Abstract
The insulin-like growth factor type I (IGF-I) receptor (IGF-IR), activated by its ligands IGF-I and IGF-II, can initiate several signal transduction pathways that mediate suppression of apoptosis, proliferation, differentiation, and transformation. Here we investigated the regulation of IGF-IR activation and function by protein tyrosine phosphatase 1B (PTP-1B). Coexpression of PTP-1B with a β-chain construct of the IGF-IR (βWT) inhibited IGF-IR kinase activity in fission yeast Schizosaccharomyces pombe, in COS cells, and in IGF-IR-deficient fibroblasts. In both spontaneously immortalized and simian virus 40 T antigen-transformed embryonic fibroblast cell lines derived from PTP-1B knockout mice, IGF-I induced higher levels of IGF-IR autophosphorylation and kinase activity than were induced in PTP-1B-expressing control cells. PTP-1B-deficient cells exhibited enhanced IGF-I-mediated protection from apoptosis in response to serum withdrawal or etoposide killing, as well as enhanced plating efficiency and IGF-I-mediated motility. Reexpression of PTP-1B in spontaneously immortalized fibroblasts resulted in decreased IGF-IR and AKT activation, as well as decreased protection from apoptosis and decreased motility. These findings demonstrate that PTP-1B can regulate IGF-IR kinase activity and function and that loss of PTP-1B can enhance IGF-I-mediated cell survival, growth, and motility in transformed cells.
The type I insulin-like growth factor (IGF-I) receptor (IGF-IR) is a transmembrane tyrosine kinase receptor that is very similar to the insulin receptor (IR) (51). These receptors are derived from a common ancestral receptor, Daf-2, which is expressed on cells of the nematode Caenorhabditis elegans (24). An evolutionarily conserved signaling pathway that is activated by the IR/IGF-IR via recruitment of the IR substrate (IRS) proteins and that leads to activation of phosphatidylinositol (PI) 3-kinase and AKT (3, 13) was recently shown to control cell survival, stress responses, and life span in yeast, C. elegans, and Drosophila melanogaster (9, 49, 58).
In mammalian cells both the IGF-IR and IR are widely expressed, and while the IR has a well-documented role in regulating glucose metabolism (23, 43), the IGF-IR is essential for normal embryonic growth and development and mediates signals for suppression of apoptosis, differentiation, and mitogenesis (for reviews see references 1, 35, and 54). Both receptors can recruit the IRS proteins and activate the PI 3-kinase/AKT pathway (52). IGF-IR is permissive for the transformation of cells by certain oncogenes and viruses (47), and circulating IGF-I and IGF-II are also associated with the transformation and progression of several types of cancer (59). Interestingly, domains in the C terminus of the IGF-IR that are not conserved in the IR are required for the antiapoptotic activity and transforming activity of the IGF-IR (36), which suggests that the C terminus of the IGF-IR may have evolved to regulate some of the events controlling cellular transformation.
A number of signaling pathways that mediate proliferation, suppression of apoptosis, and transformation can be activated by the IGF-IR. In addition to the PI 3-kinase/AKT pathway (13), the IGF-IR can activate the mitogen-activated protein kinase (MAPK) pathways (28), it can translocate c-Raf to the mitochondria (47), and it can transiently activate c-Jun N-terminal kinases (JNKs) (26).
By contrast, very little is known about the reciprocal dephosphorylation events that serve to terminate IGF-IR activation and consequently control its downstream signaling pathways. There is increasing evidence that regulation of growth and survival signaling pathways by phosphatases (50) contributes significantly to tumor growth and development. PTEN, which regulates AKT activation by dephosphorylating the phospholipid products of PI 3-kinase, is absent or mutated in advanced stages of several cancers, and this is associated with enhanced tumor cell survival and angiogenesis (12, 57). MKP-1, which regulates MAPKs, in particular JNK, has decreased expression in advanced stages of esophageal, prostate, colon, and bladder cancers (5, 6, 29). In addition, PTP-1B has been shown to antagonize the transforming activity of the BCR-Abl protein in cell lines (27).
Protein tyrosine phosphatase 1B (PTP-1B) and leukocyte antigen-related protein are well-characterized regulators of IR kinase activity and signaling (19, 46, 55, 60), and PTP-1B substrate-trapping mutants have been shown to also interact with the IGF-IR in vitro (22). However, regulation of IGF-IR kinase or its signaling pathways by either PTP-1B or other phosphatases has not been demonstrated in vivo. PTP-1B knockout mice exhibited enhanced insulin sensitivity in certain tissues and resistance to obesity (14, 25), but intriguingly they exhibited no apparent defects associated with IGF-IR function, being of normal size and having no increased incidence of cancer. This raises the possibility that PTP-1B does not regulate IGF-IR activity in vivo during development or during the lifetimes of these mice and that specific phosphatases might differentially regulate the IR and the IGF-IR. Knowledge of IGF-IR regulatory phosphatases is important because they could potentially control cell survival and differentiation as well as play a role in limiting cancer progression.
In an effort to identify regulators of IGF-IR kinase activity and in particular to investigate the role of PTP-1B in IGF-IR function, we first utilized the fission yeast Schizosaccharomyces pombe as a model system (48) to identify IGF-IR regulatory tyrosine phosphatases. Using this approach we found that the IGF-IR β chain, expressed in S. pombe as an active kinase, is inhibited by coexpression of PTP-1B. PTP-1B also inhibited IGF-IR kinase activity in COS cells and fibroblasts. We then examined the functional consequences of PTP-1B inhibition of IGF-IR kinase activity in fibroblastic cell lines derived from PTP-1B knockout mice. In response to IGF-I stimulation, cells lacking PTP-1B had increased IGF-IR autophosphorylation and kinase activity, enhanced protection from apoptosis, greater plating efficiency, and enhanced motility compared with control PTP-1B+/+ cells. Reexpression of PTP-1B in the knockout fibroblasts resulted in decreased IGF-IR autophosphorylation as well as AKT activation and also retarded IGF-I-induced antiapoptotic activity and motility. These findings demonstrate that PTP-1B can regulate IGF-IR kinase activity and that lack of PTP-1B can augment IGF-IR function in transformed cells.
MATERIALS AND METHODS
IGF-IR β-chain cloning and subcloning.
IGF-IR β-chain expression plasmids encoding the intracellular portion of the IGF-IR were constructed as follows. The sequences encoding the N-termini of βWT and βKD were amplified from the coding sequence for full-length wild-type IGF-IR and IGF-IR with the K1003R mutation, respectively, by using oligonucleotide primers MyBxb (5′-CGGCCTCGAGGATCCGCCACCATGGGGAGCAGCAAGAGCAAGCCCAAGGACCCCAGCCAGCGCCGGCGCAGAAAGAGAAATAACAGCAGG), which contains restriction sites for XhoI and BamHI and which incorporates the coding sequence for the first 16 amino acids of the Src myristoylation sequence, and HIII rev (5′-CATCACAGAAGCTTCGTTGAG), which corresponds to the region in the IGF-IR kinase domain containing a HindIII restriction site. The PCR products were subcloned into pKSβH3-C (which contains the coding sequence for the wild-type IGF-IR β chain from the HindIII site to the stop codon for the C terminus) via XhoI-HindIII digestion and subsequent ligation to generate IGF-IR β-chain constructs βWT and βKD from pKS. The entire coding sequences for βWT and βKD were then excised from pKS-βWT and pKS-βKD by BamHI digestion and ligated into S. pombe expression vector pRSP. The sequences of all DNA fragments amplified by PCR were confirmed by DNA sequence analysis.
For βWT studies with mammalian cells βWT was subcloned into the pIRES vector (Clontech, Basingstoke, Hampshire, United Kingdom). The coding sequence for human PTP-1B (hPTP-1B) was subcloned from pKS-hPTP-1B via SalI-NotI digestion into S. pombe constitutive expression vector pADH to generate pADH PTP-1B. To study the effects of PTP-1B on βWT in mammalian cells, coding sequences for wild-type PTP-1B and the D181A mutant were first subcloned from pWZL-hPTP-1B-WT or pWZL-hPTP-1B-D181A into pKS via BamHI-EcoRI and subsequently subcloned from the pKS vectors into pIRES via NotI-EcoRI to generate pIRES-hPTP-1B-WT and pIRES-hPTP-1B-D181A.
S. pombe expression system.
S. pombe strain SP200 (his leu1-32 ura4 ade6-210) was used in these studies and was cultured as described by Moreno et al. (33). Yeast cell transformation was performed by either the lithium acetate method (37) or electroporation (42). Transformants were selected based on their ability to grow on selective media, Leu− and Ura− for pRSP and pADH, respectively. To induce expression of proteins under the control of the nmt promoter, transformants were seeded in selective medium containing thiamine (5 μg/ml), grown to mid-log phase, and then washed five times in thiamine-free medium, followed by resuspension in medium with or without thiamine. Cultures were maintained in log phase following induction and were harvested by centrifugation after 16, 20, or 24 h for lysis and Western blot analysis.
Preparation of yeast cell lysates.
Cell pellets from S. pombe induction experiments were washed once in ice-cold Tris-buffered saline (TBS) containing tyrosine phosphatase inhibitor Na3VO4 (1 mM) and protease inhibitors phenylmethylsulfonyl fluoride (1 mM), pepstatin (1 μM), and aprotinin (1.5 μg/ml). Cells were then resuspended in an appropriate volume of modified radioimmunoprecipitation assay (RIPA) buffer (10 mM sodium phosphate buffer [pH 7.0], 150 mM NaCl, 50 mM NaF, 0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100, 0.5% deoxycholate) plus the above inhibitors. Lysis was achieved by the addition of glass beads (425 to 600 μm in diameter; Sigma-Aldrich Ireland Ltd.) and rigorous agitation at 4°C for 2 h. Lysates were retrieved from the beads by aspiration and a single washing with the starting volume of RIPA buffer and then cleared of debris and unlysed cells by centrifugation at 2,700 × g for 2 min.
Cell lines.
Wild-type (PTP-1B+/+) and knockout fibroblasts (PTP-1B−/−) immortalized by infection with the simian virus 40 (SV40) large T antigen were previously described (11). These are referred to as T murine embryonic fibroblasts (MEFs). To distinguish the effects of the SV40 large T antigen, we also generated spontaneously immortalized cell lines from primary PTP-1B wild-type and knockout fibroblasts by continual passaging of the cells. These are referred to as S MEFs. SR1 and SR5 cells are clones of S MEFs that reexpress PTP-1B (as described below). SR9 and SR10 cells are S MEF cells that serve as vector controls for SR1 and SR5 cells. All mammalian cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (FCS) and 5 mg of penicillin-streptomycin/ml (all from BioWhittaker, Verviers, Belgium).
Transfections and preparation of cell lysates.
For coexpression of PTP-1B and the IGF-IR β chain, COS cells and R− cells were transiently transfected with plasmids by using Lipofectamine Plus (Life Technologies). Cells were cultured for 24 h and then lysed by scraping them into an appropriate volume of NP-40 lysis buffer consisting of 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 1% NP-40 plus the above inhibitors.
Stable transfectants of the 1S MEF PTP-1B−/− cell line reexpressing PTP1B were generated by infection with a retrovirus vector encoding murine PTP-1B fused to the epitope tag Myc. Following infection the cells were subjected to selection in medium containing hygromycin (75 μg/ml), and the resulting clones were isolated following verification of PTP-1B expression. SR1 and SR5 clones express PTP-1B, and SR9 and SR10 cells do not express PTP-1B.
Cells were serum starved for 6 to 8 h and were either stimulated with 100 ng of IGF-I/ml for 15 min or left unstimulated. Cells were lysed by scraping them into an appropriate volume of RIPA buffer (10 mM sodium phosphate buffer [pH 7.2], 50 mM NaCl, 1% NP-40, 1% SDS, and 0.5% deoxycholate) plus the above inhibitors.
For each of the mammalian cell lines crude lysates were incubated for 15 min on ice and then cleared by centrifugation at 20,800 × g for 15 min. The protein concentration in lysates was determined by a Bradford microassay (Bio-Rad Laboratories GmbH, Munich, Germany).
Immunoprecipitations and IGF-IR kinase assays.
Prior to immunoprecipitation experiments, protein G-agarose beads were precoated with 1% (wt/vol) bovine serum albumin in 1% TBS-Triton X-100. Precoated beads (10 μl) were then added to 200 μg of total protein from stimulated or unstimulated PTP-1B+/+ or PTP-1B−/− MEF cell lysates and incubated for 1 to 2 h with gentle rocking at room temperature. The precleared lysates were retrieved from the beads by centrifugation at 960 × g for 3 min and incubated with 2 μg of an anti-IGF-IR-β antibody (Santa Cruz Biotechnology) overnight at 4°C with gentle rocking. The protein G-immunoprecipitated complexes were pelleted by centrifugation at 960 × g for 3 min at 4°C, washed three times in 500 μl of ice-cold RIPA buffer, and then either used for kinase assays or boiled for 5 min in 15 μl of 2× SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer for Western blot analysis.
For IGF-IR kinase assays the protein G complexes were washed in kinase buffer (50 mM HEPES [pH 7.4], 10 mM MgCl2, 10 mM MnCl2) and then resuspended in 25 μl of a kinase reaction mixture containing ATP (final concentration, 0.03 mM), 2 μl of [32P]ATP (5 μCi/μl), and 2 μl of poly(Glu-Tyr) (Sigma). Following a 20-min incubation period samples of the reaction mixture (5 μl) were removed to new tubes containing 9 μl of H2O and 35 μl of 20 mM EDTA, pH 7.4. Triplicate samples were then transferred to glass microfiber filters in 24-well plates and washed extensively with ice-cold trichloroacetic acid (10%) containing 10 mM Na2HPO4. Following a final wash with 70% ethanol, the filters were dried and 32P was measured with a scintillation counter (Beckman).
Western blot analysis.
All protein samples for Western blot analysis were resolved by 4 to 15% gradient SDS-PAGE. Following separation, proteins were transferred to nitrocellulose membranes and blocked for 1 h at room temperature in 5% milk (wt/vol) in TBS containing 0.5% Tween-20 (TBS-T). Primary-antibody incubations for phospho-specific antibodies were overnight at 4°C; those for most other primary antibodies were for 1 h at room temperature. Membranes were stripped by incubation in 62.5 mM Tris-Cl-1% SDS-0.7% β-mercaptoethanol for 30 min at 50°C, followed by extensive washing in TBS-T. Antiphosphotyrosine antibodies PY20 (Transduction Laboratories, Lexington, Ky.) and 4G10 (Upstate Biotechnology, Lake Placid, N.Y.) were used for S. pombe and mammalian-cell experiments, respectively. Expression of transfected hPTP-1B was confirmed with anti-hPTP-1B antibody (FG6; CN Biosciences, Nottingham, United Kingdom). Anti-phospho-AKT, anti-AKT, anti-phospho-p42/44 MAPK, anti-phospho-JNK, anti-JNK, and anti-phospho-p38 antibodies were all from Cell Signaling Technology (Beverly, Mass.). Anti-MAPK-2 and anti-SHP-1 antibodies were from Upstate Biotechnology. The anti-mouse PTP-1B (14) and anti-p130 Cas antibodies (4) have been described previously. Secondary antibodies conjugated with horseradish peroxidase (DAKO, Glostrup, Denmark) were used, and detection was by chemiluminescence (Super Signal; Pierce, Rockford, Ill.) for S. pombe experiments or ECL+ (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, United Kingdom) for mammalian-cell experiments.
Assessment of plating efficiency of the different cell lines.
Cells were seeded at 500 cells per well in 3 ml of Dulbecco's modified Eagle's medium-10% FCS in a six-well plate. The plates were incubated for 12 days at 37°C in 5% CO2, after which time the medium was removed and the resultant colonies were stained with Giemsa stain.
Cell survival assays.
Cells from each of the S MEF cell lines were plated in medium supplemented with 10% FCS at 6 × 104 cells per well of a 24-well plate. Twelve hours later the medium was replaced with serum-free medium alone or serum-free medium containing either 100 nM IGF-I alone, 5 μM etoposide alone, or both IGF-I and etoposide. The number of viable cells per well was determined after 36 h as follows. Each well was washed with 1× phosphate-buffered saline to remove dead cells and debris and then was coated with trypsin-EDTA to detach viable cells. These cells were then transferred to an Eppendorf tube and centrifuged at 960 × g for 3 min. The resulting cell pellets were resuspended in 100 μl of medium. Viable cells were then counted by using a hemocytometer and trypan blue exclusion. Each condition was tested in triplicate cultures, and duplicate samples from each culture were analyzed.
Monolayer wound repair to assess motilities of different cell lines.
Cells were seeded in multiple wells of 24-well plates at 4 × 104 to 6 × 104 cells per well and grown to 85 to 95% confluence. The medium was then removed, and a wound was scored in each well using a sterile 1-ml pipette tip. The cultures were reincubated with medium as outlined in the text, and the movement of cells across the wound was monitored by microscopic examination at regular intervals. At the indicated time points cultures were stained with Giemsa dye and multiple fields were photographed with a Nikon TE300 inverted microscope equipped with a SPOT digital camera and a 10× magnification objective.
RESULTS
Coexpression of PTP1B with the IGF-IR β chain in yeast and mammalian cells results in decreased IGF-IR kinase activity.
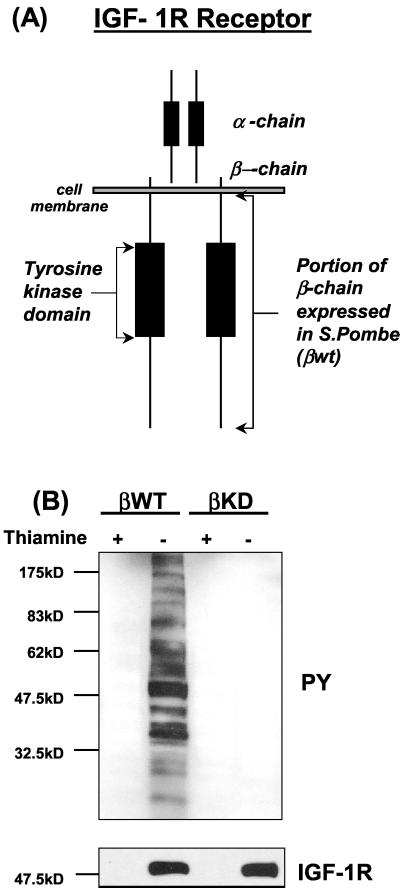
Analysis of the interactions of phosphatases with the IR and IGF-IR in vivo are complicated by the many tyrosine phosphorylation events taking place in a cell at any one time. One way around this is to use simpler eukaryotic cellular models such as yeast. The fission yeast S. pombe is a good model because it has homology with mammalian cells (61) but lacks endogenous tyrosine kinase activity. Therefore, we explored the use of the S. pombe as a host to investigate IGF-IR kinase regulation by phosphatases. Initial studies demonstrated that the β chain of the IGF-IR (βWT; residues 930 to 1337) could be expressed as an active tyrosine kinase in S. pombe (10). As shown in Fig. 1B, upon withdrawal of thiamine from the medium, βWT was expressed as an autophosphorylated protein at an approximate molecular mass of 50 kDa and this was accompanied by extensive tyrosine phosphorylation of endogenous yeast proteins. In contrast an IGF-IR β chain containing the kinase inactivating mutation K1003R (βKD) induced no phosphorylation of endogenous yeast proteins when expressed at similar levels (Fig. 1B). These data demonstrate that the IGF-IR β chain exhibits tyrosine kinase activity in S. pombe.
FIG. 1.
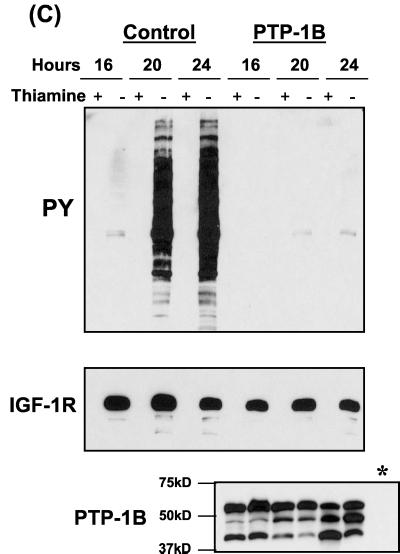
Coexpression of PTPs with the βWT protein in S. pombe. (A) Cytoplasmic region of the IGF-IR β chain (residues 930 to 1337) used in this study, illustrated in the context of the full-length receptor. (B) βWT or kinase-inactive mutant βKD was transformed into S. pombe cells as outlined in Materials and Methods. Protein expression in the cultures was induced by thiamine withdrawal. After 24 h protein extracts were prepared and separated by SDS-PAGE followed by Western blot analysis with an antiphosphotyrosine (PY) antibody (top) and then stripped and reprobed with an anti-IGF-IR antibody (bottom). (C) S. pombe cells were cotransformed with plasmids pRSP-βWT and either pADH (control) or pADH-PTP-1B and cultured in the presence or absence of thiamine (protein induction). Protein extracts were prepared at the indicated time points and analyzed for phosphotyrosine content and βWT expression by Western blotting, as for panel A. Constitutive expression of PTP-1B was confirmed by probing identical blots using the appropriate antibodies. A sample of control culture lysate (∗) was included as a negative control.
We next investigated the regulation of IGF-IR tyrosine kinase activity by PTP-1B, which was chosen as a candidate because of its role in regulation of the IR and other receptor tyrosine kinases (14, 19, 60). A cDNA encoding PTP-1B was subcloned into yeast expression vector pADH. This was then cotransformed with βWT into S. pombe cells. Proteins were extracted from cells selected to express both plasmids at three different time points and then analyzed for phosphotyrosine content by immunoblotting. Results in Fig. 1C show that PTP-1B caused a marked inhibition of βWT autophosphorylation and a complete elimination of βWT-induced yeast protein phosphorylation. Immunoblotting with the anti-IGF-IR β-chain antibody and the anti-PTP-1B antibody confirmed that comparable levels of βWT protein expression were present in the induced cultures (thiamine−) from both control and phosphatase-expressing cells and that levels of PTP-1B in the cotransformed cultures were equal (Fig. 1C).
The possibility that PTP-1B directly regulates the IGF-IR as well as the IR has potentially important implications for the effects on cell survival of modulating PTP-1B activity. PTP-1B has been shown to act directly on the tyrosine cluster in the IR kinase domain, which is also conserved in the IGF-IR (44). However, inhibition of IGF-IR kinase activity by PTP-1B has not previously been demonstrated in mammalian cells, and effects of PTP-1B overexpression on the IGF-IR are obscured by comigrating phosphorylated bands at the same size as the endogenous IGF-IR β chain (22). We were therefore interested in determining if the inhibition of the βWT protein by PTP-1B that was observed in S. pombe would also be evident in mammalian cells.
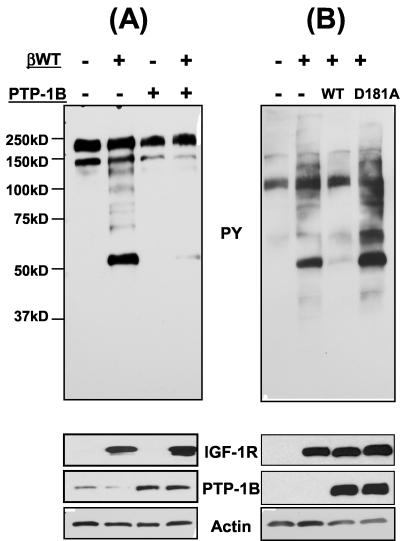
COS cells were transiently transfected with βWT or PTP-1B or cotransfected with both proteins, and the cellular phosphotyrosine content was examined 24 h later by immunoblotting. When expressed alone, the 50-kDa βWT was expressed as a constitutively active tyrosine kinase in these cells, which resulted in a considerable increase in tyrosine phosphorylation of intracellular proteins (Fig. 2A). Overexpression of PTP-1B was detected with an anti-human PTP-1B antibody, which also detected endogenous PTP-1B levels in these cells (Fig. 2A). PTP-1B overexpression alone resulted in a minimal decrease in tyrosine phosphorylation of endogenous COS cell proteins, and coexpression of PTP-1B with βWT significantly reduced the intracellular tyrosine phosphorylation induced by expression of the βWT tyrosine kinase (Fig. 2A). This indicates that PTP-1B can inhibit βWT autophosphorylation and tyrosine kinase activity in mammalian cells. Coexpression of PTB-1B with βWT in IGF-IR null (R−) fibroblasts also inhibited βWT activity (Fig. 2B). This was specifically due to PTP-1B phosphatase activity, because the catalytically impaired PTP-1B D181A mutant, as expected, did not alter βWT activity (Fig. 2B). Altogether, these results demonstrate that PTP-1B significantly inhibits phosphorylation of cellular substrates by the IGF-IR β-chain in both S. pombe cells and mammalian cells.
FIG. 2.
Inhibition of IGF-IR β-chain tyrosine kinase activity by wild-type (WT), but not catalytically impaired, PTP-1B in mammalian cells. COS cells (A) or R fibroblasts (B) were transfected with either the pIRES vector (as a control) or pIRES-βWT, pIRES-PTP-1B-WT, or pIRES-PTP-1B-D181A, as indicated, and cells were cultured for 24 h. Cell lysates were prepared and analyzed by Western blotting for phosphotyrosine (PY) content and expression of the βWT protein. PTP-1B levels and actin levels are also shown.
IGF-I-mediated activation of IGF-IR is increased in PTP-1B knockout cells.
PTP-1B is a known regulator of IR activity. However, if PTP-1B also regulates IGF-IR tyrosine kinase activity in vivo, as suggested by our data, it could influence the functions associated with the IGF-IR such as suppression of apoptosis, proliferation, and differentiation. Cells deficient in PTP-1B activity are predicted to have enhanced IGF-IR function. To address this question we asked whether IGF-IR function was altered in cells derived from PTP-1B null mice.
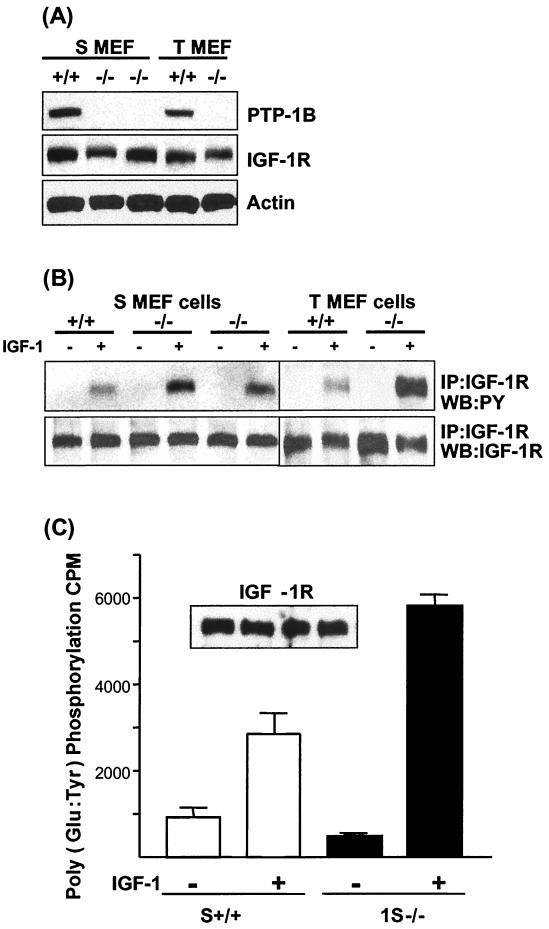
Fibroblasts derived from wild-type or PTP-1B−/− mice were immortalized by continuous passaging (S MEFs) or transformed by infection with a retrovirus encoding SV40 T-antigen (T MEFs) as previously described (11). Two clones of the S MEF PTP-1B−/− cells (1S−/− and 2S−/−) and one of the previously described T MEF−/− cells were first assessed for their levels of endogenous IGF-IR and compared with their PTP-1B+/+ counterparts. As can be seen in immunoblots of total-cell lysates (Fig. 3A), both the S and T MEF−/− cell lines express levels of endogenous IGF-IR similar to that expressed by PTP-1B+/+ cells. The levels of IGF-IR are much lower (at least 10- to 100-fold less) than levels of IGF-IR in R+ fibroblasts or MCF7 cells (not shown). Levels of PTP-1B in the S and T MEF+/+ cells are equivalent (Fig. 3A) and are, as we previously reported for the T MEFs, approximately five times higher in the cell lines than in primary fibroblasts derived from these mice.
FIG. 3.
IGF-IR expression and activity in PTP-1B knockout cells. (A) To measure IGF-IR expression levels, PTP-1B+/+ and PTP-1B−/− cells were lysed in RIPA buffer and the protein was separated by SDS-PAGE followed by Western blotting with anti-mPTP-1B or anti-IGF-IR β antibodies. Equal protein loading in the anti-IGF-IR β immunoblot was confirmed by reprobing with an antiactin antibody. (B) To assess IGF-IR activation, MEFs from each cell line were serum starved for 6 h and then stimulated with IGF-I for 15 min or left unstimulated. Cell lysates were prepared, and the IGF-IR β chain was immunoprecipitated (IP) from 200 μg of total cellular protein per sample followed by Western blotting with the antiphosphotyrosine antibody. The membrane was stripped and reprobed with the anti-IGF-IR antibody to confirm that comparable levels of endogenous IGF-IR β chain were immunoprecipitated from each sample. WB, Western blotting; PY, phosphotyrosine. (C) To assess IGF-IR kinase activity in S MEF PTP-1B+/+ cells and S MEF PTP-1B−/−, the cells were stimulated with IGF-I and the IGF-IR was immunoprecipitated as described for panel B and in vitro kinase assays were carried out as described in Materials and Methods. Poly(Glu-Tyr) was used as a substrate, and incorporated [32P]ATP was measured following trichloroacetic acid precipitation of the peptides by scintillation counting. Data represent counts per minute for triplicate samples from kinase reactions. Samples of the immunoprecipitates were assessed for IGF-IR content by Western blotting with anti-IGF-IR antibodies (inset).
We next compared levels of tyrosine phosphorylation of the IGF-IR in response to IGF-I stimulation in the PTP-1B−/− cells and the PTP-1B+/+ cells. The IGF-IR protein was immunoprecipitated from cells that had been stimulated with IGF-I for 15 min or left unstimulated, and the phosphorylation of the receptor was assessed with antiphosphotyrosine immunoblots. As shown in Fig. 3B, the level of IGF-I-induced tyrosine phosphorylation of the IGF-IR in all of the PTP-1B−/− cells (both S and T MEF cells) is consistently higher than that in the PTP-1B+/+ control cells. The relative increases were higher in the SV40 T antigen-transformed cells than in the spontaneously immortalized cells, which suggests that the presence of the T antigen could enhance IGF-IR phosphorylation in cells that lack PTP-1B. To confirm that the increase in IGF-IR autophosphorylation was associated with an increase in IGF-IR kinase activity, activated IGF-IR from S MEF PTP-1B+/+ and PTP-1B−/− cells was assessed for its ability to phosphorylate the peptide substrate poly(Glu-Tyr) in in vitro kinase assays. As can be seen in Fig. 3C, incorporation of 32P-labeled phosphate into the substrate is greatly enhanced in the S MEF PTP-1B−/− cells compared to that in the PTP-1B+/+ cells. Overall, these results indicate that in the absence of PTP-1B there is diminished negative regulation of the IGF-IR kinase. This suggests that PTP-1B negatively regulates the IGF-IR, at least in cultured transformed cells.
PTP-1B knockout cells display increased plating efficiency.
As shown above, PTP-1B can negatively regulate IGF-IR kinase activity and PTP-1B−/− cells have enhanced IGF-IR kinase activity. This suggests that lack of PTP-1B could have an impact on IGF-IR function in different kinds of cells. We were particularly interested to determine whether loss of PTP-1B could enhance IGF-IR-mediated protection from apoptosis and enhance its ability to support a transformed phenotype. There is extensive evidence of a role for IGF-IR signaling in cellular transformation, including the observations that IGF-IR null cells are refractory to transformation by a series of oncogenes (47) and that overexpression of the IGF-IR allows cells to grow as colonies in soft agarose or to form foci when plated at low cell density (47). Therefore, we next investigated whether cells lacking PTP-1B had an increased capacity for focus formation, suppression of apoptosis, and motility.
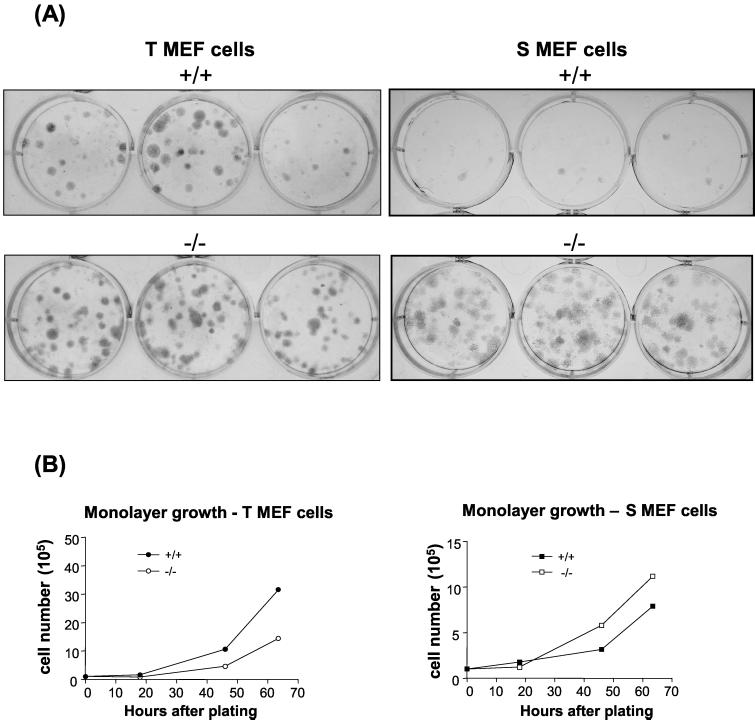
To investigate the relative plating efficiencies of PTP-1B−/− cells, both the T MEF and S MEF PTP-1B−/− cells lines were compared to PTP-1B+/+ matched control cell lines. Cells were plated at low cell density and monitored for the formation of foci over a period of 2 weeks. As shown in Fig. 4A, the T MEF cell lines always produced higher colony numbers than the S MEF cell lines, indicating, as expected, that cells transformed with the SV40 large T-antigen have enhanced plating efficiency. The S MEF PTP-1B+/+ cells had barely any detectable colony formation when plated at low cell density. However, at this same cell density, the plating efficiency of the S MEF PTP-1B−/− cells was significantly greater than that of their PTP-1B+/+ counterparts, as was the plating efficiency of the T MEF PTP-1B−/− cells compared with that of the PTP-1B+/+ controls. The T MEF PTP-1B+/+ cell lines had growth rates in monolayer culture that were approximately two times faster than their PTP-1B−/− counterparts, and the 1S MEF PTP-1B−/− cells grew slightly faster than the S MEF PTP-1B+/+ cells (Fig. 4B). This indicates that lack of PTP-1B enhances the ability of immortalized or transformed fibroblasts to form foci irrespective of the growth rate of the cells. The observation that both the S and T MEF PTP-1B −/− cells exhibited an increase in plating efficiency suggests that loss of PTP-1B activity contributes to the expression of a more transformed phenotype in either the presence or absence of an active oncogene.
FIG. 4.
Plating efficiency of transformed PTP-1B knockout cells. (A) Cells of the T and S MEF cell lines (both PTP-1B+/+ and PTP-1B−/−) as indicated were seeded in triplicate wells in medium supplemented with 10% FCS at a density of 500 cells per well and incubated for 12 days to allow colonies to form. Cells were then stained with Giemsa solution, and the entire plates were photographed. (B) Cells were seeded in multiple wells of a six-well plate at a density of 2 × 105 cells/well. At the indicated time points the cells were trypsinized and the total cell numbers per well were determined with a hemocytometer.
PTP-1B knockout cells are more sensitive to serum withdrawal than PTP-1B-expressing cells and display increased IGF-I-mediated protection from apoptosis.
The above results demonstrate that cells lacking PTP-1B activity have enhanced IGF-IR kinase activity and better ability to form colonies at low density than their PTP-1B-expressing counterparts. Another important aspect of IGF-IR function that we wanted to investigate was IGF-I-mediated protection from apoptosis. For these experiments we focused on the spontaneously immortalized S MEF cells to rule out interference from antiapoptotic signals coming from the SV40 T antigen.
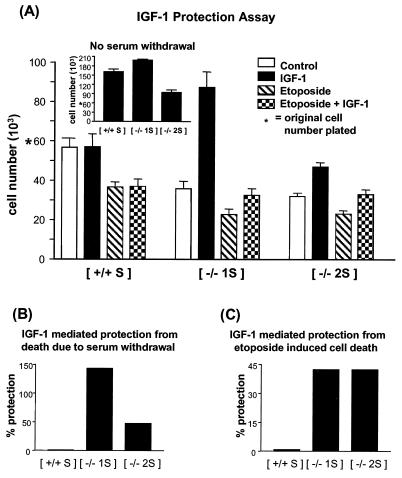
Equal numbers of the S MEF cells were plated in medium supplemented with 10% FCS. Twelve hours later the medium was replaced with serum-free medium alone or serum-free medium containing IGF-I alone, etoposide alone, or both. Cells were cultured for a further 24 or 36 h, after which they were trypsinized and assessed for viability. The data presented in Fig. 5A represent the number of viable cells per well after 36 h, although similar results were obtained in assays scored after 24 h (data not shown). Compared to the original number of cells plated, the control PTP-1B+/+ cells displayed a minimal reduction in viable-cell number in response to serum withdrawal (5.5%). However, the PTP-1B−/− cell lines displayed a greater reduction in response to serum withdrawal, with a reduction in viable-cell numbers of 40.3 and 46.7% for the 1S−/− and 2S−/− cell lines, respectively (Fig. 5A). IGF-I had no significant effect on the number of viable control PTP-1B+/+ cells (0.9% increase over control). However, IGF-I increased the numbers of PTP-1B−/− cells compared to the number of control cells (by 143.7 and 47.5% for the 1S−/− and 2S−/− cells, respectively) (Fig. 5A and B). This indicates that IGF-I protects the PTP-1B−/− cells from death due to serum withdrawal and for 1S−/− cells, also causes increased proliferation. The 1S−/− and 2S−/− cells exhibit different growth rates in monolayer culture (Fig. 5A, inset), so the apparent differences in the levels of protection afforded by IGF-I in the 1S−/− and 2S−/− cell lines are probably due to the fact that the 2S−/− cell line grows slower in monolayer culture than either the PTP-1B+/+ cells or 1S−/− cells.
FIG. 5.
Measurement of IGF-I-mediated protection from cell death in PTP-1B knockout cells. (A) Cells were seeded at 6 × 104 cells per well in triplicate in 24-well plates, and after 12 h the medium was removed and replaced with serum-free medium either alone (control) or containing IGF-I (100 nm), etoposide (5 μM), or both IGF-I and etoposide. The total viable-cell numbers in triplicate wells were determined after 36 h and are represented graphically. To assess their relative growth rates, cells were cultured in parallel in medium supplemented with 10% FCS and the total viable-cell number of each culture was determined (inset). A representative of several experiments that gave similar results is shown. (B) To demonstrate the degree of protection afforded by IGF-I, the viable-cell numbers of the IGF-I cultures in panel A were expressed as percentages of the viable-cell numbers from control cultures (serum-free medium). (C) Similarly the percent protection from etoposide killing afforded by IGF-I was expressed as a percentage of the corresponding number of cells grown in medium containing etoposide alone.
Treatment of the cells with 5 μM etoposide resulted in similar levels of cell death (28 to 36% reduction in cell viability compared with control) in all of the cell lines (Fig. 5A). IGF-I could protect the two PTP-1B−/− cell lines from this apoptotic stimulus (42.4% protection), but no significant IGF-I-mediated protection was observed in the control PTP-1B+/+ cells (Fig. 5A and C). It must be noted that the levels of IGF-IR expression are very low in these cells compared with, for example, those in the tumor cell lines and IGF-IR-transfected cells that we routinely use to study IGF-I survival signaling. Taking this into account, the levels of IGF-I-mediated protection observed in these experiments are quite substantial.
These data demonstrate that PTP-1B−/− cells are more sensitive than control cells to serum withdrawal, are equally sensitive to etoposide killing, and display increased IGF-I-mediated protection from apoptosis in response to serum withdrawal or etoposide killing.
PTP-1B−/− cells display enhanced IGF-I-mediated motility.
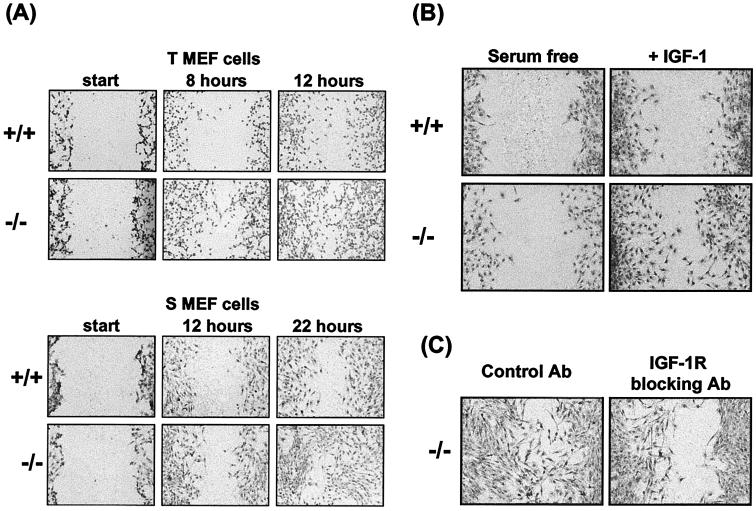
Cell motility is a function of immortalized or transformed cells that is influenced by IGF-I and that has been shown to involve cross talk between IGF-IR-mediated and integrin signaling (30, 32). To compare cellular motility in the PTP-1B−/− cells with that in controls, a wound assay was first performed on cells cultured in medium supplemented with 10% fetal bovine serum (FBS). Both the T and S MEF PTP-1B−/− cells displayed significantly increased motility in the presence of serum (10% FBS) (Fig. 6A). Eight hours after the wound was scored T MEF PTP-1B−/− cells had moved further into the wound than their PTP-1B+/+ counterparts, and 4 h later (time 12) the PTP-1B−/− cells had partially filled the wound, unlike their PTP-1B+/+ counterparts. Motility in these cells does not correlate with growth rate because the T MEF PTP-1B+/+ cells grow faster than the T MEF PTP-1B−/− cells (Fig. 4B). The migration rates of the S cell lines were overall slower than those of the T MEF cells, but the same differences between PTP-1B−/− and PTP-1B+/+ cells were observed on a different time scale. The PTP-1B−/− cells moved farther than PTP-1B+/+ cells within 12 h, and 10 h later (time 22) the PTP-1B−/− cells had almost completely filled the wound.
FIG. 6.
Monolayer wound repair assay to demonstrate motility of PTP-1B knockout cells. (A) To compare the relative motilities of the PTP-1B−/− and PTP-1B+/+ cells, each of the indicated cell lines was grown to confluence in medium supplemented with 10% FCS and a wound was then scored in each culture. Migration into the wound was monitored by microscopic visualization, and at the indicated time points (hours) cells were stained with Giemsa and photographed at 10× magnification. For each condition a representative of multiple similar fields is presented. (B) To assess the contribution of IGF-I activity to motility, the assay was performed as for panel A except that after the wound was scored cells were incubated in serum-free medium or in medium supplemented with IGF-I (100 ng/ml) and photographs were taken at 22 h. (C) To assess dependence on IGF-IR signaling, cells cultured in medium supplemented with FCS were treated with the IGF-IR blocking antibody (20 μg/ml) or an isotype-matched control antibody and the cultures were photographed 22 h later.
To determine the extent to which the motility differences observed in medium supplemented with FBS were due to IGF-IR signaling, we measured motility in serum-free medium with and without IGF-I. As can be seen in Fig. 6B, there was reduced motility of the S MEF cells in serum-free medium compared with that of cells cultured in FBS-supplemented medium (Fig. 6A). However, in IGF-I-supplemented medium the S MEF PTP-1B−/− cells displayed more migration into the wound than their PTP-1B+/+ counterparts. To further confirm that the enhanced motility detected in medium supplemented with FBS was IGF-I mediated, we tested whether an IGF-IR blocking antibody could inhibit the migration of the S MEF PTP-1B−/− cells. As shown in Fig. 6C, migration of these cells was substantially inhibited in the presence of the IGF-IR blocking antibody. These results further demonstrate that the increased motility of PTP-1B−/− cells is largely due to enhanced IGF-IR signaling.
Taken together with the results in Fig. 4 and 5, these data demonstrate that three functions that are associated with IGF-IR action in transformed cells, plating efficiency, suppression of apoptosis, and migratory capacity, are all increased in cells that lack PTP-1B activity.
IGF-I-mediated activation of PI 3-kinase and MAPK pathways is altered in PTP-1B−/− cells.
In response to IGF-I stimulation a number of signaling complexes are recruited to the activated IGF-IR via adapter proteins. In particular, activation of the PI 3-kinase, MAPK, and JNK pathways is mediated through IRS proteins or Shc proteins, all of which are dependent on kinase activity and phosphorylation of tyrosine 950 in the juxtamembrane region of the receptor.
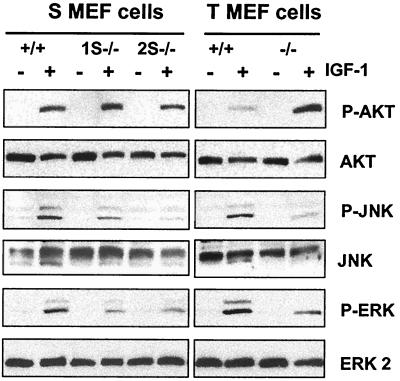
As shown above, cells that lack PTP-1B have enhanced IGF-I-mediated survival and motility, as well as enhanced plating efficiency, all functions that can be mediated through known IGF-IR signaling pathways. We next investigated whether some of these signaling pathways were altered in PTP-1B−/− cells. Lysates from unstimulated or IGF-I-stimulated PTP-1B−/− and PTP-1B+/+ cells were immunoblotted with phospho-specific antibodies directed against AKT to assess activation of this pathway, which is dependent on PI 3-kinase activity, against phospho-ERK to assess MAPK activity, and against phospho-JNK and phospho-p38 to assess stress-activated protein kinase activity (Fig. 7). Equal protein loading was confirmed in each case with antibodies specific for the nonphosphorylated forms of these proteins. In response to IGF-I stimulation, AKT phosphorylation in one of the S MEF PTP-1B−/− cell lines was increased compared to that in the PTP-1B+/+ control cells (Fig. 7, top). An even greater increase in IGF-I-mediated activation of AKT phosphorylation was observed in the T MEF PTP-1B−/− cells compared to that in the T MEF PTP-1B+/+ cells. This suggests that PI 3-kinase activity and the AKT pathway are enhanced in the PTP-1B−/− cells. IGF-I-mediated phosphorylation of ERK was decreased, and this is in agreement with our previous study on ERK activity in response to adhesion in these cells (11). JNK phosphorylation was also decreased in each of the PTP-1B−/− cell lines tested. This indicates that, although IGF-IR activity is increased in PTP-1B−/− cells, IGF-I-mediated MAPK activity and JNK activity are diminished in the absence of PTP-1B. However, because adhesion-dependent MAPK activity is also diminished in these cells, it is likely that a block in activation of these pathways occurs downstream of the IGF-IR. IGF-I did not induce p38 phosphorylation in any of the cell lines tested (data not shown). Altogether these results indicate that there is a change in the profile of the signaling pathways activated by the IGF-IR in PTP-1B−/− cells and that activation of the AKT pathway correlates with increased IGF-IR activity, whereas activation of the MAPK and JNK pathways does not.
FIG. 7.
Examination of PI 3-kinase, MAPK, and stress-activated protein kinase pathways activated in response to IGF-I in PTP-1B knockout cells. Serum-starved cells were stimulated with IGF-I for 15 min or left unstimulated. Lysates (25 μg) were analyzed by Western blotting for phospho-AKT or phospho-ERK and for phospho-JNK. Equal loading was assessed by stripping the blots and reprobing with antibodies against the nonphosphorylated forms of AKT, JNK, and ERK.
Reexpression of PTP-1B in S MEFs reduces IGF-I-mediated receptor autophosphorylation, AKT activation, and cellular motility.
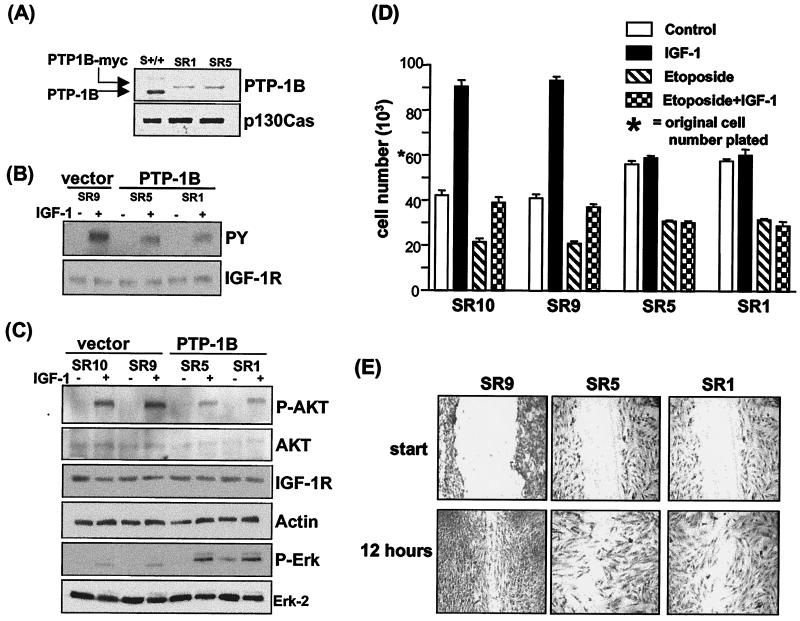
All of the above data indicate that PTP-1B can regulate IGF-IR kinase activity, activate signaling pathways that control key functions, and enhance the function of the IGF-IR in immortalized or transformed fibroblasts. To investigate whether the effects on IGF-IR function that we have measured are truly due to decreased PTP-1B activity rather than to PTP-1B-independent mechanisms associated with the immortalization or transformation procedure, we sought to rescue the cells from the PTP-1B−/− phenotype by reexpressing the PTP-1B protein. To do this, 1S MEF PTP-1B−/− cells stably reexpressing PTP-1B were generated by retrovirus infection. Levels of PTP-1B in two independent clones (SR1 and SR5) compared with those in S MEF PTP-1B+/+ cells are shown in Fig. 8A. SR1 and SR5 cells have similar levels of PTP-1B, but these are substantially lower than those in the S MEF PTP-1B+/+ cells. However, PTP-1B levels in these cells are quite comparable to the levels detected in primary PTP-1B+/+ fibroblasts (not shown). As controls we used SR9 and SR10 cells that were similarly infected and subcloned but that expressed no PTP-1B.
FIG. 8.
Reexpression of PTP-1B in S MEF PTP-1B−/− cells results in decreased IGF-IR and AKT activation. (A) SR1 and SR5 cells were clones isolated after infection of the 1S MEF cells with a retrovirus vector encoding PTP-1B-myc. To compare PTP-1B levels in SR1 and SR5 cells with those in S MEF PTP-1B+/+ cells, the cells were lysed in RIPA buffer and analyzed by Western blotting with an anti-PTP-1B antibody. Arrows, positions of endogenous PTP-1B in the PTP-1B+/+ cells and PTP-1B-myc in the infected clones. The blot was reprobed with the anti-p130 Cas antibody to control for loading. (B) IGF-IR activation in the SR9 control cells as well as SR1 and SR5 cells was assessed by starving the cells and stimulating with IGF-I for 15 min or not stimulating. Cell lysates were prepared, and the IGF-IR was immunoprecipitated, followed by Western blotting with an antiphosphotyrosine antibody (PY). The blot was then stripped and reprobed with an anti-IGF-IR antibody. (C) To assess AKT activation in SR1 and SR5 cells compared with that in two control cell lines that do not express PTP-1B, SR9 and SR10, cells were starved and stimulated with IGF-I for 15 min or not stimulated, lysed, and analyzed by Western blotting for phospho-AKT. The blot was then stripped and reprobed with antibodies against AKT, IGF-IR, and actin. An identical blot was probed with antibodies against phospho-ERK and ERK-2. (D) To measure IGF-I-mediated antiapoptotic activity, cells were seeded at 6 × 104 cells per well in triplicate in 24-well plates and after 12 h the medium was removed and replaced with serum-free medium either alone (control) or containing IGF-I (100 nM), etoposide (5 μM), or both IGF-I and etoposide. The total viable-cell numbers in triplicate wells were determined after 36 h and are represented graphically. (E) To assess cell migration, cells were grown to confluence in medium supplemented with 10% FCS and a wound was then scored in each culture. Migration into the wound was monitored by microscopic visualization, and at 12 h cells were stained with Giemsa and photographed at 10× magnification. For each condition a representative of multiple similar fields is presented. We noted that the PTP-1B-expressing SR1 and SR5 cells have a larger cytoplasm-to-nucleus ratio than the SR9 cells.
We next compared the activity of the IGF-IR in PTP-1B-reexpressing SR1 and SR5 cells with that in control SR9 cells. As can be seen in Fig. 8B, the levels of IGF-I-induced IGF-IR autophosphorylation in both PTP-1B-expressing SR5 and SR1 cells are decreased compared to those in vector control SR9 cells. In parallel with this, IGF-I-mediated AKT phosphorylation in SR1 and SR5 cells was decreased compared to that in the vector controls. By contrast IGF-I-mediated activation of ERK was restored in the PTP-1B-expressing cells (Fig. 8C). All of these cells expressed equal levels of IGF-IR (Fig. 8B and C).
To assess whether the decrease in IGF-IR activity in SR1 and SR5 cells is associated with a decrease in IGF-IR-mediated function, we measured the antiapoptotic activity and motility of these PTP-1B-expressing cells compared with the SR9 and SR10 vector control cells. As can be seen in Fig. 8D, the IGF-I-mediated response to serum withdrawal and etoposide killing was reversed by reexpression of PTP-1B. Similarly, as can be seen in Fig. 8E the motility of SR1 and SR5 cells is retarded compared with that of SR9 cells. Altogether these data demonstrate that reexpression of PTP-1B in spontaneously immortalized PTP-1B−/− fibroblasts rescues the cells from the PTP-1B deficiency phenotype and restores negative regulatory activity with respect to IGF-IR activation and function. The data suggest that PTP-1B is a regulator of IGF-IR function.
DISCUSSION
The identification of phosphatases that specifically regulate the activity of receptor tyrosine kinases or oncogenic kinases would provide a powerful tool for understanding and manipulating these pathways in normal and transformed cells (8). Although several oncogenic kinase-activated signaling pathways are known, there is still relatively little known about phosphatase regulation of these pathways. The importance of these regulatory phosphatases is illustrated by the lipid phosphatase PTEN, which dephosphorylates the products of PI 3-kinase activity that are necessary for AKT activation. PTEN exhibits loss of heterozygosity or is mutated in the advanced stages of several cancers (12). The IGF-IR is a potent mediator of survival activity and gene expression in tumor cells, and therefore phosphatases that regulate its activity can also be predicted to play an important role in tumor progression.
Our awareness of the established role for PTP-1B in IR regulation (44), the total inhibition of IGF-IR activity by PTP-1B observed with S. pombe prompted us to investigate this further. PTP-1B also suppressed IGF-IR kinase activity in mammalian cells in the context of overexpression of a constitutively active IGF-IR β chain. The consequences of lack of PTP-1B on IGF-IR activity were very striking in the fibroblast cell lines derived from PTB-1B knockout mice. These cells, whether spontaneously immortalized or transformed with SV40 T antigen, had enhanced phosphorylation of the IGF-IR as well as enhanced plating efficiency and enhanced IGF-I-mediated cell survival and motility. These cells also exhibited enhanced AKT phosphorylation, which suggests that the PI 3-kinase/AKT pathway is involved in all of these IGF-IR functions. Reintroduction of PTP-1B into one of the spontaneously immortalized cell lines resulted in reversal of this phenotype. This indicates that PTP-1B regulates IGF-IR function and that lack of PTP-1B enhances IGF-IR activity.
SV40 T antigen has previously been shown to stimulate IGF production and to cooperate with IGF-IR signaling in immortalized fibroblasts (16, 31, 41). However, in this study the PTP-1B−/− T antigen-transformed cells did not express higher IGF-IR levels, but they did demonstrate more-pronounced IGF-I-induced kinase activity than the spontaneously immortalized cells. They also exhibited better plating efficiency and motility and appeared to be morphologically more transformed, all of which could be attributed to the presence of T antigen and potential cooperation with IGF-IR. However, each of these features of transformed cells was still further enhanced in the PTP-1B−/− cells.
Although the MEF cell lines used in this study express lower levels of IGF-IR than most cultured tumor cell lines, the PTP-1B−/− cells were more sensitive to cell death in response to serum withdrawal and exhibited an IGF-I-mediated protective response from induction of apoptosis. In contrast, the control PTP-1B+/+ cells with similar numbers of IGF-I receptors were less sensitive to serum withdrawal and had no detectable IGF-I antiapoptotic response. This strongly suggests that loss of PTP-1B makes cells more dependent on IGF-IR survival signaling. This is consistent with our previous observation that T MEF cells have decreased integrin signaling, which would also contribute to decreased survival in the absence of growth factors (11). Enhanced dependence on IGF-IR survival signaling could be significant for transformed cells in vivo, because it has been proposed that tumor cells expressing active oncogenes and growing out of their normal location are more dependent on survival factors such as IGF-I than normal cells, which receive multiple survival signals from their environment (15). The enhanced plating efficiency and enhanced IGF-I-mediated motility in the PTP-1B−/− MEFs provide evidence that lack of IGF-IR regulation by PTP-1B contributes to the transformed phenotype of these cells in culture. It also suggests that signals from the IGF-IR can rescue the integrin signaling defects in the PTP-1B−/− cells that caused reduced spreading on fibronectin matrices (11).
It is clear from the decrease in IGF-I-induced activation of ERK and JNK that we observed in PTP-1B−/− cells and from our previous report of decreased ERK activation associated with integrin signaling in these cells (11) that loss of PTP-1B down-regulates MAPK signaling while other pathways such as the IGF-IR/AKT pathway are up-regulated. In addition to regulating integrin signaling, PTP-1B has previously been shown to regulate the activity of the epidermal growth factor receptor (18) and cadherins (5a), and the cross talk between all these pathways is likely to have complementary or antagonistic affects on IGF-IR signaling. Since ERK activation was attenuated in response to adhesion signals as well as in the response to IGF-I in PTP-1B−/− cells, it is likely that the MAPK pathway in these cells is regulated downstream of these stimuli by cellular kinases or phosphatases whose activity is also controlled by PTP-1B. One candidate for this could be Src, which is known to activate the MAPK pathway and which we have found to be hyperphosphorylated at an inhibitory tyrosine in PTP-1B−/− cells (11). Src has also been shown to phosphorylate the IGF-IR (39), and the transforming activity of c-Src is thought to be dependent on its interaction with the IGF-IR (53). However, decreased Src activity apparently does not inhibit IGF-IR activity or IGF-I-induced AKT activation in PTP-1B knockout cells, so if it is responsible for the decreased ERK activation it would have to act downstream of the IGF-IR.
The observation that the PTP-1B knockout mice did not present with any abnormalities or tumors (14) that could be associated with enhanced IGF-IR activity may be due to several reasons. The blunted responses of the MAPK and JNK pathways to diverse stimuli in PTP-1B knockout cells could inhibit tumor development. There may also be compensating events in these mice caused by lack of PTP-1B that could make cells resistant to transformation. These could include altered levels of IGF binding proteins that regulate IGF-I and IGF-II actions through the receptor (1). Alternatively, the phenotype of these mice may be associated with the particular role of the IGF-IR in faciliting the outgrowth of transformed cells rather than in initiating the transformation event (7). As is elegantly outlined in a number of tumor development models (20, 21, 34, 38), several abnormal events are required for cancer to develop. These include one or more active oncogenes, enhanced survival mechanisms, and angiogenesis- and metastasis-promoting activities. In the case of PTP-1B knockout mice, it is possible that they have not experienced enough oncogenic events during their lifetimes in a controlled environment, and therefore enhanced IGF-IR activity would have no effect on cancer development. On the contrary, lack of PTP-1B and enhanced IGF-IR function may confer an advantage to these animals in maintaining the survival of normal cells of the immune and nervous systems (17, 56). It will be of interest to analyze IGF-IR function in primary tissues derived from these mice.
It could also be hypothesized that acquisition of an activated oncogene by or diminished p53 function in cells of PTP-1B-deficient mice would be more likely to lead to cancer than a similar event in cells of PTP-1B+/+ mice. First, due to enhanced IGF-IR antiapoptotic activity, the likelihood that a cell with an activated oncogene would survive and develop into a tumor would be increased. Second, lack of PTP-1B could promote the progression of a tumor into a more aggressive vascularized or metastatic form through the ability of the IGF-IR to promote angiogenesis and metastasis (2, 45). An extension of this hypothesis also predicts that the offspring of PTP-1B knockout mice crossed with transgenic mice expressing an oncogene such as c-myc would develop tumors at a higher rate than their parents.
In conclusion, we have demonstrated that PTP-1B can inhibit IGF-IR tyrosine kinase activation and that IGF-IR kinase activity, cell survival, and IGF-IR functions associated with the maintenance of a transformed phenotype are enhanced in cells that lack PTP-1B. These results establish PTP-1B as a potential regulator of IGF-IR activity and suggest that PTP-1B may have a role in limiting IGF-I-promoted cancer progression.
Acknowledgments
We thank Guilio Superti-Furga for providing the SP200 yeast strain and the pRSP and pADH expression vectors, Benjamin G. Neel for providing the PTP-1B plasmids, John Wagner for help in the construction of the retroviral vector encoding the PTP-1B cDNA, and Suzanne Floyd for her assistance with cell culture.
This work was supported by grants from Enterprise Ireland, the Wellcome Trust, and the Higher Education Authority. A.C. is a recipient of an M.R.C. studentship. M.L.T. is a scientist from the Canadian Institutes for Health Research (CIHR).
D. A. Buckley and A. Cheng are joint first authors.
REFERENCES
- 1.Adams, T. E., V. C. Epa, T. P. Garrett, and C. W. Ward. 2000. Structure and function of the type 1 insulin-like growth factor receptor. Cell. Mol. Life Sci. 57:1050-1093. [DOI] [PMC free article] [PubMed]
- 2.Akagi, Y., W. Liu, B. Zebrowski, K. Xie, and L. M. Ellis. 1998. Regulation of vascular endothelial growth factor expression in human colon cancer by insulin-like growth factor-I. Cancer Res. 58:4008-4014. [PubMed] [Google Scholar]
- 3.Alessi, D. R., M. Deak, A. Casamayor, F. B. Caudwell, N. Morrice, D. G. Norman, P. Gaffney, C. B. Reese, C. N. MacDougall, D. Harbison, A. Ashworth, and M. Bownes. 1997. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Biol. 7:776-789. [DOI] [PubMed] [Google Scholar]
- 4.Angers-Loustau, A., J. F. Côte, A. Charest, D. Dowbenko, S. Spencer, L. A. Lasky, and M. L. Tremblay. 1999. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J. Cell Biol. 144:1019-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assefa, Z., A. Vantieghem, W. Declercq, P. Vandenabeele, J. R. Vandenheede, W. Merlevede, P. de Witte, and P. Agostinis. 1999. The activation of the c-Jun N-terminal kinase and p38 mitogen-activated protein kinase signaling pathways protects HeLa cells from apoptosis following photodynamic therapy with hypericin. J. Biol. Chem. 274:8788-8796. [DOI] [PubMed] [Google Scholar]
- 5a.Balsamo, J., C. Arregui, T. Leung, and J. Lilien. 1998. The nonreceptor protein tyrosine phosphatase PTP1B binds to the cytoplasmic domain of N-cadherin and regulates the cadherin-actin linkage. J. Cell Biol. 143:523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barry, O. P., B. Mullan, D. Sheehan, M. G. Kazanietz, F. Shanahan, J. K. Collins, and G. C. O'Sullivan. 2001. Constitutive ERK1/2 activation in esophagogastric rib bone marrow micrometastatic cells is MEK-independent. J. Biol. Chem. 276:15537-15546. [DOI] [PubMed] [Google Scholar]
- 7.Baserga, R., and A. Morrione. 1999. Differentiation and malignant transformation: two roads diverged in a wood. J. Cell. Biochem. 32-33(Suppl.):68-75. [DOI] [PubMed] [Google Scholar]
- 8.Blume-Jensen, P., and T. Hunter. 2001. Oncogenic kinase signalling. Nature 411:355-365. [DOI] [PubMed] [Google Scholar]
- 9.Brogiolo, W., H. Stocker, T. Ikeya, F. Rintelen, R. Fernandez, and E. Hafen. 2001. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 11:213-221. [DOI] [PubMed] [Google Scholar]
- 10.Buckley, D. A., G. Loughran, G. Murphy, C. Fennelly, and R. O'Connor. 2002. Identification of an IGF-IR regulatory phosphatase using the fission yeast Schizosaccharomyces pombe and a GFP-tagged IGF-IR. Mol. Pathol. 55:46-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng, A., S. B. Gurjeet, B. P. Kennedy, and M. L. Tremblay. 2001. Attenuation of adhesion dependent signalling and cell spreading in transformed fibroblasts lacking protein tyrosine phosphatase-1B. J. Biol. Chem. 276:25848-25855. [DOI] [PubMed] [Google Scholar]
- 12.Dahia, P. L. 2000. PTEN, a unique tumor suppressor gene. Endocr. Relat. Cancer 7:115-129. [DOI] [PubMed] [Google Scholar]
- 13.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 14.Elchebly, M., P. Payette, E. Michaliszyn, W. Cromlish, S. Collins, A. L. Loy, D. Normandin, A. Cheng, J. Himms-Hagen, C. C. Chan, C. Ramachandran, M. J. Gresser, M. L. Tremblay, and B. P. Kennedy. 1999. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283:1544-1548. [DOI] [PubMed] [Google Scholar]
- 15.Evan, G. I., and K. H. Vousden. 2001. Proliferation, cell cycle and apoptosis in cancer. Nature 411:342-348. [DOI] [PubMed] [Google Scholar]
- 16.Fei, Z. L., C. D'Ambrosio, S. Li, E. Surmacz, and R. Baserga. 1995. Association of insulin receptor substrate 1 with simian virus 40 large T antigen. Mol. Cell. Biol. 15:4232-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman, E. L., K. A. Sullivan, B. Kim, and J. W. Russell. 1997. Insulin-like growth factors regulate neuronal differentiation and survival. Neurobiol. Dis. 4:201-214. [DOI] [PubMed] [Google Scholar]
- 18.Flint, A. J., T. Tiganis, D. Barford, and N. K. Tonks. 1997. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 94:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein, B. J., F. Ahmad, W. Ding, P. M. Li, and W. R. Zhang. 1998. Regulation of the insulin signalling pathway by cellular protein-tyrosine phosphatases. Mol. Cell. Biochem. 182:91-99. [PubMed] [Google Scholar]
- 20.Hager, J. H., and D. Hanahan. 1999. Tumor cells utilize multiple pathways to down-modulate apoptosis. Lessons from a mouse model of islet cell carcinogenesis. Ann. N. Y. Acad. Sci. 887:150-163. [DOI] [PubMed] [Google Scholar]
- 21.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 22.Kenner, K. A., E. Anyanwu, J. M. Olefsky, and J. Kusari. 1996. Protein-tyrosine phosphatase 1B is a negative regulator of insulin- and insulin-like growth factor-I-stimulated signaling. J. Biol. Chem. 271:19810-19816. [DOI] [PubMed] [Google Scholar]
- 23.Kido, Y., J. Nakae, and D. Accili. 2001. Clinical review 125: the insulin receptor and its cellular targets. J. Clin. Endocrinol. Metab. 86:972-979. [DOI] [PubMed] [Google Scholar]
- 24.Kimura, K. D., H. A. Tissenbaum, Y. Liu, and G. Ruvkun. 1997. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277:942-946. [DOI] [PubMed] [Google Scholar]
- 25.Klaman, L. D., O. Boss, O. D. Peroni, J. K. Kim, J. L. Martino, J. M. Zabolotny, N. Moghal, M. Lubkin, Y. B. Kim, A. H. Sharpe, A. Stricker-Krongrad, G. I. Shulman, B. G. Neel, and B. B. Kahn. 2000. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 20:5479-5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krause, D., A. Lyons, C. Fennelly, and R. O'Connor. 2001. Transient activation of jun n-terminal kinases and protection from apoptosis by the insulin-like growth factor i receptor can be suppressed by dicumarol. J. Biol. Chem. 276:19244-19252. [DOI] [PubMed] [Google Scholar]
- 27.LaMontagne, K. R., Jr., G. Hannon, and N. K. Tonks. 1998. Protein tyrosine phosphatase PTP1B suppresses p210 bcr-abl-induced transformation of rat-1 fibroblasts and promotes differentiation of K562 cells. Proc. Natl. Acad. Sci. USA 95:14094-14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawlor, M. A., X. Feng, D. R. Everding, K. Sieger, C. E. Stewart, and P. Rotwein. 2000. Dual control of muscle cell survival by distinct growth factor-regulated signaling pathways. Mol. Cell. Biol. 20:3256-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loda, M., P. Capodieci, R. Mishra, H. Yao, C. Corless, W. Grigioni, Y. Wang, C. Magi-Galluzzi, and P. J. Stork. 1996. Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. Am. J. Pathol. 149:1553-1564. [PMC free article] [PubMed] [Google Scholar]
- 30.Manes, S., E. Mira, C. Gomez-Mouton, Z. J. Zhao, R. A. Lacalle, and A. C. Martinez. 1999. Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol. Cell. Biol. 19:3125-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin, D. C., J. L. Fowlkes, B. Babic, and R. Khokha. 1999. Insulin-like growth factor II signaling in neoplastic proliferation is blocked by transgenic expression of the metalloproteinase inhibitor TIMP-1. J. Cell Biol. 146:881-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mira, E., S. Manes, R. A. Lacalle, G. Marquez, and A. C. Martinez. 1999. Insulin-like growth factor I-triggered cell migration and invasion are mediated by matrix metalloproteinase-9. Endocrinology 140:1657-1664. [DOI] [PubMed] [Google Scholar]
- 33.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795-823. [DOI] [PubMed] [Google Scholar]
- 34.Naik, P., J. Karrim, and D. Hanahan. 1996. The rise and fall of apoptosis during multistage tumorigenesis: down-modulation contributes to tumor progression from angiogenic progenitors. Genes Dev. 10:2105-2116. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor, R. 1998. Survival factors and apoptosis. Adv. Biochem. Eng. Biotechnol. 62:137-166. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor, R., A. Kauffmann-Zeh, Y. Liu, S. Lehar, G. I. Evan, R. Baserga, and W. A. Blattler. 1997. Identification of domains of the insulin-like growth factor I receptor that are required for protection from apoptosis. Mol. Cell. Biol. 17:427-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okazaki, K., N. Okazaki, K. Kume, S. Jinno, K. Tanaka, and H. Okayama. 1990. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 18:6485-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelengaris, S., T. Littlewood, M. Khan, G. Elia, and G. Evan. 1999. Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol. Cell 3:565-577. [DOI] [PubMed] [Google Scholar]
- 39.Peterson, J. E., G. Kulik, T. Jelinek, C. W. Reuter, J. A. Shannon, and M. J. Weber. 1996. Src phosphorylates the insulin-like growth factor type I receptor on the autophosphorylation sites. Requirement for transformation by src. J. Biol. Chem. 271:31562-31571. [DOI] [PubMed] [Google Scholar]
- 40.Playford, M. P., D. Bicknell, W. F. Bodmer, and V. M. Macaulay. 2000. Insulin-like growth factor 1 regulates the location, stability, and transcriptional activity of beta-catenin. Proc. Natl. Acad. Sci. USA 97:12103-12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porcu, P., A. Ferber, Z. Pietrzkowski, C. T. Roberts, M. Adamo, D. LeRoith, and R. Baserga. 1992. The growth-stimulatory effect of simian virus 40 T antigen requires the interaction of insulinlike growth factor 1 with its receptor. Mol. Cell. Biol. 12:5069-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prentice, H. L. 1992. High efficiency transformation of Schizosaccharomyces pombe by electroporation. Nucleic Acids Res. 20:621.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rother, K. I., and D. Accili. 2000. Role of insulin receptors and IGF receptors in growth and development. Pediatr. Nephrol. 14:558-561. [DOI] [PubMed] [Google Scholar]
- 44.Salmeen, A., J. N. Andersen, M. P. Myers, N. K. Tonks, and D. Barford. 2000. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Mol. Cell 6:1401-1412. [DOI] [PubMed] [Google Scholar]
- 45.Samani, A. A., and P. Brodt. 2001. The receptor for the type I insulin-like growth factor and its ligands regulate multiple cellular functions that impact on metastasis. Surg. Oncol. Clin. N. Am. 10:289-312. [PubMed] [Google Scholar]
- 46.Schaapveld, R. Q., J. T. Schepens, G. W. Robinson, J. Attema, F. T. Oerlemans, J. A. Fransen, M. Streuli, B. Wieringa, L. Hennighausen, and W. J. Hendriks. 1997. Impaired mammary gland development and function in mice lacking LAR receptor-like tyrosine phosphatase activity. Dev. Biol. 188:134-146. [DOI] [PubMed] [Google Scholar]
- 47.Sell, C., G. Dumenil, C. Deveaud, M. Miura, D. Coppola, T. DeAngelis, R. Rubin, A. Efstratiadis, and R. Baserga. 1994. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol. Cell. Biol. 14:3604-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Superti-Furga, G., K. Jonsson, and S. A. Courtneidge. 1996. A functional screen in yeast for regulators and antagonizers of heterologous protein tyrosine kinases. Nat. Biotechnol. 14:600-605. [DOI] [PubMed] [Google Scholar]
- 49.Tissenbaum, H. A., and L. Guarente. 2001. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410:227-230. [DOI] [PubMed] [Google Scholar]
- 50.Tonks, N. K., and B. G. Neel. 2001. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr. Opin. Cell Biol. 13:182-195. [DOI] [PubMed] [Google Scholar]
- 51.Ullrich, A., A. Gray, A. W. Tam, T. Yang-Feng, M. Tsubokawa, C. Collins, W. Henzel, T. Le Bon, S. Kathuria, E. Chen, et al. 1986. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 5:2503-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urso, B., C. U. Niesler, S. O'Rahilly, and K. Siddle. 2001. Comparison of anti-apoptotic signalling by the insulin receptor and IGF-I receptor in preadipocytes and adipocytes. Cell Signal. 13:279-285. [DOI] [PubMed] [Google Scholar]
- 53.Valentinis, B., A. Morrione, S. J. Taylor, and R. Baserga. 1997. Insulin-like growth factor I receptor signaling in transformation by src oncogenes. Mol. Cell. Biol. 17:3744-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valentinis, B., G. Romano, F. Peruzzi, A. Morrione, M. Prisco, S. Soddu, B. Cristofanelli, A. Sacchi, and R. Baserga. 1999. Growth and differentiation signals by the insulin-like growth factor 1 receptor in hemopoietic cells are mediated through different pathways. J. Biol. Chem. 274:12423-12430. [DOI] [PubMed] [Google Scholar]
- 55.Walchli, S., M. L. Curchod, R. P. Gobert, S. Arkinstall, and R. Hooft van Huijsduijnen. 2000. Identification of tyrosine phosphatases that dephosphorylate the insulin receptor. A brute force approach based on “substrate-trapping” mutants. J. Biol. Chem. 275:9792-9796. [DOI] [PubMed] [Google Scholar]
- 56.Walsh, P. T., and R. O'Connor. 2000. The insulin-like growth factor-I receptor is regulated by CD28 and protects activated T cells from apoptosis. Eur J. Immunol 30:1010-1018. [DOI] [PubMed] [Google Scholar]
- 57.Wen, S., J. Stolarov, M. P. Myers, J. D. Su, M. H. Wigler, N. K. Tonks, and D. L. Durden. 2001. PTEN controls tumor-induced angiogenesis. Proc. Natl. Acad. Sci. USA 98:4622-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolkow, C. A., K. D. Kimura, M. S. Lee, and G. Ruvkun. 2000. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 290:147-150. [DOI] [PubMed] [Google Scholar]
- 59.Yu, H., and T. Rohan. 2000. Role of the insulin-like growth factor family in cancer development and progression. J. Natl. Cancer Inst. 92:1472-1489. [DOI] [PubMed] [Google Scholar]
- 60.Zabolotny, J. M., Y. B. Kim, O. D. Peroni, J. K. Kim, M. A. Pani, O. Boss, L. D. Klaman, S. Kamatkar, G. I. Shulman, B. B. Kahn, and B. G. Neel. 2001. Overexpression of the LAR (leukocyte antigen-related) protein-tyrosine phosphatase in muscle causes insulin resistance. Proc. Natl. Acad. Sci. USA 98:5187-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao, Y., and H. B. Lieberman. 1995. Schizosaccharomyces pombe: a model for molecular studies of eukaryotic genes. DNA Cell Biol. 14:359-371. [DOI] [PubMed] [Google Scholar]