), mouse monoclonal anti-hemagglutinin (HA) tag antibody 12CA5 (1:200; Roche Applied Science) or rhodamine-labeled phalloidin (Molecular Probes, Eugene, OR). Fluorescently labeled (fluorescein isothiocyanate and tetramethylrhodamine B isothiocyanate) secondary antibodies were obtained from Dianova (Hamburg, Germany). Samples were examined using an LSM510 confocal microscope or an Axioplan fluorescence microscope equipped with a cooled charge-coupled device camera (Zeiss, Jena, Germany).
Kinase Preparations and Assays
Recombinant MARK2 was expressed in Escherichia coli (BL21, DE3 pLys) with a C-terminal His-tag. Cells were lysed in buffer A (50 mM Tris-HCl, pH 7.5, 200 mM NaCl, 50 mM imidazole, 5 mM 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate, 2 mM benzamidine, 1 mM β-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride [PMSF]) with a French press. The supernatant was loaded onto a Ni2+-NTA column (QIAGEN). After washing with 10 column volumes of buffer A the protein was eluted with a short gradient (5 column volumes) to buffer B (as buffer A, but 500 mM imidazole). Fractions that contained MARK2 were pooled and dialyzed against buffer C (50 mM Tris-HCl pH 7.5, 200 mM NaCl, 2 mM benzamidine, 1 mM β-mercaptoethanol, 1 mM PMSF, 50% glycerol) and stored at −20°C. The purity of the obtained kinase was higher than 95%. Kinase activities were assayed in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 2 mM EGTA, 0.5 mM PMSF, 0.5 mM dithiothreitol, 0.5 mM benzamidine for 30 min at 30°C. The final concentration of [32P]ATP (7.4 × 105 MBq/mmol) and substrate peptides was 15 μM. As substrates we used the synthetic tau-repeat1-peptide 255NVKSKIGSTENLK276 (Drewes et al. 1997) for recombinant MARK2 and the pCreb-peptide (prephosophorylated by PKA) for GSK3β (Upstate Biotechnology, Lake Placid, NY). Reactions were stopped by addition of one-half the reaction volume of 30% (wt/vol) trichloroacetic acid. After centrifugation, the supernatant was applied to phosphocellulose-paper discs, five times washed with 0.1 M phosphoric acid, dried by air, and counted in a scintillation counter. Blank values were subtracted and activities expressed in percentage of the maximal activity, i.e., without inhibitors. IC50 values were estimated from the dose-response curves. Brain extract kinase activity for phosphorylating and activating MARK2 was prepared as described previously (Biernat et al., 1993).
In Vivo Labeling of Tau Derivatives in Sf9 Cells and Phosphopeptide Mapping
Metabolic labeling of Sf9 cells with 32P was performed using 0.5 mCi of 32Pi/ml TNM FH cell medium lacking phosphate and supplemented with dialyzed fetal calf serum. Sf9 cells were infected with recombinant baculovirus at an MOI of 10. At 36 h postinfection cells were supplemented with 0.5 mCi of 32Pi per milliliter of medium and incubated for 3 h. After labeling cells were washed, resuspended in lysis/boiling buffer, and immediately boiled. After centrifugation the supernatant was subjected for SDS-PAGE and radioactive tau bands were eluted and precipitated by trichloroacetic acid. The radioactive Tau derivatives were further used for peptide mapping. Two-dimensional (2D) phosphopeptide mapping (on thin layer cellulose plates) was performed according to Boyle et al. (1991) and Illenberger et al. (1998). Note that the absolute degree of tau phosphorylation in Sf9 cells cannot be obtained from the radioactive labeling experiments because the efficiency of conversion of 32P to [γ-32P]ATP in the cells is not known; however, it can be estimated from the molecular weight shifts and the known specificities of the phosphorylation-sensitive antibodies.
RESULTS
Neurite Outgrowth in N2a Cells Requires Phosphorylation at KXGS Motifs in the Repeat Domain of Tau
One aim of our studies was to determine the role of tau protein, its phosphorylation, and its protein kinases on the elaboration of neurites. To achieve this one needs an experimental system that allows both cell biological and biochemical observations. We focused on the mouse neuroblastoma cell line N2a because it can be readily differentiated with retinoic acid after serum deprivation to form neurite-like cell processes (Figure 2). Differentiation of neuronal cells requires endogenous tau (Drubin and Kirschner 1986; Caceres and Kosik, 1990; Esmaeli-Azad et al., 1994); in N2a cells tau is only present at low concentrations, below the detectability by immunofluorescence. We transfected human fetal tau isoform htau23 (352 residues) transiently into wild-type N2a cells that induces them to develop long neurites after a differentiation stimulus. In the example of Figure 2a, ∼60% of transfected cells have processes longer than twice the cell body diameter. As a control, among cells not expressing exogenous htau23 only 20% develop extended processes (Figure 2, a, b, and f).
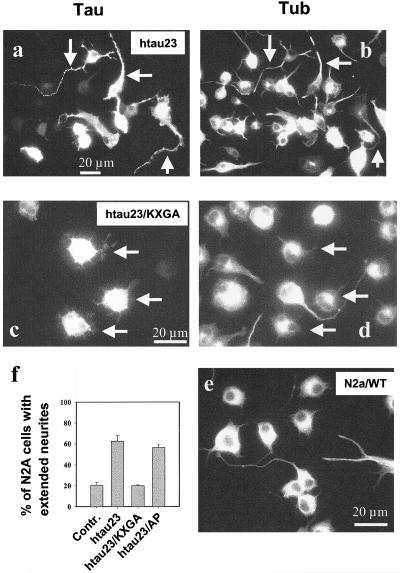
Figure 2.
Neurite outgrowth by N2a cells and dependence on tau phosphorylation. N2a cells were differentiated by serum withdrawal and retinoic acid and then transiently transfected with tau or tau mutants, and scored for the development of extended neurites longer than two cell body diameters (>40 μm). (a and c) Tau staining (antibody K9JA). (b and d) Tubulin staining (DM1A). (a and b) Cells transfected transiently with htau23 (50% efficiency). Among the cells not expressing exogenous tau, only 20% develop extended neurites (control; Figure 2e). In contrast, among the cells expressing exogenous htau23 the fraction with extended neurites rises to 60% (Figure 2, a and b, arrows, and f). (c and d) Cells transfected with the KXGA mutant of htau23: Among the cells expressing exogenous tau mutant (three cells highlighted by arrows in 2, c and d) only few develop neurites longer than two cell body diameters (compare other untransfected cells in d). This shows that the phosphorylation at KXGS motifs is important for neurite outgrowth. (e) Differentiated control N2a cells (no transfection with tau). (f) Histogram of neurite formation with different tau constructs: Only 20% of control cells show extended neurites, but 60% of cells transfected with htau23. Cells transfected with the KXGA mutant of htau23 are comparable with controls (20%, no phosphorylation by MARK2 possible). Cells transfected with the AP mutant are comparable with transfection by htau23 (60%, no phosphorylation by proline-directed kinases). The data show that transfection with htau23 and the AP mutant are similarly efficient in promoting neurite outgrowth, whereas transfection with the KXGA mutant has no effect. Error bars show SE.
Tau contains many Ser or Thr residues that can be phosphorylated by several kinases. To probe whether the outgrowth of neurites depends on the phosphorylation of tau we generated several tau constructs in which certain Ser or Thr residues were mutated into Ala, thus making them inaccessible to phosphorylation. Among the phosphorylation sites one can distinguish different classes: 1) The S-P or T-P motifs (14 in isoform htau23, mostly in the domains flanking the repeats; Figure 1) can be phosphorylated by proline-directed kinases such as GSK-3β and cdk5. They are thought to play a role in neurodegeneration (Imahori and Uchida, 1997; Mandelkow and Mandelkow, 1998), they induce the epitopes of several antibodies characteristic of Alzheimer tau (e.g., AT-8, AT-100, AT-180, and PHF-1), but have only a modest influence on tau-microtubule interactions (Biernat et al., 1993). 2) The KXGS motifs (one per repeat, three in htau23, four in htau40; Figure 1) are targets of nonproline-directed kinases, primarily MARK (and less efficiently PKA; Biernat et al., 1993; Drewes et al., 1997), which has a pronounced effect on detaching tau from microtubules (particularly Ser262 in the first repeat) and render them dynamic in vitro and in vivo (Ebneth et al., 1999). We therefore made modified constructs of htau23, one with all 14 S-P or T-P motifs mutated into A-P (AP-tau), and one with the KXGS motifs mutated into KXGA (nonphosphorylatable KXGA-tau) (Figure 1). When AP-tau was transfected into N2a cells, the effect was similar to that of wild-type tau, i.e., a strong induction of neurites, with about one-half of the transfected cells displaying extended processes (Figure 2f). Because proline-directed kinases are active in the cells (see below), this result means that the phosphorylation at SP or TP motifs is of lesser importance for neurite outgrowth.
In contrast, when repeating the experiment with KXGA-tau the outgrowth of extended neurites after a differentiation stimulus was nearly abolished. As a control, the effect was limited to the cells that actually express the tau mutant (Figure 2c, cells with arrows), whereas the others obtain normal processes after differentiation (Figure 2d, cells without arrows). Thus, the KXGA mutations in the repeats essentially abrogate tau's ability to induce neurites (Figure 2f). We note that the KXGA mutant has the same ability to bind and polymerize microtubules as wild-type unphosphorylated tau, in contrast to tau phosphorylated at KXGS motifs (Biernat et al., 1993; our unpublished data). These results suggest that N2a cells contain active kinase(s) that phosphorylate the KXGS motifs and that the phosphorylation at these motifs is important for neurite outgrowth.
Neurite Outgrowth Is Promoted by Activity of MARK2
Given that the KXGS motifs of tau are important for neurite outgrowth, the next question was to identify the responsible kinase(s). We had shown previously that the kinase that phosphorylates the KXGS motifs in tau and related MAPs most efficiently is MARK (Drewes et al., 1995; Illenberger et al., 1996). We therefore approached the identification of the kinase by asking how MARK influences neurite outgrowth and the phosphorylation of tau in N2a cells. Surprisingly, transient transfection of MARK2 into N2a cells caused differentiation without further stimuli (such as serum withdrawal and retinoic acid; Figure 3a, b, and e), although the transfection rate was low (1–2%). In contrast, the majority of N2a cells expressing the dominant negative mutant of MARK2 (Figure 3, c, d, and e) were not able to form extended neurites, even after a differentiation stimulus. As shown previously (Drewes et al., 1997), this mutant was rendered inactive in vitro and in cells by replacing phosphorylatable residues in the regulatory loop by alanines (T208A and S212A). Wild-type N2a cells contain endogenous tau only at the low level of 24 ng/107 cell (using an enzyme-linked immunosorbent assay developed by Ackmann et al., 2000). We therefore generated N2a cells stably expressing htau40, the largest tau isoform in the CNS, at a ∼40-fold higher level (∼1 μg/107 cells), to analyze the phosphorylation state of tau biochemically. These cells were transiently transfected with dnMARK2. Cells expressing the inactive mutant of MARK2 could not form neurites (Figure 4, a–d, arrows), whereas cells not expressing dnMARK2 were able to differentiate (Figure 4, a–d, asterisks). This argues that MARK2 or a closely related isoform might be important for neurite outgrowth and for the phosphorylation of the KXGS motifs in tau. To verify that MARK2 operates via tau phosphorylation, N2a cells were cotransfected with GFP-MARK2 and KGXA-htau23 (Figure 4, e–h). In the cases where the cells express the KXGA mutant of tau, or both MARK2 and the KXGA mutant, formation of extended neurites is strongly reduced. In other words, the neurite-promoting effects of tau can be obliterated either by mutating the KXGS target motif on tau (KXGA-tau; Figures 2c and 4, e–g), or by inactivating the kinase MARK2 (Figure 3, c–e).
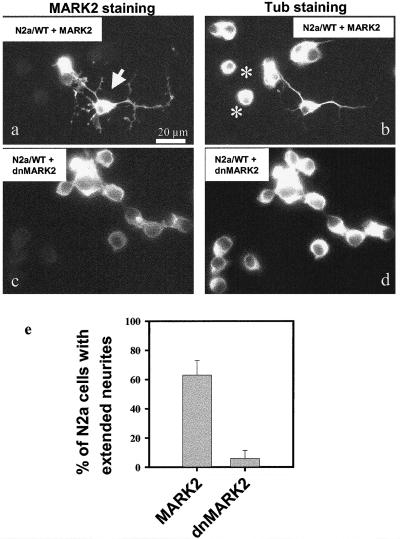
Figure 3.
Transfection of N2a cells with MARK2 promotes neurite formation, and dominant negative MARK2 inhibits it. N2a cells were transiently transfected with MARK2 (a and b) or a dominant negative mutant of MARK2 (c and d). (a and c) Staining for MARK2 (antibody 12CA5). (b and d) Staining for tubulin (antibody DM1A). (a and b) Although cells containing only the low levels of endogenous tau show no neurites in serum before differentiation (see rounded-up cells in b, asterisks) and would require differentiation conditions to develop processes (serum withdrawal, retinoic acid, etc), transfection with MARK2 overcomes this barrier and allows 60% of MARK2 transfected cells even without serum withdrawal and retinoic acid to differentiate and extend neurites (see cell at center in a and b, arrow and see histogram e). (c and d) When N2a cells are transfected with dnMARK2, only a small fraction of the cells expressing dnMARK2 (6%) form extended neurites after differentiation (Figure 3e).
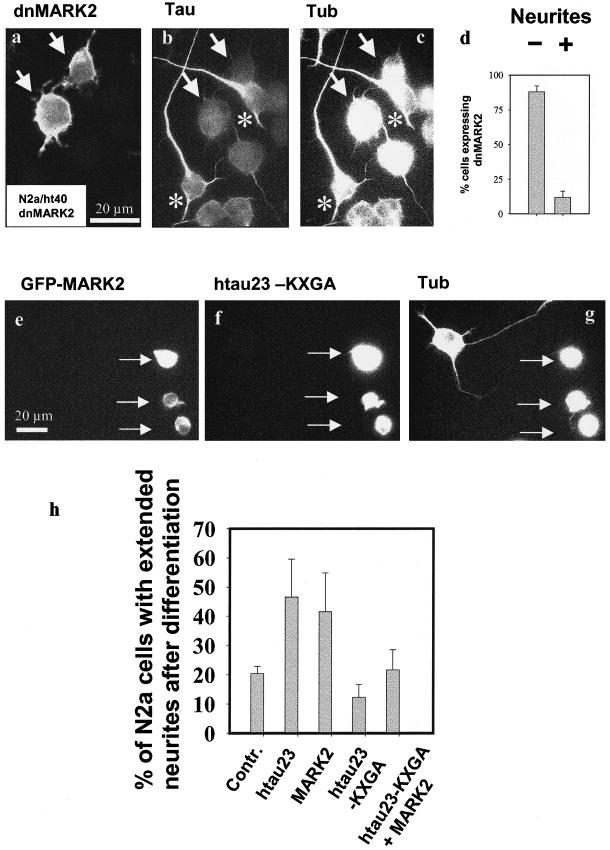
Figure 4.
Inhibition of neurite outgrowth by inactive MARK2 (dnMARK2) or nonphosphorylatable Tau (KXGA). N2a cells stably transfected with htau40 can be differentiated by serum withdrawal and retinoic acid (see b and c, asterisks). However, dnMARK2 prevents neurite formation (see a, arrows). (d) Histogram showing that dnMARK2 suppresses neurites (ca. 90% of transfected cells fail to develop neurites). (a–c) Expression of a dominant negative mutant (dnMARK2) in N2a/htau40 cells. (a) Staining for dnMARK2 (antibody 12CA5). (b) Staining for tau (K9JA). (c) Staining for tubulin (DM1A). (e–g) N2a cells cotransfected transiently with GFP-MARK2 and KXGA/htau23 and then differentiated. The doubly transfected cells (arrows) show no neurites, whereas the untransfected cell has extended neurites. (h) Histogram showing the fraction of N2a with extended neurites after transfection: control N2a cells, cells transfected with htau23, MARK2, htau23-KXGA mutant, double transfection with htau23-KXGA and MARK2. The data illustrate that htau23 and MARK2 promote neurite outgrowth, htau23-KXGA abolishes this activation, and even MARK2 is not able to rescue the inhibitory effect of the KXGA-tau mutant on neurite formation after differentiation, because the phosphorylation of tau at its KXGS motifs is blocked.
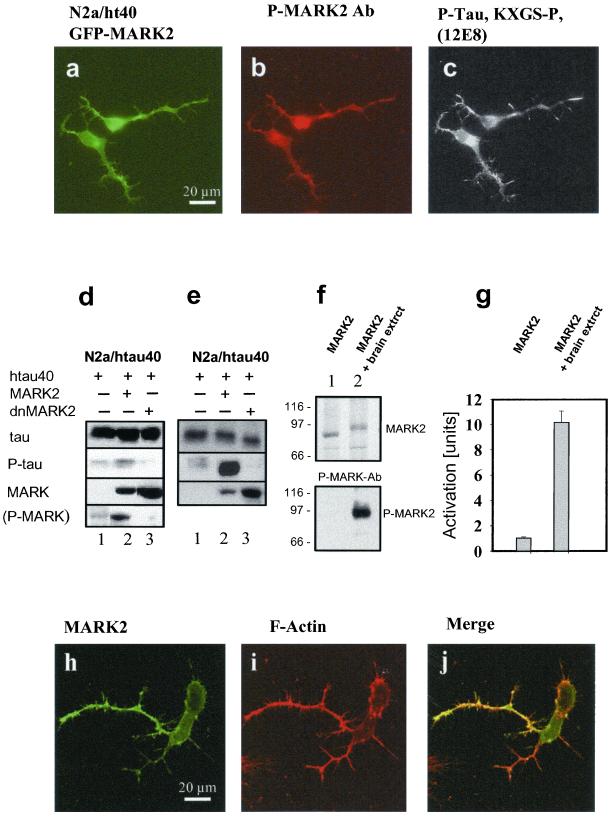
To show that MARK2 indeed phosphorylates the KXGS motifs of tau in living cells we transiently transfected GFP-MARK2 (or its dominant negative mutant) into N2a cells stably transfected with tau. Differentiated cells were analyzed by GFP fluorescence (showing MARK2; Figure 5a), immunofluorescence with the antibody p-MARK against active MARK2 (Figure 5b), and phospho-KXGS motifs in tau (antibody 12E8; Seubert et al., 1995; Figure 5c). The three patterns were similar, suggesting that active MARK2 localizes in the same compartments as tau throughout the cell and would therefore be able to phosphorylate it. In contrast, transfection with dnMARK2 showed no differentiation, no staining for active MARK2, and no phospho-KXGS tau (our unpublished data). To corroborate these findings the proteins were isolated from the N2a cells, and phosphorylation sites were determined by phospho-sensitive antibodies of known specificities in Western blots (Figure 5, d and e). The pan-tau antibody K9JA detects tau regardless of phosphorylation and serves as a standard for the protein concentration. Antibody 12E8 detects the phosphorylated KXGS motifs in the tau-repeats 1 and 4 (containing S262 and S356). Its signal is enhanced when MARK2 is transfected (Figure 5d, lane 2) but weak with no transfection or after transfection with dnMARK2 (Figure 5d, lanes 1 and 3). The weak reaction in lane 1 presumably reflects the residual activity of the endogenous MARK isoforms. As a control, the expression of MARK2 (active or inactive) is revealed by the antibody against the HA-tag (Figure 5, d and e). Finally, the state of activation of MARK2 is shown by the rabbit polyclonal peptide antibody p-MARK (SA6941) raised against a peptide of the phosphorylated activating loop of MARK2 (with phosphorylated T208 and S212). This antibody was affinity purified and characterized in detail; Figure 5g shows that recombinant MARK2 can be phosphorylated and activated 10-fold by a kinase activity in brain extract (Drewes et al., 1997). This phosphorylation of MARK causes a shift in the SDS gel and the reaction with p-MARK antibody (Figure 5f). The p-MARK antibody shows a pronounced signal in the blot after transfection of the cells with active MARK2, but only a weak signal without MARK2 transfection or with dnMARK2 (Figure 5d, lanes 1–3). The data argue that the elaboration of neurites is achieved by MARK2 phosphorylating the KXGS motifs on tau. The effect can be demonstrated even more clearly when the experiment is repeated, but cells are incubated with the phosphatase inhibitor okadaic acid (0.2 μM, 30 min) before harvesting. In this case the phosphorylation at the KXGS motifs seen by antibody 12E8 (against p-Tau) in blots is particularly pronounced after transfection with MARK2 (Figure 5e, lane 2), but not with dnMARK2 (Figure 5e, lane 3), indicating that other kinases do not phosphorylate the KXGS motifs in the presence of okadaic acid.
Figure 5.
Phosphorylation of tau induced by MARK2. (a–c) N2a/htau40 cells transiently transfected with GFP-MARK2, differentiated, fixed after 24 h, and analyzed by fluorescence microscopy. (a) Distribution of GFP-MARK2. (b) Immunofluorescence of p-MARK antibody against active MARK2 (phosphorylated T-loop). (c) Antibody 12E8 against phospho-KXGS motifs of tau. Note that active MARK2 and phosphorylated tau at KXGS sites colocalize (see below). (d and e) N2a/htau40 cells were transiently transfected with MARK2 or a dominant negative mutant (dnMARK2), differentiated for 6 h, and analyzed by preparing a cell lysate and probing by Western blotting with a panel of antibodies against phosphorylated MARK2 and tau. The data show that MARK2 causes the phosphorylation of the KXGS motifs of tau and dnMARK2 inhibits this. (d) Lane 1, control N2a/htau40 cell extract. Lane 2, transfection with MARK2. Lane 3, transfection with dominant negative MARK2 (T208A and S212A). Antibody K9JA (“Tau”) stains tau independently of phosphorylation. The three samples contain the same amount of tau. Antibody 12E8 against the phosphorylated KXGS motifs in tau repeats 1 and 4 (phospho-S262 and -S356, “p-Tau”) shows stronger staining in the case of MARK2 transfection (lane 2), but only weak staining without MARK2 transfection (lane 1), and no staining with dnMARK2 (lane 3). The antibody against hemagglutinin-tag (“MARK”) reveals the expression of exogenous MARK2 or dnMARK2 (lanes 2 and 3) but is absent from untransfected cells (lane 1). The antibody SA6941 against phosphorylated T-loop peptide on MARK2 (pT208, pS212, corresponding to activated MARK2) shows pronounced staining after transfection of exogenous MARK2 (lane 2) but only weak staining in controls (lane 1), corresponding to the activity of endogenous MARK-like kinases, and no staining in the presence of dnMARK. (e) N2a/htau40 cells were transiently transfected with MARK2 or a dominant negative mutant, dnMARK2, differentiated for 6 h, and before harvesting treated for 30 min with 0.2 μM okadaic acid. Note that only the sample expressing the transfected wt MARK2, but not dnMARK2, has clearly increased phosphorylation at KXGS motifs shown by reaction with antibody 12E8 (“p-Tau”, lane 2). This means that MARK2 but not other endogenous kinases such as PKA are responsible for tau phosphorylation at KXGS motifs in these conditions. (f and g) Western blot analysis demonstrating the specificity of the p-MARK antibody (polyclonal antibody SA6941) against the posphorylation sites T208 and S212 in the activating loop of kinase MARK2. Top, MARK2 protein before (lane 1) and after (lane 2) phosphorylation by brain extract kinase activity (see MATERIALS AND METHODS). Bottom, antibody p-MARK (SA6941) recognizes exclusively the phosphorylated MARK2 (lane 2) whose increased activity is demonstrated in the histogram (g). (h–j) N2a/htau40 cells transiently transfected with HA-tagged MARK2, fixed 24 h after differentiation and analyzed by confocal fluorescence microscopy. (h) Distribution of MARK2 by using fluorescent HA antibody. (i) Fluorescence of phalloidin-labeled actin. (j) Merge of h and i. Note that MARK2 and actin largely colocalize.
As seen in Figure 5, a–c, the differentiated N2a cells showed numerous filopodia and microspikes emanating from the cell body and the neurites, suggesting that active MARK2 and phospho-tau might colocalize with the actin network. We therefore transfected MARK2 transiently into N2a-htau40 cells and checked the distribution of MARK2 and actin by immunofluorescence. Figure 5, h–j, shows by confocal microscopy that the pattern of MARK2 coincides largely with that of actin. We hypothesize that tau phosphorylated at KXGS motifs detaches from microtubules and partly translocates to the actin network during neurite outgrowth, consistent with recent observations with MAP2 phosphorylated at KXGS motifs (Ozer and Halpain, 2000).
MARK Is Potently Inhibited by Hymenialdisine
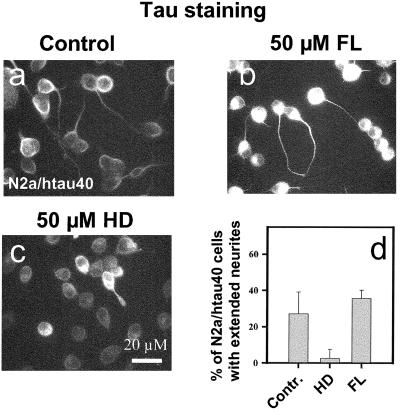
Tau contains multiple phosphorylation sites that could affect its function in different ways. The majority (95%) of tau's endogenous phosphorylation in cells occurs on SP or TP motifs in the regions flanking the repeats (Illenberger et al., 1998; Biernat and Mandelkow, 1999). These sites are phosphorylated by proline-directed kinases such as cdk5 and GSK-3β, which are known to play a role in differentiating neurons. To analyze the role of these kinases in process outgrowth we used two novel potent inhibitors of both cdk5 and GSK-3β, FL and HD (Meijer et al., 2000; Leclerc et al., 2001), and applied them to N2a cells stably transfected with htau40. Figure 6a shows that control cells develop extended neurites (∼25%) in differentiation medium. When the cells were exposed to 50 μM FL, neurite outgrowth was similar or somewhat enhanced (Figure 6, b and d), consistent with our previous observation that phosphorylation of tau at SP or TP motifs was neutral or somewhat inhibitory (Biernat and Mandelkow, 1999). In contrast, when N2a/htau40 cells were treated with the kinase inhibitor HD (50 μM), neurite outgrowth was strongly inhibited (∼3%; Figure 6, c and d). How can this apparent discrepancy between the two kinase inhibitors be explained? To answer this question the affected phosphorylation sites on tau had to be determined in more detail. This cannot be achieved with N2a or N2a/htau40 cells because their level of tau is too low for biochemical analysis. We therefore turned to the Sf9 cell system that generates sufficient quantities of protein for the biochemical analysis of transfected tau. The justification is the observation that the interplay between tau and tau kinases during process formation is qualitatively similar between neuronal and nonneuronal cells (Biernat and Mandelkow, 1999).
Figure 6.
Effect of kinase inhibitors FL and HD on tau-induced neurite outgrowth in N2a cells stably transfected with htau40. (a) Control N2a/htau40 cells were differentiated and stained for immunofluorescence with tau antibody K9JA; ∼25% of cells develop extended cell processes. (b) N2a/htau40 cells differentiated and treated with 50 μM FL, a strong inhibitor of cdk5 and GSK-3β (Leclerc et al., 2001). Neurite outgrowth is somewhat enhanced (∼35%). (c) N2a/htau40 cells differentiated and treated with 50 μM HD, also a strong inhibitor of cdk5 and GSK-3β (Meijer et al., 2000). Neurite outgrowth is strongly inhibited (∼3%). (d) Histogram illustrating the fraction of N2a/htau40 cells bearing extended neurites after differentiation and treatment with kinase inhibitors HD and FL. HD strongly reduces the extent of neurite formation, and FL causes a slight increase.
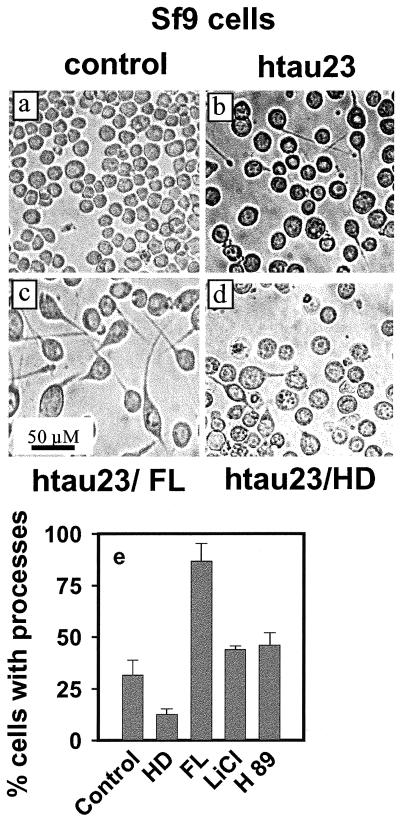
Figure 7 illustrates untreated Sf9 cells (diameter around 20 μm, no cell processes; Figure 7a) and cells transfected with htau23 (Figure 7b). After 30 h of transfection, ∼25% of these cells were enlarged and developed a single cell process of uniform diameter. Next, we exposed the cells to the drug FL (50 μM). This resulted in a pronounced threefold increase in cells with processes (∼75%; Figure 7, c and e), arguing that proline-directed phosphorylation of tau by cdk5 and GSK-3β is inhibitory for cell processes. However, when the cells were exposed to HD, process formation was strongly reduced (∼10%; Figure 7, d and e). The results showed that both N2a cells and tau-transfected Sf9 cells had a similar response to the kinase inhibitors FL and HD.
Figure 7.
Effect of kinase inhibitors FL and HD on tau-induced process outgrowth in Sf9 cells. (a) Untreated Sf9 cells (control), showing round shapes) and no cell processes. (b) Sf9 cells transfected with htau23 by baculovirus vector; ∼30% grow extended cell processes up to 100 μm. They also have larger cell bodies (2- to 3-fold). (c) Sf9 cells transfected with htau23 and treated with the kinase inhibitor FL (50 μM). The number of cells with processes increases strongly (∼3-fold). (d) Sf9 cells transfected with htau23 and treated with the kinase inhibitor HD (50 μM). The number of cells with processes drops to ∼10%. (e) Histogram displaying the efficiency of process formation by Sf9 cells in the presence of kinase inhibitors. HD inhibits them strongly, LiCl and H89 promote modestly, and FL promotes strongly.
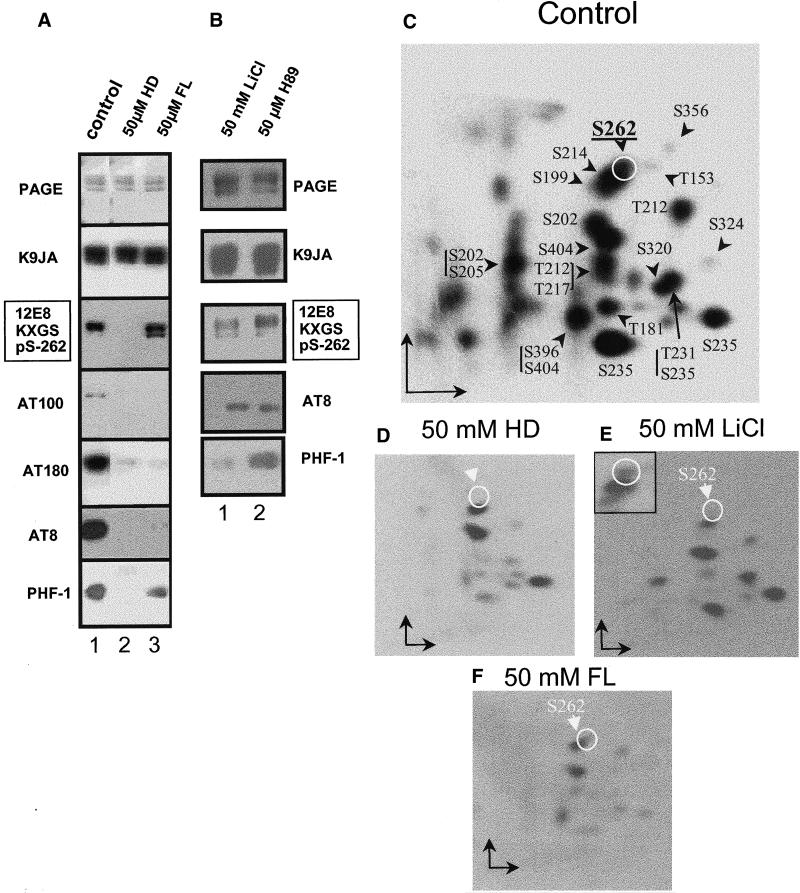
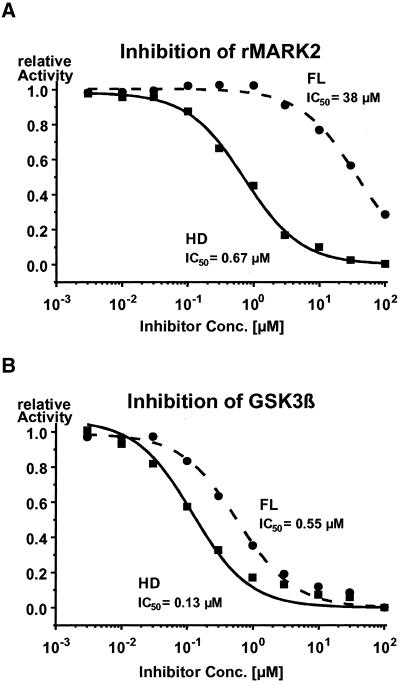
The phosphorylation sites on tau were determined using site-specific antibodies on Western blots, and by 2D phosphopeptide mapping (Figure 8). As shown previously (Illenberger et al., 1998; Godemann et al., 1999), major targets of GSK-3β on tau are S404, followed by S396 (which together make up the epitope of PHF-1), and to a lesser extent S202 and T205 (antibody AT-8). The major targets of cdk5 are S235 followed by T231 (epitope of antibody AT-180), S202/T205 (AT-8 epitope), whereas the reaction with PHF-1 is very weak because S404 is a major site but not S396. The Sf9 cells contain very active kinases so that all phosphorylation-dependent antibody reactions are observed on the transfected tau (Biernat and Mandelkow, 1999). Figure 8a (lane 1) illustrates this for the antibodies 12E8 (pS262 and pS356), AT-100 (pT212 and pS214), AT-180 (pT231 and pS235), AT-8 (pS202 and pT205), and PHF-1 (pS396 and pS404). When the inhibitor FL is added to the cells (Figure 8a, lane 3), the reactions of tau in Western blots with antibodies AT-100, AT-180, AT-8, and PHF-1 are strongly suppressed. The same is true for the inhibitor HD (Figure 8a, lane 2). However, there was an unexpected difference with regard to antibody 12E8, diagnostic for the KXGS motifs containing phospho-S262/S356. FL allows phosphorylation at these sites, and HD inhibits it, as seen in the Western blot (Figure 8a, lanes 2 and 3). Because neither GSK-3β nor cdk5 phosphorylate S262 or S356 (Godemann et al., 1999), the result suggests that HD is also an inhibitor of MARK2, the kinase phosphorylating these sites most efficiently. This was tested directly by kinase activity assays performed with recombinant MARK2 and GSK-3β in vitro (Figure 9). In the case of GSK-3β, we found comparably strong inhibition by HD (IC50 = 0.13 μM) and FL (IC50 = 0.55 μM; Figure 9). In the case of MARK2, only HD is a strong inhibitor (IC50 = 0.67 μM).
Figure 8.
Effect of kinase inhibitors on phosphorylation of htau23 in Sf9 cells. (a and b) Antibody blots. (c and f) 2D phosphopeptide maps. (a) Lane 1, no inhibitor (control). Lane 2, 50 μM HD. Lane 3, 50 μM FL. From top to bottom: Coomassie-stained gel (control) showing roughly equal amounts of tau; Western blot with antibody K9JA that recognizes tau independently of phosphorylation; antibody 12E8 (against phosphorylated KXGS motifs). The inhibitor HD prevents phosphorylation (lane 2) because it inhibits MARK and related kinases, and not because of its inhibition of GSK-3β (see below). LiCl and PKA inhibitors have no pronounced effect (Figure 8b, lanes 1 and 2). Antibody AT-100 (against phospho-T212 and S214). This epitope requires the activity of GSK-3β (to phosphorylate T212), and PKA (to phosphorylate S214; Zheng-Fischhofer et al., 1998). Therefore, the epitope is blocked by both HD and FL (lanes 2 and 3). Antibody AT-180 (against phospho-T231 and S235). This is an epitope induced by cdk5 and GSK-3β, and thus the signal is blocked by both HD and FL (lanes 2 and 3). Antibody AT-8 (against phospho-S202 and T205). This is an epitope induced mainly by cdk5, and therefore the signal is blocked by both HD and FL (lanes 2 and 3). Antibody PHF-1 (against phospho-S396 and S404). This epitope is phophorylated mainly by GSK-3β, and therefore the signal is inhibited by HD, but less by FL. (b) Lane 1, 50 mM LiCl. Lane 2, 50 μM H89, a PKA inhibitor. (c–f) Two-dimensional phosphopeptide maps of htau23 protein expressed in Sf9 cells and phosphorylated by endogenous kinases without (c), or in the presence of kinase inhibitors (d–f). (c) Control, tau expressed in Sf9 cells. (d) Inhibition by 50 μM HD to inhibit cdk5, GSK-3β, and MARK. (e) Inhibition by 50 mM LiCl to inhibit GSK-3β. (f) Inhibition by 50 μM FL to inhibit cdk5 and GSK3β; note that c, e, and f show spots for phosphorylated S262 (circle, also see insert in e at higher exposure), d does not because HD inhibits MARK activity.
Figure 9.
In vitro inhibition assay of kinases of tau by inhibitors HD and FL. (a) MARK2. (b) GSK3β. Note that GSK-3β is inhibited by HD (IC50 = 0.13 μM) and FL (IC50 = 0.55 μM), but MARK2 is inhibited mainly by HD (IC50 = 0.67 μM), whereas FL is only a weak inhibitor (IC50 = 38 μM).
Additional control experiments on Sf9 cells were done with LiCl, a specific inhibitor of GSK-3β (Stambolic et al., 1996), and H89, an inhibitor of PKA (Chijiwa et al., 1990). These experiments were prompted by our previous observations that PKA can phosphorylate the KXGS motifs of tau in vitro, albeit with low efficiency (Schneider et al., 1999), and by conflicting reports that GSK-3β could also phosphorylate KXGS motifs (Moreno et al., 1995; Godemann et al., 1999). We tested these two kinases with their inhibitors (LiCl for GSK-3β, H89 for PKA) and found that the reaction with the antibody 12E8 against pSer262 was not affected (Figure 8b, lanes 1 and 2, compare with control in Figure 8a, lane 1); in contrast, 50 μM HD suppressed the reaction with antibody 12E8 (Figure 8a, lane 2). This was confirmed by phosphopeptide mapping of tau in Sf9 cells after treatment with 50 mM LiCl to inhibit GSK-3β (see below, Figure 8e, circled spot). Furthermore, the quantification of tau-induced cell processes of Sf9 cells after treatment with inhibitors LiCl or H89 showed a slight increase, rather than the pronounced decrease observed with the MARK inhibitor HD (Figure 7e). This suggests that GSK-3β and PKA are not responsible for the phosphorylation of KXGS motifs in tau during cell process formation.
Finally, we performed metabolic labeling of Sf9 cells with 32P and analyzed the phosphorylation state of tau by phosphopeptide mapping (Figure 8, c–f). The reference map of tryptic phosphopeptides of tau expressed in Sf9 cells is shown in Figure 8c; the identification of the spots is described in detail elsewhere (Illenberger et al., 1998; Biernat and Mandelkow, 1999). The spots of pS262 and pS356 are rather weak compared with those of the SP or TP motifs. Nevertheless, the spot of pS262 is clearly visible in the maps obtained with LiCl or FL (Figure 8, e and f, circles, see inset), but not with HD (Figure 8d, circle). This confirms that HD is also an inhibitor of MARK2, and that GSK-3β or cdk5 are not responsible for the phosphorylation of tau at S262 in Sf9 cells.
The results with kinase inhibitors explain the antibody reactions (disappearance of 12E8 staining in the presence of inhibitor HD), but more importantly they explain the response of the cells to inhibitor treatment. As noted above, phosphorylation at KXGS motifs is essential for process formation. Because this is achieved by MARK2, the inhibitor HD (but not FL) suppresses the cell processes (Figure 7, d and e) because it is also an inhibitor of MARK2 and not because it is also an inhibitor of GSK-3β and cdk5.
DISCUSSION
In this study, we asked how the function of tau, a protein known to initiate axonal outgrowth, is regulated by phosphorylation, and what kinases are responsible for it. We argue that the phosphorylation of tau at its KXGS motifs by the kinase MARK is critical in neurons and nonneuronal cell models (N2a and Sf9 cells). Because these cell types contain little or no endogenous tau, cell processes can be strongly enhanced by transfection with exogenous tau. The evidence for the importance of MARK is based on experiments where we changed either the activity of the regulator kinase MARK or the sites on its effector protein tau. The function of tau was changed by point mutations at the target sites of MARK by replacing the KXGS motifs with nonphosphorylatable KXGA motifs. KXGA-tau was not able to stimulate neurite outgrowth in N2a cells. The activity of MARK was changed in three ways: 1) transient transfection of cells with MARK2, one of the MARK isoforms, which can be activated by phosphorylation at the activating loop (Drewes et al., 1997); 2) transfection with a dominant negative MARK lacking the regulatory phosphorylation sites (T208A and S212A); and 3) inactivation of MARK by hymenialdisine, a new kinase inhibitor (Meijer et al., 2000). When MARK was inactivated, no stimulation of neurite outgrowth occurred.
Because tau contains many phosphorylation sites, an obvious issue is to distinguish their effects on the functions of tau. This is a common problem in the field because tau's phosphorylation in cells is often detected by antibodies that may vary in affinity and specificity. Many of the “Alzheimer-diagnostic” antibodies are directed against phospho-SP or -TP motifs, suggesting the activity of proline-directed kinases (e.g., MAP kinase, GSK-3β, or cdk5). In our case, we can rule out a major role of these kinases for tau-induced neurite outgrowth because the AP-tau mutant (where all SP or TP motifs were turned into AP) shows a similar or greater stimulation of neurites than normal tau, contrary to the KXGA-mutant of tau. This agrees with previous observations on Sf9 cells (Biernat and Mandelkow, 1999). Other, non-KXGS or non-SP/TP phosphorylation sites are also of minor importance because they represent a minor fraction of cellular phosphorylation sites (Watanabe et al., 1993, Illenberger et al., 1998), and they are not phosphorylated by MARK so that they cannot explain the effects of MARK inhibition (Drewes et al., 1995). A special issue is the possible phosphorylation of KXGS motifs by two other kinases, GSK-3β or PKA. In one study GKS-3β was thought to phosphorylate tau at S262 (Moreno et al., 1995), but our subsequent analysis showed that this was due to other activities in the kinase preparation (Godemann et al., 1999). This is confirmed herein by the LiCl experiment that inhibits the GKS-3β targets on tau (SP or TP motifs; Figure 8b, lane 1, PHF1 staining) without affecting the KXGS sites (Figure 8b, lane 1, staining with 12E8, and Figure 8e, spot of phospho-S262 in inset). PKA remains another possibility; it indeed phosphorylates tau at several sites, including the KXGS motifs (albeit much less efficiently than MARK; Drewes et al., 1995; Schneider et al., 1999). However, inhibition of PKA by H89 did not inhibit process formation (Figure 7e), contrary to inhibition of MARK by HD and did not inhibit the phosphorylation of KXGS motifs (Figure 8b, lane 2, staining with 12E8). We also showed in an in vitro activity assay (Figure 9) that hymenialdisine is able to inhibit MARK, but not PKA. Furthermore, the treatment of N2a/htau40 cells with okadaic acid led to the increase in phosphorylation at KXGS motifs only after transfecting the cells with MARK2 (but not with dnMARK2), nor in cells without transfected MARK2 (Figure 5e, lanes 1–3). This suggests that no other kinase phosphorylates the KXGS-motifs of tau in differentiating N2a cells even under the conditions of okadaic acid, pointing to MARK2 as the responsible kinase. Finally, in living cells GFP-MARK2 (but not dnMARK2) colocalizes with MARK2 activity (as shown by the p-MARK antibody) and phospho-KXGS tau (Figure 5, a–c). Collectively, the biochemical and immunofluorescence evidence suggests that MARK2 or a kinase of the MARK family is the kinase phosphorylating tau at KXGS motifs in differentiating N2a cells.
Microtubule stability is considered to be necessary for neurites because they provide mechanical strength and tracks for the intracellular transport. The phosphorylation of the KXGS motifs of tau during neurite outgrowth tends to detach tau from microtubules and thus renders microtubules less stable. This is the opposite of what one would expect intuitively, and therefore the question arises why this should be important for neurite outgrowth. The answer may lie in a more subtle role of microtubules: As the growth cone advances, actin filaments prepare the ground in the form of transient lamellipodia and filopodia. This ground is probed by pioneering microtubules that make temporary excursions into the actin meshwork and retract again, until finally a decision is made to stabilize them, form bundles, and allow the growth cone to advance (reviewed in Borisy and Svitkina, 2000; Bradke and Dotti, 2000; Goode et al., 2000). Thus, microtubules must be dynamically instable before neurites can extend. This explains why the suppression of dynamic instability suffices to block the growth of neurites, even when the polymer mass is not changed (Baas and Ahmad, 1993; Liao et al., 1995; Tanaka et al., 1995; Rochlin et al., 1996; Kaverina et al., 1998). The cell seems to use tau or related MAPs for this regulation (Drubin and Kirschner, 1986; Panda et al., 1999). Tau is present in the growth cone, but it is largely detached from microtubules (Black et al., 1996). Why should this pool of tau not stabilize microtubules so that they can advance out of the shaft of the neurite? We suggest that tau is locally phosphorylated at the KXGS motifs, is therefore unable to bind to microtubules (thus rendering them unstable), and relocates to the actin network (Figure 5c; Ozer and Halpain, 2000, for the analogous case of MAP2). Only a small fraction of microtubules are highly dynamic (Waterman-Storer and Salmon, 1997; Kabir et al., 2001); this explains why the overall extent of KXGS-phosphorylation is low in normal cells, in spite of its crucial role (Biernat and Mandelkow, 1999).
It is interesting to compare our results with two other studies on the phosphorylation of tau in neurons. Mandell and Banker (1996) argued that tau phosphorylation was lowest at the distal end of the neurite where microtubules are most dynamic. This seemed puzzling because unphosphorylated tau is often considered tantamount to stable microtubules. The finding can be explained by noting that the authors had used the antibody AT-8, which senses two phosphorylated SP and TP motifs outside the repeat domain (S202 and T205), but does not recognize the phosphorylation at KXGS motifs. We have shown elsewhere that the phosphorylation at the SP or TP motifs sites has only a modest effect on the tau-microtubule binding (Biernat et al., 1993), and indeed all proline-directed sites combined have no major influence on neurite outgrowth (Figure 2f), in contrast to the KXGS motifs. Overall, the role of proline-directed phosphorylation of tau is poorly understood and may be important in other contexts, for example, protection against degradation (Litersky and Johnson, 1995), cell division (Ookata et al., 1997; Illenberger et al., 1998), and compartmentalization in neurons (Binder et al., 1985; Hirokawa et al., 1996).
In a related study, Ozer and Halpain (2000) investigated the phosphorylation of MAP2 in HeLa cells. This MAP is similar to tau and the endogenous HeLa MAP4 by having homologous repeats, including KXGS motifs, and is sorted into the somatodendritic compartment of neurons (in contrast to axonal tau). The authors found that the KXGS motifs of MAP2 could be phosphorylated by PKA, resulting in the dissociation of MAP2 from microtubules and translocation to the actin network. This would be consistent with the fact that PKA binds to MAP2 through its regulatory RII subunit (Obar et al., 1989), which is not the case for tau. The common denominator for their results and ours would be that the cell needs to control the dynamics of microtubules by phosphorylating the locally available MAPs at their KXGS motifs. This could be tau in axons, MAP2 in dendrites, and presumably MAP4 in other cell types. The phosphorylation could be achieved by different kinases (e.g., MARK or PKA), with the same result of detaching the MAPs from the microtubules. Alternatively, it is possible that there is a more complex mechanism involving activating kinases and/or phosphatases. In our experimental system we found no evidence for a major role of PKA in initiating neurite outgrowth, consistent with a role of kinases of the MARK/PAR-1 family in establishing cell polarity (Drewes et al., 1998; Kemphues, 2000, Shulman et al., 2000; Tomancak et al., 2000).
Neurons contain several MAPs with different distributions and partially overlapping functions. For example, tau and MAP1b can substitute for one another during axonal growth in different extracellular environments (DiTella et al., 1996). A transgenic mouse lacking only tau is viable, but a mouse model lacking both tau and MAP1b shows severe defects in axonogenesis (Harada et al., 1994; Takei et al., 2000). The complementarity extends even to molecular properties: Phosphorylation of tau at certain sites detaches tau from microtubules and makes them more dynamic, whereas certain phosphorylations of MAP1b promote the binding to microtubules, and in both cases there is a gradient along axons, with the lower affinity species near the growth cone (Ulloa et al., 1994). Down-regulation of GSK-3β by the WNT signaling pathway or inhibition by lithium induces the dephosphorylation of MAP1b, its detachment from microtubules, and hence axonal remodeling such as increased growth cone size and branching (Lucas et al., 1998). Because hymenialdisine is an inhibitor not only of MARK but also of GSK-3β (Meijer et al., 2000), one could argue that its effect on neurite outgrowth might be mediated by GSK-3β and possibly MAP1b. This can however be ruled out because two other inhibitors of GSK-3β, lithium and flavopiridol, do not reproduce the effects of HD. Furthermore, the effects of MARK inhibition mediated by tau (i.e., inhibition of neurite outgrowth) are opposite from the effects of GSK-3β inhibition mediated by MAP1b (growth cone remodeling). In other words, the inhibition of MARK reduces microtubule dynamics, whereas the inhibition of GSK-3β enhances dynamics.
Finally, we note that there is growing evidence for an interplay between microtubule and actin dynamics mediated by phosphorylation of accessory components during neurite outgrowth. Several kinases have been described that are docked on one or both of these fiber systems and promote their organization, e.g., c-Abl, PAK5, or protein kinase C (Kabir et al., 2001; Dan et al., 2002; Woodring et al., 2002), and MARK would be another case in point. Usually, this takes place in tight coordination with small G proteins of the rho family (Daub et al., 2001; Palazzo et al., 2001). The phosphorylation of MAPs could regulate different subaspects of microtubule behavior in the growth cone, such as microtubule bundling in the shaft, dynamic instability of the pioneer microtubules, activation of tubulin scavengers such as stathmin, and anchoring at focal contacts. Furthermore, MAPs can interact with components other than microtubules; such as the actin cytoskeleton (Griffith and Pollard, 1982; DiTella et al., 1994; Cunningham et al., 1997; Ozer and Halpain, 2000). This may explain why different kinases have overlapping effects on growth cone behavior, presumably in response to different external signals whose nature remains to be elucidated.
ACKNOWLEDGMENTS
We thank Kerstin Neumann and Natalie Habbe for excellent technical assistance, Guangxun Meng for help with transfection experiments, Peter Davies (Albert Einstein College) for antibody PHF-1, Peter Seubert (Elan Pharma) for antibody 12E8, and George Pettit (University of Arizona) for the kinase inhibitor hymenialdisine. This work was supported by the Deutsche Forschungsgemeinschaft.
Abbreviations used:
- Cdk5
cdc2-like protein kinase-5
- HD
hymenialdisine
- FL
flavopiridol
- MAPK
mitogen-activated protein kinase
- MARK
microtubule affinity-regulating kinase
- MOI
multiplicity of infection
- PKA
protein kinase A
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–03–0046. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–03–0046.
REFERENCES
- Ackmann M, Wiech H, Mandelkow E. Non-saturable binding indicates clustering of tau on the microtubule surface in a paired helical filament-like conformation. J Biol Chem. 2000;275:30335–30343. doi: 10.1074/jbc.M002590200. [DOI] [PubMed] [Google Scholar]
- Baas P, Ahmad F. The transport properties of axonal microtubules establish their polarity orientation. J Cell Biol. 1993;111:495–509. doi: 10.1083/jcb.120.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Pienkowski TP, Kosik KS. Processes induced by tau expression in Sf9-cells have an axon-like microtubule organization. J Cell Biol. 1991;115:1333–1344. doi: 10.1083/jcb.115.5.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow S, Gonzalez-Garay ML, West R, Olmsted JB, Cabral F. Stable expression of heterologous microtubule-associated proteins (MAPs) in Chinese hamster ovary cells: evidence for differing roles of MAPs in microtubule organization. J Cell Biol. 1994;126:1017–1029. doi: 10.1083/jcb.126.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernat J, Gustke N, Drewes G, Mandelkow E-M, Mandelkow E. Phosphorylation of serine 262 strongly reduces the binding of tau protein to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993;11:153–163. doi: 10.1016/0896-6273(93)90279-z. [DOI] [PubMed] [Google Scholar]
- Biernat J, Mandelkow E-M. The development of cell processes induced by tau protein requires phosphorylation of serine 262 and 356 in the repeat domain and is inhibited by phosphorylation in the proline-rich domains. Mol Biol Cell. 1999;10:727–740. doi: 10.1091/mbc.10.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder LI, Frankfurter A, Rebhun L. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM, Slaughter T, Moshiach S, Obrocka M, Fischer I. Tau is enriched on dynamic microtubules in the distal region of growing axons. J Neurosci. 1996;16:3601–3619. doi: 10.1523/JNEUROSCI.16-11-03601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm H, Brinkmann V, Drab M, Henske A, Kurzchalia TV. Mammalian homologues of C. elegans PAR-1 are asymmetrically localized in epithelial cells and may influence their polarity. Curr Biol. 1997;7:603–606. doi: 10.1016/s0960-9822(06)00260-0. [DOI] [PubMed] [Google Scholar]
- Borisy GG, Svitkina TM. Actin machinery: pushing the envelope. Curr Opin Cell Biol. 2000;12:104–112. doi: 10.1016/s0955-0674(99)00063-0. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr Opin Neurobiol. 2000;10:574–581. doi: 10.1016/s0959-4388(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Brandt R, Leger J, Lee G. Interaction of tau with the neural plasma membrane mediated by tau's amino-terminal projection domain. J Cell Biol. 1995;131:1327–1340. doi: 10.1083/jcb.131.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Hutchings C, Burke JF, Mayne LV. Application of a rapid method (targeted display) for the identification of differentially expressed mRNAs following NGF-induced neuronal differentiation in PC12 cells. Mol Cell Neurosci. 1999;13:119–130. doi: 10.1006/mcne.1999.0736. [DOI] [PubMed] [Google Scholar]
- Butner KA, Kirschner MW. Tau-protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol. 1991;115:717–730. doi: 10.1083/jcb.115.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A, Kosik KS. Inhibition of neurite polarity by tau antisense oligonucleotide in primary cerebellar neurons. Nature. 1990;343:461–463. doi: 10.1038/343461a0. [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- Cleveland DW, Hwo S-Y, Kirschner MW. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol. 1977;116:227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Leclerc N, Flanagan L, Lu M, Janmey P, Kosik K. Microtubule-associated protein 2c reorganizes both microtubules and microfilaments into distinct cytological structures in an actin-binding protein-280-deficient melanoma cell line. J Cell Biol. 1997;136:845–857. doi: 10.1083/jcb.136.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan C, Nath N, Liberto M, Minden A. PAK5, a brain-specific kinase, promotes neurite outgrowth in N1E-115 cells. Mol Cell Biol. 2002;22:567–577. doi: 10.1128/MCB.22.2.567-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. Rac/cdc42 and p65PAK regulated the microtubule-destablizing protein stathmin through phosphorylation at serine 16. J Biol Chem. 2001;276:1677–1680. doi: 10.1074/jbc.C000635200. [DOI] [PubMed] [Google Scholar]
- Delacourte A, Buee L. Tau pathology: a marker of neurodegenerative disorders. Curr Opin Neurol. 2000;13:371–376. doi: 10.1097/00019052-200008000-00002. [DOI] [PubMed] [Google Scholar]
- DiTella MC, Feiguin F, Carri N, Kosik KS, Caceres A. MAP-1B/TAU functional redundancy during laminin-enhanced axonal growth. J Cell Sci. 1996;109:467–477. doi: 10.1242/jcs.109.2.467. [DOI] [PubMed] [Google Scholar]
- DiTella M, Feiguin F, Morfini G, Caceres A. Microfilament-associated growth cone component depends upon Tau for its intracellular localization. Cell Motil Cytoskeleton. 1994;29:117–130. doi: 10.1002/cm.970290204. [DOI] [PubMed] [Google Scholar]
- Drewes G, Trinczek B, Illenberger S, Biernat J, Schmitt-Ulms G, Meyer HE, Mandelkow E-M, Mandelkow E. MAP/microtubule affinity regulating kinase (P110/mark): a novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site Serine 262. J Biol Chem. 1995;270:7679–7688. doi: 10.1074/jbc.270.13.7679. [DOI] [PubMed] [Google Scholar]
- Drewes G, Ebneth A, Mandelkow E-M. MAPs, MARKs, and microtubule dynamics. Trends Biochem Sci. 1998;23:307–311. doi: 10.1016/s0968-0004(98)01245-6. [DOI] [PubMed] [Google Scholar]
- Drewes G, Ebneth A, Preuss U, Mandelkow E-M, Mandelkow E. MARK: a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Drubin D, Kirschner M. Tau protein function in living cells. J Cell Biol. 1986;103:2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebneth A, Drewes G, Mandelkow E-M, Mandelkow E. Phosphorylation of MAP2c and MAP4 by MARK kinases leads to the destabilization of microtubules in cells. Cell Motil Cytoskeleton. 1999;44:209–224. doi: 10.1002/(SICI)1097-0169(199911)44:3<209::AID-CM6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E-M, Mandelkow E. Overexpression of tau protein alters kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer's disease. J Cell Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson K, Weisshaar B, Matus A. Actin depolymerization induces process formation on MAP2-transfected nonneuronal cells. Development. 1993;117:689–700. doi: 10.1242/dev.117.2.689. [DOI] [PubMed] [Google Scholar]
- Esmaeli-Azad B, Mccarty JH, Feinstein SC. Sense and antisense transfection analysis of tau-function: tau influences net microtubule assembly, neurite outgrowth and neuritic stability. J Cell Sci. 1994;107:869–879. doi: 10.1242/jcs.107.4.869. [DOI] [PubMed] [Google Scholar]
- Godemann R, Biernat J, Mandelkow E, Mandelkow E-M. Phosphorylation of tau protein by recombinant GSK-3β: pronounced phosphorylation at select Ser/Thr-Pro motifs but no phosphorylation at Ser262 in the repeat domain. FEBS Lett. 1999;454:157–164. doi: 10.1016/s0014-5793(99)00741-3. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini M, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein-tau: sequences and localization in neurofibrillary tangles of Alzheimers-disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol. 2000;12:63–71. doi: 10.1016/s0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Griffith LM, Pollard TM. The interaction of actin filaments with microtubules and microtubule-associated proteins. J Biol Chem. 1982;257:9143–9151. [PubMed] [Google Scholar]
- Guo S, Kemphues KJ. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell. 1995;81:611–620. doi: 10.1016/0092-8674(95)90082-9. [DOI] [PubMed] [Google Scholar]
- Gustke N, Trinczek B, Biernat J, Mandelkow E-M, Mandelkow E. Domains of tau protein and interactions with microtubules. Biochemistry. 1994;33:9511–9522. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase catalytic domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N. Altered microtubule organization in small-caliber axons of mice lacking tau protein. Nature. 1994;369:488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Funakoshi T, Satoharada R, Kanai Y. Selective stabilization of tau in axons and microtubule-associated protein 2c in cell-bodies and dendrites contributes to polarized localization of cytoskeletal proteins in mature neurons. J Cell Biol. 1996;132:667–679. doi: 10.1083/jcb.132.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illenberger S, Drewes G, Trinczek B, Biernat J, Meyer HE, Olmsted JB, Mandelkow E-M, Mandelkow E. Phosphorylation of microtubule-associated proteins MAP2 and MAP4 by the protein kinase p110-MARK: phosphorylation sites and regulation of microtubule dynamics. J Biol Chem. 1996;271:10834–10843. doi: 10.1074/jbc.271.18.10834. [DOI] [PubMed] [Google Scholar]
- Illenberger S, Zheng-Fischhofer Q, Preuss U, Stamer K, Baumann K, Trinczek B, Biernat J, Godemann R, Mandelkow E-M, Mandelkow E. The endogenous and cell-cycle dependent phosphorylation of tau protein in living cells: implications for Alzheimer's disease. Mol Biol Cell, 1998;9:1495–1512. doi: 10.1091/mbc.9.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imahori K, Uchida T. Physiology and pathology of tau protein kinases in relation to Alzheimer's disease. J Biochem. 1997;121:179–188. [PubMed] [Google Scholar]
- Jenkins SM, Johnson GVW. Phosphorylation of microtubule-associated protein tau on Ser 262 by an embryonic 100 kDa protein kinase. Brain Res. 1997;767:305–313. doi: 10.1016/s0006-8993(97)00615-x. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Wilson L. Use of drugs to study role of microtubule assembly dynamics in living cells. Methods Enzymol. 1998;298:252–76. doi: 10.1016/s0076-6879(98)98024-7. [DOI] [PubMed] [Google Scholar]
- Kabir N, Schaefer AW, Nakhost A, Sossin WS, Forscher P. Protein kinase C activation promotes microtubule advance in neuronal growth cones by increasing average microtubule growth lifetimes. J Cell Biol. 2001;152:1033–1044. doi: 10.1083/jcb.152.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Takemura R, Oshima T, Mori H, Ihara Y, Yanagisawa M, Masaki T, Hirokawa N. Expression of multiple tau isoforms and microtubule bundle formation in fibroblasts transfected with a single tau cDNA. J Cell Biol. 1989;109:1173–1184. doi: 10.1083/jcb.109.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I, Rottner K, Small JV. Targeting, capture, and stabilization of microtubules in early focal adhesions. J Cell Biol. 1998;142:181–190. doi: 10.1083/jcb.142.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues K. PARsing embryonic polarity. Cell. 2000;101:345–348. doi: 10.1016/s0092-8674(00)80844-2. [DOI] [PubMed] [Google Scholar]
- Knops J, Kosik K, Lee G, Pardee J, Cohen-Gould L, McConlogue L. Overexpression of tau in a nonneuronal cell induces long cellular processes. J Cell Biol. 1991;114:725–733. doi: 10.1083/jcb.114.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R, Leclerc N, Kosik KS. Organization of actin and microtubules during process formation in tau-expressing Sf9 cells. Cell Motil Cytoskeleton. 1994;28:256–264. doi: 10.1002/cm.970280308. [DOI] [PubMed] [Google Scholar]
- Leclerc S, et al. Indirubins inhibit glycogen synthase kinase-3b and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer's disease: a property common to most CDK inhibitors? J Biol Chem. 2001;276:251–260. doi: 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
- Lee VMY, Balin BJ, Otvos L, Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Lee G, Cowan N, Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988;239:285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- Lee G, Newman ST, Gard DL, Band H, Panchamoorthy G. Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci. 1998;111:3167–3177. doi: 10.1242/jcs.111.21.3167. [DOI] [PubMed] [Google Scholar]
- Leger JG, Brandt R, Lee G. Identification of tau protein regions required for process formation in PC12 cells. J Cell Sci. 1994;107:3403–3412. doi: 10.1242/jcs.107.12.3403. [DOI] [PubMed] [Google Scholar]
- Levin DE, Bishop JM. A putative protein-kinase gene (kin1+) is important for growth polarity in Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1990;87:8272–8276. doi: 10.1073/pnas.87.21.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Li Y, Brautigan DL, Gundersen GG. Protein phosphatase 1 is targeted to microtubules by the microtubule-associated protein tau. J Biol Chem. 1998;273:21901–21908. doi: 10.1074/jbc.273.34.21901. [DOI] [PubMed] [Google Scholar]
- Liao G, Nagasaki T, Gundersen GG. Low concentrations of nocodazole interfere with fibroblast locomotion without significantly affecting microtubule level: implications for the role of dynamic microtubules in cell locomotion. J Cell Sci. 1995;108:3473–3483. doi: 10.1242/jcs.108.11.3473. [DOI] [PubMed] [Google Scholar]
- Litersky JM, Johnson GVW. Phosphorylation of tau in-situ: inhibition of calcium-dependent proteolysis. J Neurochem. 1995;65:903–911. doi: 10.1046/j.1471-4159.1995.65020903.x. [DOI] [PubMed] [Google Scholar]
- Lopez LA, Sheetz MP. A microtubule-associated protein (MAP2) kinase restores microtubule motility in embryonic brain. J Biol Chem. 1995;270:12511–12517. doi: 10.1074/jbc.270.21.12511. [DOI] [PubMed] [Google Scholar]
- Lucas F, Goold R, Gordon-Weeks P, Salinas P. Inhibition of GSK-3β leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J Cell Sci. 1998;111:1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- Mandell JW, Banker GA. A spatial gradient of tau-protein phosphorylation in nascent axons. J Neurosci. 1996;16:5727–5740. doi: 10.1523/JNEUROSCI.16-18-05727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow E-M, Mandelkow E. Tau in Alzheimer's disease. Trends Cell Biol. 1998;8:425–427. doi: 10.1016/s0962-8924(98)01368-3. [DOI] [PubMed] [Google Scholar]
- Meijer L, et al. Inhibition of cyclin-dependent kinases, G.S.K-3b and casein kinase 1 by hymenialdisine, a marine sponge constituent. Chem Biol. 2000;7:51–63. doi: 10.1016/s1074-5521(00)00063-6. [DOI] [PubMed] [Google Scholar]
- Moreno FJ, Medina M, Perez M, Degarcini EM, Avila J. Glycogen-synthase kinase-3 phosphorylates recombinant human tau protein at Serine-262 in the presence of heparin (or tubulin) FEBS Lett. 1995;372:65–68. doi: 10.1016/0014-5793(95)00934-2. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, Ihara Y. Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem. 1995;270:823–829. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Grindstaff KK. Cell polarity: par for the polar course. Curr Biol. 1997;7:R562–R564. doi: 10.1016/s0960-9822(06)00282-x. [DOI] [PubMed] [Google Scholar]
- Obar RA, Dingus J, Bayley H, Vallee RB. The RII subunit of cAMP-dependent protein-kinase binds to a common amino-terminal domain in microtubule-associated proteins 2a, 2b, and 2c. Neuron. 1989;3:639–645. doi: 10.1016/0896-6273(89)90274-2. [DOI] [PubMed] [Google Scholar]
- Ookata K, Hisanaga S, Sugita M, Okuyama A, Murofushi H, Kitazawa H, Chari S, Bulinski JC, Kishimoto T. MAP4 is the in-vivo substrate for cdc2 kinase in HeLa cells: identification of an M-phase specific and a cell cycle-independent phosphorylation site in MAP4. Biochemistry. 1997;36:15873–15883. doi: 10.1021/bi971251w. [DOI] [PubMed] [Google Scholar]
- Ozer R, Halpain S. Phosphorylation-dependent localization of microtubule-associated protein MAP2c to the actin cytoskeleton. Mol Biol Cell. 2000;11:3573–3587. doi: 10.1091/mbc.11.10.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo A, Cook T, Alberts A, Gundersen GG. m-Dia mediates Rho-regulated formation, and orientation of stable microtubules. Nat Cell Biol. 2001;3:723–729. doi: 10.1038/35087035. [DOI] [PubMed] [Google Scholar]
- Panda D, Miller HP, Wilson L. Rapid treadmilling of brain microtubules free of microtubule-associated proteins in vitro and its suppression by tau. Proc Natl Acad Sci USA. 1999;96:12459–12464. doi: 10.1073/pnas.96.22.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin MW, Wickline K, Bridgman P. Microtubule stability decreases axon elongation but not axoplasm production. J Neurosci. 1996;16:3236–3246. doi: 10.1523/JNEUROSCI.16-10-03236.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow E-M. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry. 1999;38:3549–3558. doi: 10.1021/bi981874p. [DOI] [PubMed] [Google Scholar]
- Seubert P, et al. Detection of phosphorylated Ser(262) in fetal tau, adult tau, and paired helical filament tau. J Biol Chem. 1995;270:18917–18922. doi: 10.1074/jbc.270.32.18917. [DOI] [PubMed] [Google Scholar]
- Shulman J, Benton R, St. Johnston D. The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localization to the posterior pole. Cell. 2000;101:377–388. doi: 10.1016/s0092-8674(00)80848-x. [DOI] [PubMed] [Google Scholar]
- Sontag E, Nunbhakdi-Craig V, Lee G, Brandt R, Kamibayashi C, Kuret J, White C, Mumby M, Bloom G. Molecular interactions among protein phosphatase 2A, tau, and microtubules. Implications for the regulation of tau phosphorylation and the development of tauopathies. J Biol Chem. 1999;274:25490–25498. doi: 10.1074/jbc.274.36.25490. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen-synthase kinase-3 activity and mimics wingless signaling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow E-M. Tau blocks traffic of organelles, neurofilaments, and APP-vesicles in neurons and enhances oxidative stress. J Cell Biol. 2002;156:1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Ho T, Kirschner MW. Microtubule behavior in the growth cones of living neurons during axon elongation. J Cell Biol. 1995;115:345–363. doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomancak P, Piano F, Riechmann V, Gunsalus KC, Kemphues KJ, Ephrussi A. A Drosophila melanogaster homologue of Caenorhabditis elegans par-1 acts at an early step in embryonic-axis formation. Nat Cell Biol. 2000;2:458–460. doi: 10.1038/35017101. [DOI] [PubMed] [Google Scholar]
- Takei Y, Teng J, Harada A, Hirokawa N. Defects in axonal elongation and neuronal migration in mice with disrupted tau and MAP1b genes. J Cell Biol. 2000;150:989–1000. doi: 10.1083/jcb.150.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa L, Montejo de Garcini E, Gomez-Ramos P, Moran MA, Avila J. Microtubule-associated protein MAP1B showing a fetal phosphorylation pattern is present in sites of neurofibrillary degeneration in brains of Alzheimer's disease patients. Brain Res Mol Brain Res. 1994;26:113–122. doi: 10.1016/0169-328x(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J Cell Biol. 1997;139:417–434. doi: 10.1083/jcb.139.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer C, Salmon ED. Positive feedback interactions between microtubule and actin dynamics during cell motility. Curr Opin Cell Biol. 1999;11:61–67. doi: 10.1016/s0955-0674(99)80008-8. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Hasegawa M, Suzuki M, Takio K, Morishima-Kawashima M, Titani K, Arai T, Kosik KS, Ihara Y. In vivo phosphorylation sites in fetal and adult rat tau. J Biol Chem. 1993;268:25712–25717. [PubMed] [Google Scholar]
- Woodring PJ, Litwack ED, O'Leary DD, Lucero GR, Wang JY, Hunter T. Modulation of the F-actin cytoskeleton by c-Abl tyrosine kinase in cell spreading and neurite extension. J Cell Biol. 2002;156:879–892. doi: 10.1083/jcb.200110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng-Fischhofer Q, Biernat J, Mandelkow E-M, Illenberger S, Godemann R, Mandelkow E. Sequential phosphorylation of tau-protein by GSK-3b and protein kinase A at Thr212 and Ser214 generates the Alzheimer-specific epitope of antibody AT100 and requires a paired helical filament-like conformation. Eur J Biochem. 1998;252:542–552. doi: 10.1046/j.1432-1327.1998.2520542.x. [DOI] [PubMed] [Google Scholar]