Abstract
CD45 plays a critical role in T-cell receptor (TCR)-mediated signaling. In a yeast two-hybrid screen, SKAP55, the Src kinase-associated phosphoprotein of unknown function, was found as a substrate which associated with CD45 in vivo. Mutational analysis demonstrated the pivotal role of Tyr-232 in SKAP55 in the association with CD45. In Jurkat cells, anti-CD3 antibody stimulation promoted SKAP55 tyrosine phosphorylation and translocation from the cytoplasm to the membrane. Overexpression of SKAP55 in these cells induced transcriptional activation of the IL-2 promoter, while mutant SKAP55-Y232F totally suppressed the promoter activity. Furthermore, overexpression of SKAP55-Y232F also caused the tyrosine hyperphosphorylation of Fyn with a decreased kinase activity. Thus, SKAP55 is an essential adapter to couple CD45 with the Src family kinases for dephosphorylation and, thus, positively regulates TCR signaling.
CD45, also known as leukocyte common antigen (LCA), is a member of the receptor-like transmembrane protein tyrosine phosphatases (PTPases). CD45 is exclusively expressed on nucleated cells of hematopoietic origin and is critically involved in activation of hematopoietic cells through their antigen receptors. The CD45 molecule consists of a distinct extracellular domain, a transmembrane domain, and a conserved cytoplasmic tail containing two PTPase domains: domain 1 (D1) and D2 (10). The cytoplasmic D1 possesses major PTPase activity and is necessary to restore T-cell receptor (TCR) signaling in an HPB-ALL leukemic cell line (5). The role of the D2 may be to facilitate and regulate the activity of the D1, in addition to its important role in TCR-mediated interleukin-2 (IL-2) production (31). It has been shown that the epidermal growth factor receptor (EGFR)-CD45 chimera is able to restore TCR induction in CD45-deficient cell lines (7, 8), supporting the notion that the cytoplasmic domain of CD45 is necessary and sufficient for TCR signal transduction. Conclusive evidence that CD45 plays a critical role in TCR signaling comes from an analysis of a CD45-deficient cell line in which TCR signal transduction was totally abrogated (27, 30). Consistent with the abolition of TCR signaling, the loss of TCR-mediated tyrosine phosphorylation was observed in CD45-deficient cells (15). Likewise, CD45−/− mutants of mouse T-cell lines derived by immunoselection were impaired in their ability to respond to antigen, and such impaired antigen responsiveness was restored in CD45+ revertants (27, 30).
It is conceivable that to exert its regulatory function in TCR signaling CD45 needs to interact with and dephosphorylate its tyrosine-phosphorylated substrates and downstream effectors, thereby allowing TCR signaling to take place. In recent years, great efforts have been made to define signaling molecules associating with CD45 in the TCR complex. Using immunoprecipitation and/or cocapping techniques, a number of molecules have been found to associate with CD45 in the TCR complex, such as CD4/CD8, CD28, CD45-AP, the intracellular tyrosine kinases p56Lck and p59Fyn, molecules with molecular masses of 29 to 34 kDa, and others (1). However, the physiological significance of some of these associations with CD45 has been questioned due to a lack of reproducibility. More importantly, immunoprecipitation and/or cocapping techniques may reveal many signaling molecules that are indirectly associated with CD45 in the TCR multimolecular complex through third partners or adapters. It is therefore assumed that the complete spectrum of CD45 substrates and its direct downstream targets are not yet defined.
Recently, a number of immune cell restricted adapter proteins in lymphocytes have been identified (23). These adapters can be tyrosine phosphorylated and represent signaling linkers to downstream effectors. An Src kinase-associated phosphoprotein, SKAP55/SKAP55-related protein, was identified as an adapter protein binding to the SH2 domain of Fyn and other Src-like SH2 domains (17, 20). Later, it was found that a novel proline-independent motif in SKAP55 can bind to SH3 domains (13). Both Fyn and Lck are two lymphocyte-restricted members of the Src family kinases and play important roles in TCR/CD3-mediated signal transduction in mature T cells (2). SKAP55 is exclusively expressed in T lymphocytes (20). However, its biological function in T cells remains completely unknown (19). In order to identify proteins and targets directly associated with CD45, we used a CD45 substrate-trapping mutant as bait in a yeast two-hybrid screen. Here we report that the tyrosine-phosphorylated SKAP55 was identified as a substrate binding to the catalytic active site of CD45. In Jurkat cells, SKAP55 can be strongly tyrosine phosphorylated and translocated from the cytoplasm to the cell membrane in response to anti-CD3 antibody stimulation. More importantly, overexpression of SKAP55 in Jurkat cells induced transcriptional activation of the IL-2 gene promoter, whereas mutation of the CD45-binding site of Tyr-232 in SKAP55 totally suppressed anti-CD3 antibody-initiated TCR-mediated gene transcription and led to the tyrosine hyperphosphorylation of Fyn. These results suggest that SKAP55 is a substrate for CD45, which recruits this adapter to the membrane. At this location, SKAP55 in turn binds to the SH2 domain of the Src family kinases to bring CD45 to the specific proximal inhibitory site of these kinases for dephosphorylation, thus activating TCR signaling.
MATERIALS AND METHODS
Reagents and cell lines.
Both Jurkat and CD45-deficient Jurkat cells (J45.01) were obtained from American Type Culture Collection (ATCC) and cultured in RPMI 1640 medium with 10% (vol/vol) fetal bovine serum (FBS), 2 mM l-glutamine (GIBCO), and 50 mM HEPES. 293T cells (ATCC) were cultured in Dulbecco minimal essential medium (DMEM) containing 10% FBS. OKT3 hybridoma cells (ATCC) were maintained in Iscove’s modified Dulbecco’s medium (GIBCO) with 20% FBS, and anti-CD3 antibody was purified from the supernatants. Rat antihemagglutinin (anti-HA) monoclonal antibody (clone 3F10) was purchased from Boehringer GmbH (Mannheim, Germany). Anti-Myc (9E10) monoclonal and anti-Fyn p59 polyclonal antibodies were purchased from Santa Cruz Biotechnology. Antiphosphotyrosine antibody (4G10) was purchased from Upstate Biotechnology. Anti-SKAP55 and anti-CD45 antibodies were from BD Transduction Laboratory.
Yeast two-hybrid screen.
Truncated CD45 cDNA (residues 597 to 1304) containing two tandem cytoplasmic catalytic domains was amplified from plasmid pSP6.DL.LCA6 (25), and an aspartic acid (residue 819) was mutated to valine (D819V) by PCR. The mutated CD45 (D819V) is designated the substrate trapping mutant (9) and was subcloned in frame with the Lex A DNA binding domain in pBTM116/Src vector (14) as bait. The cDNA library with the activation domain of GAL4 (pACT2) was made from leukemia Jurkat cell lines (Clontech). The bait construct (pBTM116/Src-CD45-D819V) and library cDNA were sequentially transformed into yeast strain L40α and screened for growth according to the manufacturer's protocols. Positive clones were further screened by β-galactosidase assay.
Epitope-tagged constructs and mutagenesis.
The full-length CD45 cDNA was subcloned into pcDNA3.1 vector (Invitrogen). The truncated CD45 substrate-trapping mutant was subcloned in frame with an N-terminal triple HA tagged into pAcTAG2 vector (Invitrogen). SKAP55 was subcloned in frame with the C-terminal Myc-His tag into pCDA3.1 vector. Fyn (p59) in pRK5 vector was a kind gift from S. Stamm (Munich, Germany). For the cellular distribution experiment, SKAP55 and its mutants were cloned and fused in frame with the C terminus of the green fluorescence protein (GFP) gene in pEGFP-C1 vector (Clontech). For site-directed mutagenesis, the tyrosine residues of SKAP55 at 219, 232, and 271 sites were selected and mutated into phenylalanine (F) by PCR. The mutants SKAP55-Y219F, SKAP55-Y232F, and SKAP55-Y271F were also cloned into the pCDNA3.1-Myc-His vector. The mutations were confirmed by DNA sequencing.
Cell transfections, immunoprecipitation, and Western blot analysis.
293T cells (107) were transfected with 20 μg of plasmid DNA by a standard calcium phosphate precipitation method. Approximately 10 × 107 Jurkat and CD45-deficient (CD45−) Jurkat cells were transfected with 50 μg of plasmid DNA by electroporation with 300 V for 10 ms in a 300-μl volume using a BTX820 electroporator (BTX Corp., San Diego, Calif.). Cells resistant to G418 were pooled together from each independent transfection experiment. A similar, but harsher, electroporation condition was used for transient transfection in Jurkat cells, over 70% of which were killed to obtain effective transfections. For anti-CD3 antibody stimulation, cells were treated with anti-CD3 antibody (5 μg/ml) and the secondary antibody (rabbit anti-mouse antibody [20 μg/ml]) for 5 min before adding lysis buffer.
To stimulate protein tyrosine phosphorylation, 36 h following transfection, cells were serum starved for 12 h. The starved cells were treated with 20% serum for 60 min as previously described (26). Immunoprecipitation and immunoblotting were performed as described before (26).
Chemical cross-linking of proteins.
CD45-D819V and SKAP55 were cotransfected into the 293T cells. The transfected cells were cultured for 48 h with 20% serum, and 10 mM ethyl-3-(3-dimethylaminopropyl)cardodimede (EDAC) was added to the medium for chemical cross-linking of interacted proteins as described previously (6). The chemically treated cells were lysed and immunoprecipitated with either anti-Myc antibody or anti-HA antibody. The immunocomplexes were subjected to sodium dodecyl sulfate-7% polyacrylamide gel electrophoresis (SDS-7% PAGE) for immunoblotting analysis.
Immune complex kinase assay.
Immune complex in vitro kinase assays were performed as described before (22) except that the reaction was carried out for 7 min.
Subcellular localization and confocal microscopic examination of SKAP55.
Normal Jurkat and CD45-deficient Jurkat cell lines were cultured in RPMI 1640 medium as described above. Cells were transfected with 10 μg of DNA of GFP-SKAP55 (or its mutants) plus 20 μl of Superfects (Qiagen, Inc., Hilden, Germany) in 60-mm-diameter plates overnight. The transfection efficiency was approximately 50%. In a particular experiment, 50 μg of DNA of a constitutive active Fyn kinase was cotransfected with 5 μg DNA of GFP-SKAP55 in CD45-deficient Jurkat cells. For confocal microscopic examination, glass slides were pretreated and coated with 3-aminopropyltriethoxy-saline (Sigma). The transfected cells were seeded on glass slides in RPMI 1640 medium, grown overnight, and then treated with anti-CD3 antibody for 5 min or left untreated. Cell images were taken using a Multi-Probe 2001 confocal microscopy (Nikon). Confocal laser images were analyzed using CLSM Imaging Software (Molecular Dynamics, Sunnyvale, Calif.) on the Unix system.
Luciferase assay.
Approximately 8 × 106 Jurkat cells were transiently transfected by electroporation with 10 μg of constructs SKAP55 and its mutants, or vector alone, together with 10 μg of IL-2-Luc plasmid carrying the luciferase gene driven by the IL-2 promoter. Transfection efficiency was monitored by cotransfection with 1 μg of pSV-β-galactosidase vector (Promega) and normalized by β-galactosidase activity. The transfected cells were cultured with fresh medium for 24 h and then equally divided into two plates. The divided cells were cultured with fresh medium for an additional 12 h and then treated with anti-CD3 antibody plus phorbol 12-myristate (PMA) (50 ng/ml) and ionomycin (1 μM) for additional 6 h or were left untreated. The treated and untreated cells were washed with phosphate-buffered saline and lysed. Luciferase activity was determined in triplicate samples by using a luciferase assay kit (Promega). All data were normalized by β-galactosidase activity for transfection efficiency with the basal level activity obtained in unstimulated cells.
RESULTS
CD45 trapping mutant (D819V) interacts with SKAP55 in yeast two-hybrid system.
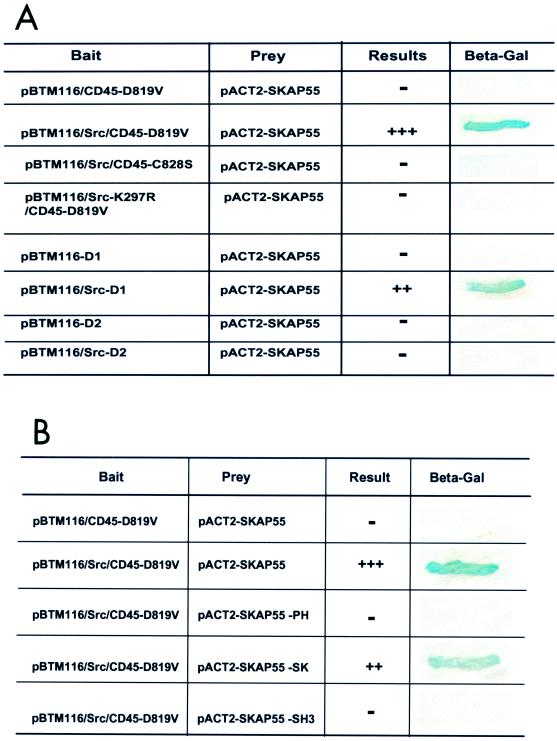
To identify proteins directly associated with CD45, we used a modified yeast two-hybrid system in which a mutated c-Src kinase was integrated into the vector pBTM116 for protein tyrosine phosphorylation in yeast (14). The entire cytoplasmic domain (597 to 1304) of CD45 with a mutation of Asp-819 to valine in D1 was used as bait. This mutant CD45-D819V is designated a substrate-trapping mutant, as it can tightly bind to its substrates but exerts no catalytic activity (9). CD45-D819V was cloned into the pBTM116/Src vector in frame. A Jurkat cell cDNA library fused to the GAL4 activation domain in pACT2 was used as target prey for screens. Sequencing analysis of positive clones revealed that, among others, three independent clones contained the full length of Src kinase-associated phosphoprotein (SKAP55), which was reported to interact with Fyn and other Src family kinases (20). The interaction of SKAP55 with the substrate-trapping mutant CD45-D819V was found to be specific because SKAP55 failed to interact with the catalytic inactive mutant CD45-C828S (Fig. 1A), in which the essential nucleophilic cysteine-828 in the phosphatase active site of CD45 was mutated to serine, thus eliminating its interaction with substrates (11). Other substrate-trapping mutants of PTPases, such as SHP-1 and SHP-2, were also incapable of interacting with SKAP55 in the system (data not shown), further suggesting the specificity of the interaction between SKAP55 and CD45.
FIG. 1.
Catalytic D1 of CD45 interacts with SK region of SKAP55, and interaction in yeast two-hybrid system requires Src kinase activity. (A) The D1 domain of CD45-D819V interacts with SKAP55 in the presence of Src kinase. Gal4-SKAP55 was cotransformed into yeast with LexA-CD45-D819V, LexA-CD45-D1 (residues 596 to 942), or LexA-CD45-D2 (residues 943 to 1304), with or without the expression of wild-type Src kinase or the kinase-dead mutant Src-K297R. The interactions were evaluated using the β-galactosidase filter assay. After 60 min, the level of blue color in transformed yeasts was used as an indicator: −, no interaction; ++, strong interaction; +++, very strong interaction. The data summarize results of three independent experiments. (B) The SK region of SKAP55 interacts with CD45-D819V. SKAP55 was dissected into three regions: PH domain (residues 1 to 205), SK domain (residues 206 to 300), and SH3 domain (residues 301 to 359). As described for panel A, the individual domain was cotransformed with CD45-D819V into yeast, and the β-galactosidase filter assay was used to evaluate the interactions as described for panel A.
Phosphatase catalytic D1 of CD45-D819V is mainly associated with SK region of SKAP55.
To define the position of the interactions, the cytoplasmic sequence of CD45-D819V was further divided into two domains, namely catalytic D1 (residues 597 to 942) and catalytic D2 (residues 943 to 1304). D1 and D2 were separately subcloned into the pBTM116/Src vector and individually cotransformed with pACT2-SKAP55 in yeast. The protein interactions in transformants were evaluated by measuring β-galactosidase activity. As shown in Fig. 1A, D1 of CD45-D819V strongly interacted with SKAP55, whereas D2 of CD45 displayed no association with SKAP55, providing evidence that catalytic D1 of CD45 is a major binding partner for SKAP55. Fig. 1A also shows that the interaction required c-Src-kinase mediated-tyrosine phosphorylation, as the interaction was not detected with the catalytic inactive mutant Src-K297R.
Similarly, SKAP55 was dissected into three domains: the PH domain (residues 1 to 205), an intervening region termed SK (residues 206 to 299), and the SH3 domain (residues 300 to 359). Each domain was cloned into pACT2 vector fused in frame with the GAL4 activation domain and cotransfected with pBTM116/Src-CD45-D819V in yeast. The expression and tyrosine phosphorylation of each GAL4-fused domain were confirmed in yeast by anti-GAL4 and antiphosphotyrosine antibodies, respectively (data not shown). β-Galactosidase assays revealed that the CD45-D819V trapping mutant interacted with the SK region of SKAP55 in yeast (Fig. 1B). Taken together, our data demonstrate that D1 of CD45 interacts with the SK region of SKAP55.
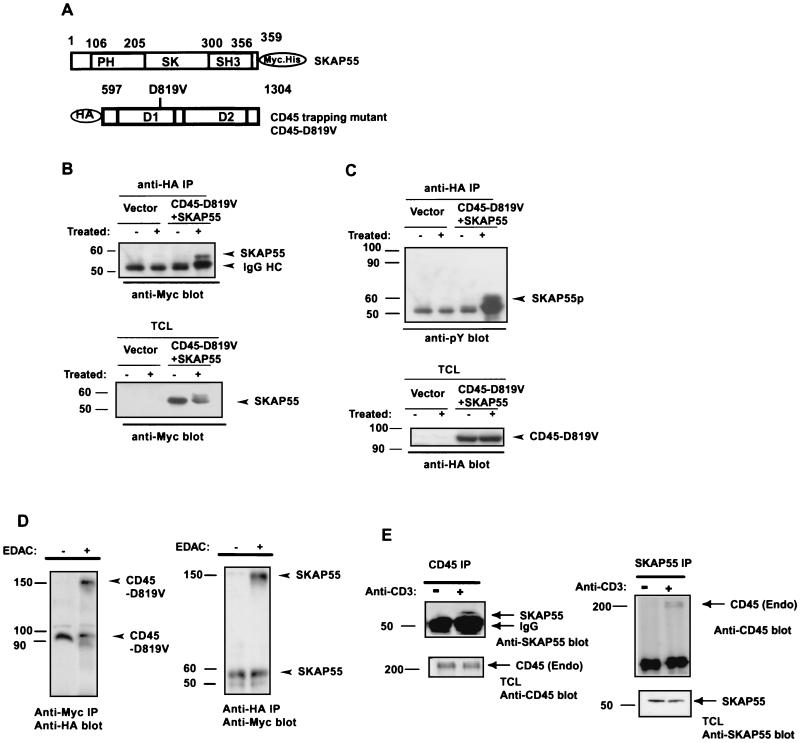
Tyrosine phosphorylation of SKAP55 is required for its interaction with CD45-D819V in vivo.
The interaction of CD45-D819V with SKAP55 identified in the yeast two-hybrid screen, was further characterized in mammalian cells. For this, SKAP55 was subcloned into vector pcDNA3.1 with an Myc tag at the C terminus, and CD45-D819V was cloned into pAcTAG2 with a HA tag at the N terminus (Fig. 2A). The two constructs were cotransfected into 293T cells. The transfected cells were treated with serum as described previously (26). The interaction of CD45-D819V with SKAP55 in vivo was assayed by performing coimmunoprecipitation in cell lysates. Figure 2B shows that SKAP55 was coimmunoprecipitated with CD45-D819V in the serum-stimulated cells but not in nontreated control cells. The same membrane was stripped and reprobed with antiphosphotyrosine antibody. As shown in Fig. 2C, a protein that migrated at the same position as SKAP55 was heavily tyrosine phosphorylated. The specificity of the interaction between CD45 and SKAP55 will be further demonstrated by the SKAP55 mutants (see below). To reveal the direct interaction of SKAP55 with CD45-D819V, protein cross-linking experiments were performed. As before, the two constructs were cotransfected into 293T cells and the transfected cells were reacted with the chemical cross-linker EDAC. The cell lysates were immunoprecipitated by anti-Myc (SKAP55) antibody and blotted by anti-HA (CD45) antibody. Figure 2D shows that an approximately 150-kDa protein band reactive to anti-HA (CD45) antibody was coimmunoprecipitated by anti-Myc (SKAP55) antibody (left). In a reciprocal experiment, this 150-kDa protein was detected by anti-Myc (SKAP55) antibody in the pellets precipitated by anti-HA (CD45) antibody (Fig. 2D, right). These results suggest that tyrosine-phosphorylated SKAP55 interacted directly with CD45-D819V in vivo as the linked molecule was only 150 kDa, approximately equal to the mass of CD45 plus SKAP55, ruling out the presence of any additional component, such as src kinase. To investigate the physiological relevance of the interaction between CD45 and SKAP55 observed in 293 cells, we further examine whether the endogenous interaction between CD45 and SKAP55 occurs in Jurkat T cells, where the two proteins are natively expressed. As before, the interaction of CD45 with SKAP55 was assayed by performing coimmunoprecipitation in Jurkat cell lysates. Figure 2E shows that the interaction between the two endogenous proteins indeed occurred, as SKAP55 could be detected in the coimmunoprecipitates with anti-CD45 antibody in anti-CD3 antibody-stimulated Jurkat cell lysates but not in unstimulated cell lysates (Fig. 2E, left). In a reciprocal experiment, the full length of CD45 was also detectable in the pellets precipitated by anti-SKAP55 antibody in anti-CD3 antibody-stimulated cells (Fig. 2E, right).
FIG. 2.
Tyrosine-phosphorylated SKAP55 interacts with CD45-D819V. (A) Full-length SKAP55 was subcloned in frame with the C-terminal Myc-His tag into the pcDNA3.1 vector. Truncated CD45-D819V was subcloned in frame with an N-terminal triple-HA tag into pAcTAG2 vector. (B) CD45-D819V interacts with SKAP55 expressed in serum-stimulated cells. 293T cells were transiently cotransfected with CD45-D819V and SKAP55. The transfected cells were either left untreated (−) or treated with serum (+). The cells (5 × 106) were lysed and immunoprecipitated (IP) by anti-HA antibody. The immunoprecipitates were subjected to SDS-7% PAGE, and blotted by anti-Myc antibody (1:2,000). The bottom panel is a Western blot of total SKAP55 from whole-cell lysates from 2 × 104 cells to ensure equal SKAP55 expression in samples. (C) Only the tyrosine phosphorylated form of SKAP55 interacts with CD45-D819V. The same membrane shown in panel B was stripped and reprobed with anti-phosphotyrosine antibody. The bottom panel is a Western blot of total CD45 from whole-cell lysates of 2 × 104 cells, indicating equal CD45 expression of samples. (D) Chemical cross-linking verifies the direct interaction of CD45 with SKAP55. The serum-stimulated cells were either treated with 10 mM EDAC or left untreated. The cell lysates of 5 × 106 cells were immunoprecipitated either by anti-Myc antibody and blotted by anti-HA antibody (left) or precipitated by anti-HA antibody and blotted by anti-Myc antibody (right) as done for panel B. Molecular mass markers (in kilodaltons) are on the left. (E) The endogenous CD45 interacts with the native SKAP55 in Jurkat cells in response to anti-CD3 antibody stimulation. Volumes of 5 × 108 Jurkat cells were equally divided into two portions, one of which was treated with anti-CD3 antibody for 5 min, and the other portion of cells was left untreated. The cell lysates were immunoprecipated either with anti-CD45 antibody or with anti-SKAP55 antibody and subjected to SDS-7% PAGE. The anti-CD45 antibody immunocomplexes were blotted by anti-SKAP55 antibody, and total CD45 protein in cell lysates was determined by anti-CD45 antibody blotting analysis (left). Reciprocally, anti-SKAP55 immunocomplexes were subjected to anti-CD45 antibody probing, and total SKAP55 protein in cell lysates was assayed by anti-SKAP55 antibody to ensure equal amounts of SKAP55 protein were used in the experiments (right).
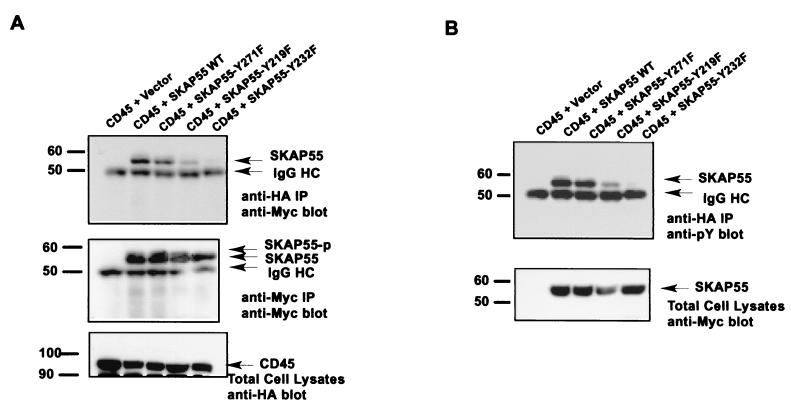
Mutant SKAP55-Y232F abolishes its association with CD45 substrate-trapping mutant in vivo.
Defining the requirement of tyrosine phosphorylation for association with CD45-D819V prompted us to identify which tyrosine phosphorylation site(s) in the SK region of SKAP55 mediates its association with CD45. There are three potential tyrosine phosphorylation sites in the SK region of SKAP55, namely Y219, Y232, and Y271. Point mutations of tyrosine (Y) to phenylalanine (F) were made for the each tyrosine residue. SKAP55 mutants with mutations of Y219F, Y232F, and Y271F were Myc tagged at the C terminus, and CD45-D819V was HA tagged at the N terminus. The epitope-tagged sequences were cloned into an expression vector. For characterizing protein interactions, each SKAP55 mutant was cotransfected with CD45-D819V into 293T cells. The transfected cells were treated with serum as described before. Figure 3A shows that while the Y271F mutation exerted no apparent effect on the interaction of SKAP55 with CD45-D819V, the Y219F mutation caused a significant reduction of the association of SKAP55 with CD45-D819 and the Y232F mutation nearly completely abolished the interaction with CD45-D819V. When this blot was stripped and reprobed with anti-phosphotyrosine antibody, the tyrosine phosphorylation pattern was similar to that observed in the protein interaction (Fig. 3B), indicating that the CD45-bound SKAP55 was tyrosine phosphorylated. Taken together, these results suggest that SKAP55 specifically interacts with CD45 through its defined tyrosine-phosphorylated sites, and both Tyr-219 and Tyr-232 residues are likely responsible for mediating the specific interaction of SKAP55 with CD45-D819V in vivo.
FIG. 3.
Tyrosine residue 232 of SKAP55 plays critical role for its interaction with CD45. (A) Mutant SKAP55-Y232F completely abolished its interaction with CD45-D819V. CD45-D819V was cotransfected with vector alone and SKAP55, SKAP55-Y271F, SKAP55-Y219F, or SKAP55-Y232F in 293T cells. The transfected cells were stimulated with serum for tyrosine phosphorylation as described for Fig. 2. The cells (7 × 106) were lysed and immunoprecipitated (IP) by anti-HA antibody, subjected to SDS-7% PAGE, and blotted with anti-Myc antibody. The middle panel is a blot made by precipitating SKAP55 and reprobing with anti-Myc antibody. The bottom panel is a Western blot of total CD45 from whole-cell lysates of 2 × 104 cells. (B) The CD45-bound SKAP55 was tyrosine phosphorylated upon serum stimulation. The same membrane shown in panel A was stripped and reprobed with anti-phosphotyrosine antibody. The bottom panel is a Western blot of total SKAP55 from whole-cell lysates of 2 × 104 cells. The molecular mass markers (in kilodaltons) are on the left.
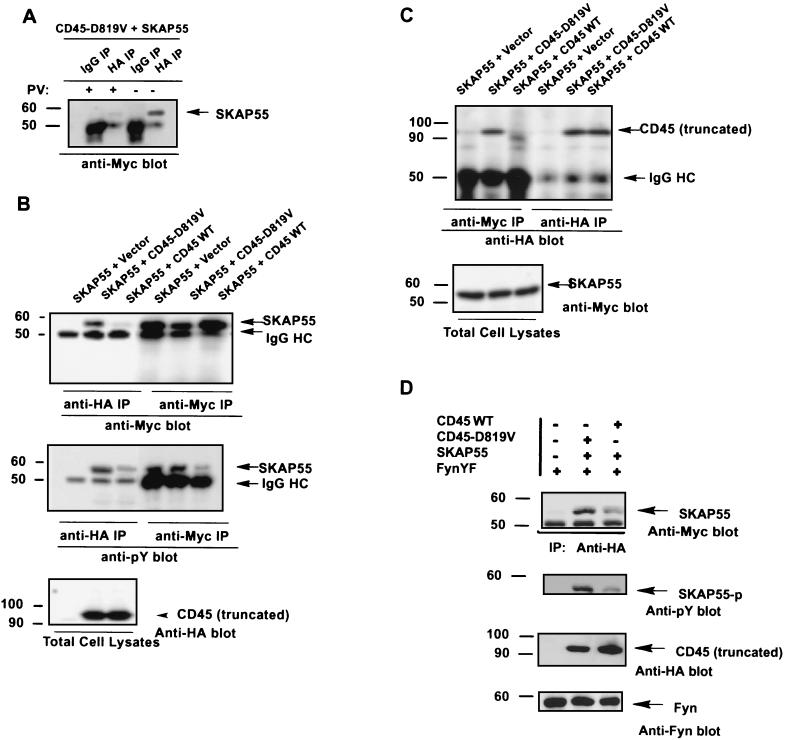
SKAP55 as a substrate binds to the phosphatase active site of CD45 in vivo.
Since the adapter protein SKAP55 associates with catalytic D1 of the CD45-D819V substrate-trapping mutant in vivo, it is expected that SKAP55 is a potential substrate for CD45. The substrate nature of SKAP55 for CD45 was first demonstrated by the yeast two-hybrid interactions (Fig. 1A) in which SKAP55 was specifically interacted with the substrate-trapping CD45-D819V, but not with the catalytic inactive mutant CD45-C828S in which the mutation of the essential nucleophilic cysteine-828 in the phosphatase active site of CD45 abolished the interaction between CD45 and its substrates (11). Further, we examined the interaction of SKAP55 with CD45-D819V in the presence of pervanadate, as it is known that this phosphatase inhibitor covalently modifies the essential nucleophilic cysteine residue in the tyrosine phosphatase active site (12, 29). Again, epitope-tagged CD45-D819V and SKAP55 were cloned into the expression vector and cotransfected into 293T cells. The transfected cells were treated with serum as before. The treated cells were lysed in the presence and absence of pervanadate. Coimmunoprecipitations were performed. Western blotting showed that in the presence of pervanadate, the interaction of SKAP55 with CD45-D819V was disrupted (Fig. 4A). These results suggest that interaction of SKAP55 with CD45-D819V is through the binding of SKAP55 to the phosphatase catalytic active sites in which the Cys-828 residue of CD45 mediates the interaction of the phosphatase with its substrate(s) (3, 29).
FIG. 4.
SKAP55 as substrate interacts with CD45-D819V through its binding to active site of phosphatase. (A) Phosphatase inhibitor pervanadate abolishes the interaction of SKAP55 with CD45-D819V in vivo. 293T cells were cotransfected with tagged CD45-D819V and SKAP55 as described for Fig. 2A. The transfected cells were stimulated with serum as in Fig. 2. The treated cells were lysed with lysis buffer with (+) or without (−) sodium pervanadate (2 mM). The cell lysates were precipitated either by anti-HA antibody or by immunoglobulin G (IgG) antibody. Immunoprecipitated (IP) complexes were subjected to SDS-7% PAGE and immunoblotted by anti-Myc antibody. (B) Association of SKAP55 with wild-type and mutant CD45-D819V in immunoprecipitates. As described for panel A, the tagged SKAP55 was cotransfected with tagged CD45 or its mutant CD45-D819V in 293T cells. The transfected cells were stimulated with serum as before. Cell lysates were immunoprecipitated by anti-HA antibody and immunoblotted by anti-Myc antibody (left) or the cells were immunoprecipitated by anti-Myc antibody and reprobed by anti-Myc antibody for the control of SKAP55 precipitation (right). The same membrane was stripped and reprobed by antiphosphotyrosine antibody (middle). The bottom panel is a Western blot of total CD45 from whole-cell lysates. (C) As described for panel B, CD45 and CD45-D918V were coprecipitated by anti-Myc antibody and immunoblotted by anti-HA antibody. The bottom panel is a Western blot of total SKAP55 from whole-cell lysates. (D) SKAP55 is a potential substrate for CD45 in T cells. CD45-deficient Jurkat cells (7 × 106) were cotransfected with the constitutively active mutant Fyn-Y531F (FynYF), wild-type CD45, mutant CD45-D819V, and SKAP55 as indicated. The cell lysates were precipitated by anti-HA antibody and probed either by anti-Myc antibody (top), by antiphosphotyrosine antibody (upper middle), or by anti-HA antibody again (lower middle). The bottom panel is a Western blot of total Fyn from whole-cell lysates. Molecular mass markers (in kilodaltons) are on the left.
To further reveal the substrate nature of SKAP55 in interaction with CD45, we examined the effect of the phosphatase activity of CD45 on the coimmunoprecipitation of the two proteins and the status of tyrosine phosphorylation of SKAP55. In this regard, both SKAP55 and the CD45 cytoplasmic domain were cloned into the expression vectors tagged with Myc and HA epitopes, respectively. SKAP55 was cotransfected either with the wild-type CD45 or with its substrate-trapping mutant CD45-D819V in 293T cells. The transfected cells were serum stimulated. Figure 4B shows that SKAP55 was readily coimmunoprecipitated by the substrate-trapping mutant CD45-D819V but hardly coprecipitated with wild-type CD45. A reciprocal experiment also demonstrated that little, if any, wild-type CD45 was coprecipitated with SKAP55 (Fig. 4C). Moreover, when the immunoprecipitated SKAP55 was blotted with antiphosphotyrosine antibody, it was found that SKAP55 was largely tyrosine dephosphorylated in coimmunoprecipitation with wild-type CD45 but was protected from tyrosine dephosphorylation in coprecipitation with the substrate-trapping mutant CD45-D819V (Fig. 4B, middle).
The substrate nature of SKAP55 for CD45 was further examined in T cells where SKAP55 is exclusively expressed. As before, both SKAP55 and the CD45 cytoplasmic domain were tagged with Myc and HA epitopes in the expression vector, respectively. In order to use the substrate-trapping mutant CD45-D819V for interaction with its substrate(s), SKAP55 was cotransfected with either wild-type CD45 or the substrate-trapping mutant CD45-D819V into CD45-deficient (CD45−) Jurkat cells. To ensure the tyrosine phosphorylation of proteins in these CD45-negative cells, the constitutive active mutant Fyn-Y531F was also cotransfected into the cells. As shown in Fig. 4D, SKAP55 was readily coimmunoprecipitated with the substrate-trapping mutant CD45-D819V but was much less associated with wild-type CD45. As with 293T cells, blotting of the immunoprecipitates with antiphosphotyrosine antibody further revealed that the tyrosine phosphorylation of SKAP55 was essentially protected when it was precipitated by the substrate-trapping mutant CD45-D819V. In CD45-positive Jurkat cells, when the transfected SKAP55 and its mutant SKAP55-Y232F were coprecipitated by the endogenous CD45, the level of tyrosine phoshorylation of SKAP55 was low but detectable (data not shown), as was observed above for the CD45-deficient cells. The tyrosine phosphorylation of SKAP55-Y232F, however, could not be detected in the precipitates under the same conditions (data not shown), a result similar to that displayed in 293T cells (Fig. 3). Hence, the use of the well-established substrate-trapping mutant (CD45-D819V) demonstrated that SKAP55 as a substrate, likely via its Tyr-232 residue, associates with CD45 in vivo.
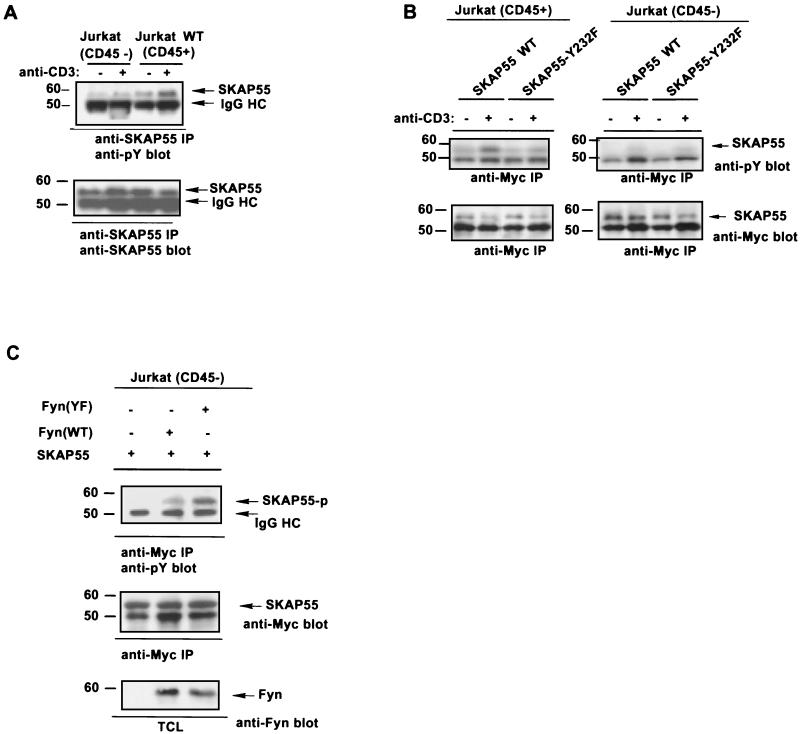
Tyrosine phosphorylation of SKAP55 is largely enhanced with anti-CD3 antibody treatment in CD45-positive cells.
Tyrosine phosphorylation of signaling proteins is the earliest event following TCR stimulation and is regulated by CD45 (28). Since both CD45 and SKAP55 are endogenously expressed in Jurkat cells, we investigated the status of SKAP55 tyrosine phosphorylation in response to anti-CD3 stimulation. To this end, both CD45-positive (CD45+) and CD45-deficient (CD45−) Jurkat cell lines were treated with an anti-CD3 antibody, and SKAP55 proteins were precipitated with an anti-SKAP55 antibody. The tyrosine phosphorylation of SKAP55 was examined by immunoblotting with an anti-phosphotyrosine antibody. Figure 5A shows that in resting CD45-positive cells, SKAP55 was weakly tyrosine phosphorylated. However, following anti-CD3 antibody stimulation, SKAP55 became heavily tyrosine phosphorylated (Fig. 5A, right), a result that was not observed in the previous report (20). In CD45-negative cells, tyrosine phosphorylation of SKAP55 was barely detectable, even with anti-CD3 antibody stimulation (Fig. 5A, left). The effect of CD45 on SKAP55 tyrosine phosphorylation was further evaluated with transiently expressed SKAP55. In this case, both Myc-tagged SKAP55 and mutant SKAP55-Y232F were transiently transfected into CD45-positive (CD45+) and CD45-deficient (CD45−) Jurkat cell lines, respectively. Again, the transfected cells were treated with anti-CD3 antibody. The tyrosine phosphorylation of SKAP55 was examined by performing immunoprecipitation with anti-Myc antibody and then blotting with antiphosphotyrosine antibody. In CD45-positive cells, similar to the endogenous SKAP55, stimulation with anti-CD3 antibody greatly induced the tyrosine phosphorylation of transiently expressed SKAP55 (Fig. 5B) but not mutant SKAP55-Y232F, in which tyrosine phosphorylation was not apparently induced following anti-CD3 antibody stimulation (Fig. 5B, left). In CD45-deficient cells, both wild-type and mutant SKAP55-Y232F displayed a comparable, but very weak, tyrosine phosphorylation (Fig. 5B, right), a pattern similar to that exhibited by mutant SKAP55-Y232F in CD45-positive cells. As a potential substrate for CD45, the tyrosine phosphorylation of SKAP55 would be protected in the CD45-negative cells. To investigate the cause of SKAP55 weak-tyrosine phosphorylation, wild-type Fyn and its constitutively active mutant Fyn-Y531F were cotransfected with SKAP55 in these CD45-negative cells. Figure 5C shows that with coexpression of the mutant Fyn-Y531F, the tyrosine phosphorylation of SKAP55 was dramatically increased in comparison with the untransfected cells. Coexpression of wild-type Fyn also led to a moderate increase of SKAP55 tyrosine phosphorylation, probably due to some non-CD45-mediated dephosphorylation of the Y531 residue on Fyn protein when it was overexpressed in the cells. This result is consistent with the notion that SKAP55 is a potential physiological substrate for CD45 in T-cells. Taken together, these results demonstrate that although both the endogenous and transiently expressed SKAP55 are constitutively tyrosine phosphorylated, stimulation with anti-CD3 antibody can substantially induce the tyrosine phosphorylation of SKAP55 in CD45-positive Jurkat cells. In CD45-negative cells, lacking of src tyrosine kinase activities is likely responsible for the weaky tyrosine phosphorylation of SKAP55.
FIG. 5.
Tyrosine phosphorylation of SKAP55 is enhanced with anti-CD3 antibody stimulation. (A) The tyrosine phosphorylation of endogenous SKAP55. Volumes of 10 × 107 cells were untreated (−) or treated (+) by anti-CD3 antibody and lysed. The endogenous SKAP55 was precipitated with anti-SKAP55 specific antibody from wild-type Jurkat (right) or CD45-deficient Jurkat (CD45−) (left) cells. Immunoprecipitates (IP) were subjected to SDS-7% PAGE and blotted with antiphosphotyrosine antibody. The immunoprecipitated SKAP55 protein was also reprobed by anti-SKAP55 antibody to indicate that an equal amount of SKAP55 was precipitated in the samples (bottom). (B) The tyrosine phosphorylation of the transfected exogenous SKAP55. SKAP55 and mutant SKAP55-Y232F were transfected into the Jurkat cells by electroporation. The cells were treated with anti-CD3 antibody (+) or were left untreated (−) and were lysed. The cell lysates were immunoprecipitated by anti-Myc antibody and subjected to SDS-7% PAGE. The membrane was probed by antiphosphotyrosine antibody. To ensure equal immunoprecipitation of SKAP55, the membrane was reprobed by anti-Myc antibody (bottom). (C) SKAP55 was tyrosine phosphorylated by coexpression of mutant Fyn in CD45-negative cells. CD45-deficient Jurkat cells (10 × 107) were cotransfected with SKAP55, wild-type Fyn, or mutant Fyn-Y531F (FynYF). The cell lysates were immunoprecipitated by anti-Myc antibody and blotted by antiphosphotyrosine antibody (top), again by anti-Myc antibody (middle), or by anti-Fyn antibody (bottom). Molecular mass markers (in kilodaltons) are on the left.
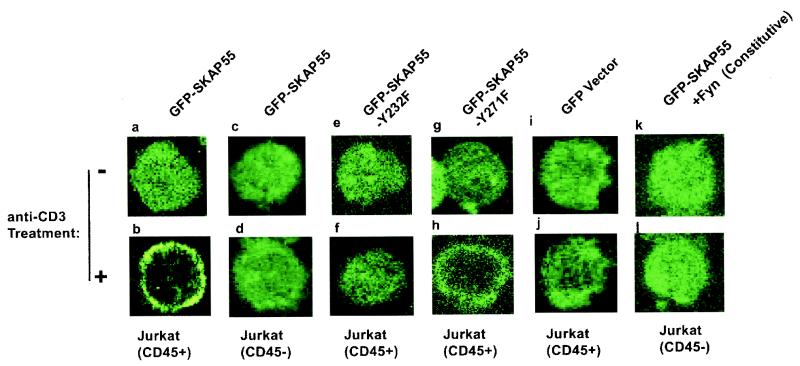
SKAP55 is translocated from cytoplasm to cell membrane upon anti-CD3 antibody stimulation.
To evaluate the localization of SKAP55 in both resting and anti-CD3 antibody-stimulated Jurkat cells, wild-type SKAP55, mutant SKAP55-Y271F, and mutant SKAP55-Y232F were fused to the C terminus of the GFP gene in frame. The constructed plasmids were transfected into either CD45-positive (CD45+) or CD45-deficient (CD45−) Jurkat cell lines. The transfected cells were either stimulated with anti-CD3 antibody or were left unstimulated. The cells were fixed and images were taken using a confocal microscope. The results show that in resting (unstimulated) cells, GFP-SKAP55 was mainly distributed evenly within the cytoplasm and partly in the nucleus (Fig. 6a). Upon stimulation with anti-CD3 antibody, GFP-SKAP55 was translocated from the cytoplasm to the cell membrane (Fig. 6b), while the control GFP alone remained in the cytoplasm of the stimulated cells (Fig. 6j). In comparison with the control, a significant portion of GFP-SKAP55-Y271F was also translocated from the cytoplasm to the cell membrane (Fig. 6h). However, GFP-SKAP55-Y232F, in which the binding site of SKAP55 to CD45 was mutated, failed to translocate from the cytoplasm to the membrane in response to anti-CD3 antibody stimulation (Fig. 6f). Consistent with this, in CD45-deficient (CD45−) cells, GFP-SKAP55 was also unable to translocate from the cytoplasm to the membrane following anti-CD3 antibody stimulation (Fig. 6d). Cotransfection of the constitutively active mutant Fyn into CD45-deficient cells did not restore the translocation of GFP-SKAP55 from the cytoplasm to the cell membrane (Fig. 6I), indicating that Fyn was incapable of recruiting its tyrosine-phosphorylated substrate SKAP55 to the membrane. These results suggest that the translocation of SKAP55 from the cytoplasm to the cell membrane in response to anti-CD3 antibody stimulation requires CD45 protein, and such CD45-regulated membrane localization of adapter SKAP55 protein potentially mediates T-cell signaling.
FIG. 6.
SKAP55 is translocated from cytoplasm to cell membrane upon anti-CD3 antibody stimulation. GFP-SKAP55, GFP-SKAP55-Y232F, and GFP-SKAP55-Y271F were transfected into CD45-positive (CD45+) and CD45-negative (CD45−) Jurkat cells. At 24 h following transfection, the transfected cells were treated with anti-CD3 antibody (+) or were left untreated (−). The translocation was visualized by confocal microscopy in a single cell as the representatives from the GFP-positive cells. (a and b) GFP-SKAP55 was transfected into CD45-positive Jurkat cells; (c and d) GFP-SKAP55 was transfected into CD45-deficient Jurkat cells; (e and f) mutant GFP-SKAP55-Y232F was transfected into CD45-positive Jurkat cells; (g and h) mutant GFP-SKAP55-Y271F was transfected into CD45-positive Jurkat cells; (i and j) GFP vector alone was transfected into CD45-positive Jurkat cells as control; (k and l) both GFP-SKAP55 and the constitutively active Fyn were transfected in CD45-deficient Jurkat cells. The ratio of the two plasmid DNA, namely Fyn to SKAP55, was 10:1 to ensure that the green cells were also expressed with the constitutively active Fyn.
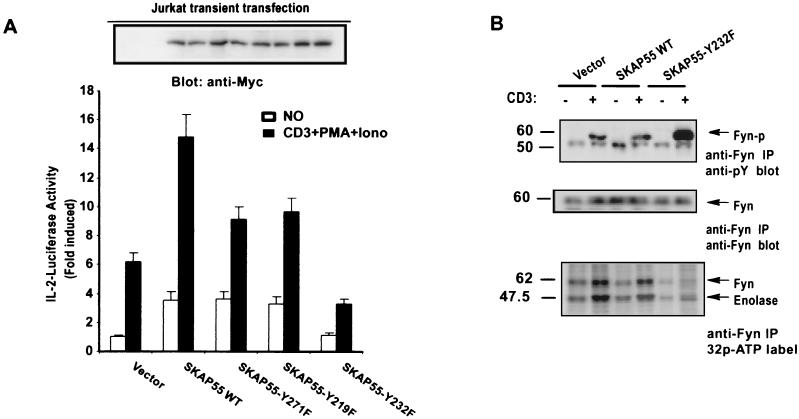
Mutant SKAP55-Y232F suppresses TCR-mediated IL-2 gene transcription.
The TCR-activated distinct signaling cascades ultimately lead to activating transcription of the IL-2 gene (32). In order to evaluate the potential role of SKAP55 in TCR-mediated IL-2 transcriptional activation, a luciferase reporter gene driven by the IL-2 promoter was used for a sensitive reporter assay. Jurkat cells were transiently transfected with SKAP55, mutant SKAP55-Y219F, SKAP55-Y232F, and SKAP55-Y271F or vector only, together with the luciferase reporter gene. The transfected cells were either stimulated with anti-CD3 antibody plus PMA and ionomycin or were left unstimulated, and luciferase activities were measured. The activity assays revealed that overexpression of wild-type SKAP55 significantly increased the activity of the IL-2 promoter when compared with transfection of the control vector in response to the stimulation of anti-CD3 antibody plus PMA and ionomycin (Fig. 7A). Both mutant SKAP55-Y219F and SKAP55-Y271F and mutant SKAP55-Y271F also slightly increased IL-2 transcriptional activity compared with the control. In contrast, however, overexpression of mutant SKAP55-Y232F greatly reduced the transcriptional activation of the IL-2 promoter when compared with transfection of the control vector following the treatment of anti-CD3 antibody plus PMA and ionomycin (Fig. 7A). These results indicate that mutant SKAP55-Y232F plays a dominant-negative function in TCR-mediated IL-2 transcription activation, and thus, SKAP55 positively regulates the transcription activation mediated by IL-2 following TCR stimulation. In CD45-deficient Jurkat cells, expression of the wild type and the three mutants of SKAP55 exerted no detectable effects on IL-2 transcriptional activation following anti-CD3 antibody plus PMA and ionomycin stimulation (data not shown).
FIG. 7.
SKAP55 functions as a positive regulator in IL-2 gene activation. (A) Activation of the IL-2 promoter by SKAP55 in Jurkat cells. Jurkat cells (107) were transiently transfected by electroporation with 10 μg of wild-type (WT) SKAP55, mutant SKAP55-Y219F, SKAP55-Y232F, or SKAP55-Y271F, or vector only, together with 10 μg of IL-2-Luc plasmid carrying the luciferase gene driven by the IL-2 promoter. For the internal control of transfection efficiency, pSV-β-galactosidase vector (1 μg) was cotransfected into Jurkat cells. Each transfected sample was divided into two portions that were either stimulated with anti-CD3 antibody plus PMA (50 ng/ml) and ionomycin (1 μM) or left unstimulated and then cultured for an additional 6 h to activate IL-2 gene transcription. Following incubation, luciferase activities were measured. Relative activity was determined after normalization, with unstimulation being defined as equal to one. The efficiency of transfections was normalized with the activity of β-galactosidase produced by a transfected reporter vector. The data represent the average and standard deviation from three independent experiments. The inset on top represents anti-SKAP55 immunoblot showing the expression of transfected SKAP55 and its mutants. (B) Overexpression of SKAP55-Y232F in Jurkat cells induces the tyrosine hyperphosphorylation of Fyn. Jurkat cells stably expressing the empty vector, SKAP55, and mutant SKAP55-Y232F were treated with anti-CD3 antibody stimulation (+) or were left untreated (−). Cell lysates were prepared and immunoprecipitated (IP) with anti-Fyn antibody. The immunoprecipitates were subjected to SDS-7% PAGE, and the membrane was probed by antiphosphotyrosine antibody. The equal amounts of precipitated Fyn protein in the samples were shown (middle) by stripping the same membrane and reprobing it with anti-Fyn antibody. In the bottom panel, Fyn kinase activity was examined in vitro by its autophosphorylation and the phosphorylation of the exogenous substrate enolase. The immunoprecipitates of Fyn were incubated with the substrate enolase in the presence of [γ-32P]ATP. Incorporation of the label in Fyn and enolase was determined by resolution on SDS-7% PAGE and autoradiography. Molecular mass markers (in kilodaltons) are on the left.
Since the activation of TCR signaling is initiated through the CD45-engaged dephosphorylation of Src-family kinases Fyn and Lck (28), we therefore further investigated the effect of mutant SKAP55-Y232F on the tyrosine phosphorylation of Fyn. In this regard, Jurkat cells stably expressing SKAP55 and mutant SKAP55-Y232F were treated with anti-CD3 antibody or were left untreated. Cell lysates were prepared from the treated and untreated cells. Fyn protein was precipitated from the lysates with anti-Fyn antibody and subjected to immunoblotting with antiphosphotyrosine antibody. Figure 7B shows that Fyn protein was modestly tyrosine phosphorylated in both Jurkat cells and the cells overexpressing wild-type SKAP55 in response to anti-CD3 stimulation. However, overexpression of mutant SKAP55-Y232F resulted in the tyrosine hyperphosphorylation of Fyn protein (Fig. 7B, top), a result similar to that observed with CD45-deficient cells (1, 22). To examine the effect of the tyrosine hyperphosphorylation on Fyn, the in vitro kinase activity of Fyn in immunoprecipitates was analyzed. Both Fyn autophosphorylation and the phosphorylation of its exogenous substrate enolase showed a significantly decreased kinase activity in cells overexpressing mutant SKAP55-Y232F (7B, bottom).
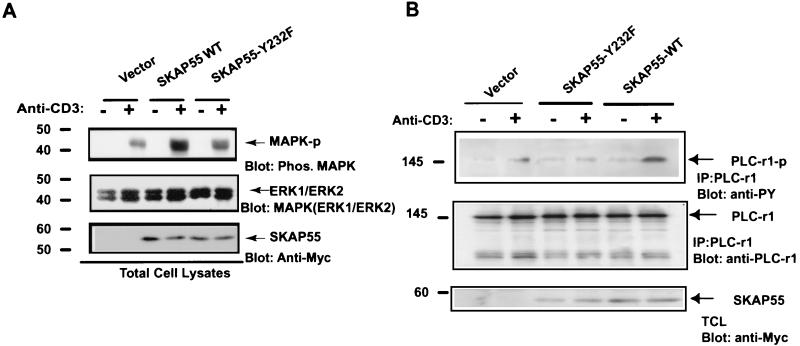
To further investigate the role of SKAP55 in TCR-mediated signaling pathway, the effects of overexpressed SKAP55 on both mitogen-activated protein (MAP) kinase activation and phospholipase γ1 (PLC-γ1) tyrosine phosphorylation were examined. Figure 8A shows that in comparison with the control vector alone, overexpression of wild-type SKAP55 in CD45-positive Jurkat cells strongly enhanced MAP kinase activity, whereas mutant SKAP55-Y232Y exerted only a moderate effect on MAP kinase activation in response to anti-CD3 antibody stimulation. Similarly, overexpression of wild-type SKAP55 in these CD45-positive cells dramatically promoted the tyrosine phosphorylation of PLC-γ1, while mutation of SKAP55 on tyrosine residue 232 resulted in nearly completely blocking the tyrosine phosphorylation of PLC-γ1 activated by anti-CD3 antibody stimulation (Fig. 8B), suggesting that SKAP55 plays a positive role in the TCR-mediated MAP kinase and PLC-γ1 signaling events. Taken together, these results clearly demonstrate that SKAP55 plays a positive role in TCR-mediated IL-2 transcriptional activation and related signaling events, and such a positive role of SKAP55 is exerted through its interaction with both CD45 and the Src family kinases.
FIG. 8.
Overexpression of SKAP55 activates MAPK kinase and induces tyrosine phosphorylation of PLC-γ1. (A) SKAP55 functions as a positive regulator in MAPK kinase pathway. Jurkat cells (2 × 107) either stably transfected with wild-type SKAP55, with mutant SKAP55-Y232F, or with vector were treated with anti-CD3 antibody for 5 min or were left untreated. Total-cell lysates were subjected to SDS-7% PAGE and blotted by anti-phospho-ERK or anti-total-ERK antibody. The total SKAP55 protein in cell lysates was determined by anti-Myc blottings. (B) SKAP55 promotes the tyrosine phosphorylation of PLC-γ1. Aliquots of 8 × 107 Jurkat cells were stablely transfected either with wild-type SKAP55, mutant SKAP55-Y232F, or vector for 24 h. The transfected cells were treated with anti-CD3 antibody for 5 min or were left untreated. The cell lysates were immunoprecipitated by anti-PLC-γ1 antibody and blotted either by antiphosphotyrosine antibody (top) or by anti-PLC-γ1 antibody (middle). The expression of SKAP55 was measured by anti-Myc blotting (bottom). Molecular mass markers (in kilodaltons) are on the left.
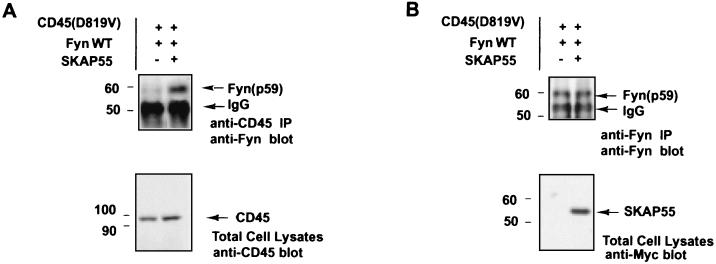
Interaction of CD45 with Fyn was greatly enhanced by SKAP55.
It has been suggested that Src family p56Lck and p59Fyn are putative substrates in vivo for CD45. To investigate how CD45 interacts with the Src family kinases, we transiently coexpressed either Fyn with CD45-D819V alone or Fyn with CD45-D819V and SKAP55 together and then performed coimmunoprecipitation in 293T cells. Since there is no TCR-multiple protein complex in 293T cells, indirect interactions of these components via a third partner contributed by the TCR-immucomplex would be excluded. Immunoblottings revealed that in the absence of SKAP55, Fyn was barely coprecipitated with CD45-D819V (Fig. 8A), a result similar to that reported for COS cells (4). However, when SKAP55 was coexpressed with these two proteins together, Fyn was readily coprecipitated with CD45-D819V (Fig. 8A). These results suggest that the adapter SKAP55 plays a linker function to promoter the interaction of CD45 with Fyn for activating the kinase through tyrosine dephosphorylation in vivo.
DISCUSSION
To identify proteins directly interacting with CD45, we have used a CD45-D819V substrate-trapping mutant as bait in a modified yeast two-hybrid screen and found that the Src kinase-associated phosphoprotein, SKAP55, directly associated with CD45. The association of SKAP55 with CD45 was further confirmed in vivo in Jurkat cells by immunoprecipitation and direct cross-linking of the two proteins (Fig. 2). Several lines of evidence suggest that this adapter protein is a substrate interacting with CD45. First, SKAP55 interacted with the substrate-trapping mutant CD45-D819V but not with the catalytic inactive mutant CD45-C828S, in which the essential nucleophilic cysteine-828 in the phosphatase active site for substrate interaction was mutated to serine. Second, the interaction of SKAP55 with mutant CD45-D819V or wild-type CD45 was interrupted by pervanadate, a PTPase inhibitor, which is known to covalently modify the essential nucleophilic cysteine residue in the phosphatase active site (12, 29). More importantly, when performing the coimmunoprecipitation of SKAP55 with CD45 in T cells, it was found that SKAP55 was largely dephosphorylated by wild-type CD45 but not by the substrate-trapping mutant CD45-D819V (Fig. 4). These results clearly demonstrate that SKAP55 as substrate in vivo associates with CD45 through its binding to the phosphatase catalytic active site. Mutational analysis revealed that mutant SKAP55-Y232F nearly completely abolished its binding to CD45, and mutation of Y219F in the adapter also greatly blocked its binding to the phosphatase, suggesting that CD45 likely binds to the two different sites. Tyr-271 does not have an appreciable contribution to the interaction (Fig. 3). Interestingly, Tyr-271 resides in the peptide sequence motif EDIYEVL (EXXYXXL) and was found to bind the SH2 domain of Fyn (data not shown) confirming the previous prediction for the interaction (20). Since elimination of either Tyr-232 or Tyr-219 led to an almost complete loss of the interaction, one explanation would be that both sites coordinately stabilize the interaction of SKAP55 with CD45. Alternatively, other unknown factor(s) may have been involved in effecting the interaction.
Although both endogenous and transiently expressed SKAP55 are constitutively tyrosine phosphorylated in Jurkat cells (19), we revealed that the level of tyrosine phosphorylation on SKAP55 was substantially increased following anti-CD3 antibody stimulation (Fig. 5). The tyrosine phosphorylation of SKAP55 in cells coexpressing wild-type CD45 was substantially reduced (Fig. 4B) due to its substrate nature in the interaction. As a potential substrate of SKAP55 for CD45, it is expected that its tyrosine phosphorylation should be largely, if not entirely, protected in CD45-negative cells. However, the tyrosine phosphorylation of SKAP55 was hardly detectable in CD45-deficient Jurkat cells (Fig. 5A). It is likely that in CD45-negative cells, the Src family kinases are inactive, as their activations in T cells are initiated by CD45-mediated dephosphorylation and the tyrosine phosphorylation of SKAP55 is thought to be done by the active Src family kinase Fyn (19). Indeed, cotransfection of the constitutively active mutant Fyn-Y531F greatly promoted the tyrosine phosphorylation of SKAP55 in these CD45-deficient cells (Fig. 5B), supporting the notion that SKAP55 is a substrate for CD45. In Jurkat cells, but not in CD45-deficient Jurkat cells, stimulation with anti-CD3 antibody also led to the translocation of SKAP55 from the cytoplasm to the cell membrane. However, such anti-CD3 antibody-triggered translocation of SKAP55 was completely blocked by mutation of Tyr-232 (Fig. 6f). At present, it is not clear why mutant SKAP55-Y232F blocked its translocation. Nevertheless, these results suggest that with anti-CD3 antibody stimulation SKAP55 can be further tyrosine phosphorylated and the phosphorylated adapter is recruited specifically via its phosphorylated Tyr-232 residue to the membrane for exerting its adapter function (see below). The function of SKAP55 in conjunction with CD45 activity in TCR-mediated signal transduction was demonstrated by its effect on gene transcription (Fig. 7), MAP kinase activation, and tyrosine phosphorylation of PLC-γ1 (Fig. 8). Overexpression of wild-type SKAP55 in Jurkat cells increased the activation of the IL-2 promoter, whereas mutant SKAP55-Y232F exhibited a strong dominant-negative effect on the transcriptional activation when compared with the control vector (Fig. 7A). Furthermore, overexpression of mutant SKAP55-Y232F also led to the tyrosine hyperphosphorylation of Fyn, a phenomenon observed in all CD45-deficient cells in which both the abolition of TCR signaling and the loss of TCR-mediated tyrosine phosphorylation were observed (15). Since Lck does not interact with SKAP55 (18), expression of mutant SKAP55-Y232F would not dramatically exert its effect on the tyrosine phosphorylation of this Src kinase. The in vitro kinase activity assay also revealed that the tyrosine-hyperphosphorylated Fyn exhibited a decreased kinase activity (Fig. 7B, bottom). Thus, through the interaction of its phosphorylated Tyr-232 residue with CD45, SKAP55 functions as an essential adapter to direct CD45 for the dephosphorylation of Src family kinase Fyn and thereby positively regulates TCR-mediated gene transcription.
Previous studies have suggested that both p56Lck and p59Fyn are potential in vivo substrates for CD45. As discussed above, this is based on the well-established observation that in all CD45-deficient cells, tyrosine phosphorylation at the inhibitory site of these Src family kinases is significantly increased and that this correlates with inefficient TCR signaling through the antigen receptor (1, 28). However, there is no clear evidence to support that these two tyrosine kinases directly interact with CD45. In the TCR-multiple protein complex, p56Lck was reported to coprecipitate with CD45 via other signaling molecules such as CD4, CD8, p34, and 30- to 32-kDa proteins (16, 24). It is also known that without third partners, p59Fyn failed to coprecipitate with CD45 in COS cells (4). Thus, it is unclear how p59 Fyn and p56Lck as putative substrates interact with CD45 for tyrosine dephosphorylation in TCR signaling. Our present results suggest that CD45 probably does not directly interact with the Src family kinase Fyn. Instead, it recruits adapter SKAP55 as a linker to access the kinase for dephosphorylation. This notion was further supported by coimmunoprecipitation following coexpression of Fyn either with CD45 alone or with CD45 plus SKAP55 in 293T cells. As demonstrated in Fig. 9, in the absence of SKAP55, the association of Fyn with CD45 was hardly detectable, and coexpression of SKAP55 dramatically promoted the interaction of Fyn with CD45. All these data strongly suggest that interaction of CD45 with Fyn in T cells for dephosphorylation is very likely mediated by the adapter protein SKAP55, rather than directly binding of CD45 to this kinase as currently viewed. At present, the mechanism by which CD45 regulates Src family kinase activities in vivo is not completely understood. It is known that in resting cells the tyrosine-phosphorylated inhibitory residue of Src family kinases at the C terminus folds back to bind the SH2 domain of the kinases and inhibits kinase activity. The interaction of the inhibitory site with the SH2 domain of the kinases is of a low affinity, and dephosphorylation of the inhibitory site is an initial event in Fyn and Lck activation (28). Since mutant SKAP55-Y232F suppressed the CD3-stimulated gene transcription and induced the tyrosine hyperphosphorylation of Fyn, it is likely that through the interaction of CD45 with the Tyr-232 residue of SAKP55, CD45 recruits this adapter to the membrane. On the membrane SKAP55, via its phosphorylated Tyr-271, further binds the SH2 domain of Fyn to replace the low-affinity bound inhibitory site of the kinase. Consequently, CD45 may have transiently disassociated with the Tyr-232 residue of SKAP55 through dephosphorylation and simultaneously interacted with the released the phosphorylated inhibitory tyrosine residue of Fyn for dephosphorylation, resulting in activation of the Src family kinase Fyn and initiation of TCR-engaged signal transduction. At present, the precise order of processive binding and dephosphorylation between CD45, SKAP55, and Fyn on the TCR complex is yet to be elucidated. It is worthy of note that the interaction of tyrosine-phosphorylated SKAP55 with Fyn occurs readily in Jurkat cells as demonstrated in our coimmunoprecipitation experiments (data not shown). Such tyrosine phosphorylation-dependent association of SKAP55 with Fyn in Jurkat cells may have been neglected in a previous report (21).
FIG. 9.
SKAP55 as an adapter bridges CD45 with Fyn for interaction. (A) Fyn was either transfected only with CD45-D819V or was cotransfected with both CD45-D819V and SKAP55 in 293T cells. At 48 h following the transfection, cell lysates were immunoprecipitated (IP) by anti-CD45 specific antibody and subjected to SDS-7% PAGE and immunoblotted by anti-Fyn antibody. The bottom panel is a Western blot of total CD45 from whole-cell lysates, indicating the equal expression of CD45-D819V in the two samples. (B) As described for panel A, the cell lysates were immunoprecipitated with anti-Fyn specific antibody. The immunoprecipitates were subjected to SDS-7% PAGE and immunoblotted with anti-Fyn antibody again, demonstrating its equal precipitation. The bottom panel is a Western blot of SKAP55 from whole-cell lysates, indicating its appropriate expression in the sample. Molecular mass markers (in kilodaltons) are on the left.
In summary, our results suggest that CD45, contrary to the current view of its direct interaction with Fyn and Lck, transiently recruits adapter SKAP55. The latter binds to the SH2 domain of the Src family kinase Fyn and brings CD45 to access the released inhibitory tyrosine-phosphorylated site of Fyn for dephosphorylation and hence positively regulates TCR signaling.
Acknowledgments
We thank Dick Mosser and Darrell D. Mousseau for valuable comments on the manuscript, Denis Banville and Zhanbao Yu for valuable discussion and advice, D. L'Abbe for technical assistance, and N. Jolicoeur for preparation of the figures.
This work was supported in part by a grant from the International Human Frontier Programs and in part by The Natural Science and Engineering Research Council of Canada Grant 0GP0183691.
REFERENCES
- 1.Altin, J. G., and E. K. Sloan. 1997. The role of CD45 and CD45-associated molecules in T cell activation. Immunol. Cell Biol. 75:430-445. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, S. J., S. D. Levin, and R. M. Perlmutter. 1994. Involvement of the protein tyrosine kinase p56lck in T cell signaling and thymocyte development. Adv. Immunol. 56:151-178. [DOI] [PubMed] [Google Scholar]
- 3.Barford, D., A. J. Flint, and N. K. Tonks. 1994. Crystal structure of human protein tyrosine phosphatase 1B. Science 263:1397-1404. [PubMed] [Google Scholar]
- 4.Bhandari, V., K. L. Lim, and C. J. Pallen. 1998. Physical and functional interactions between receptor-like protein-tyrosine phosphatase alpha and p59fyn. J. Biol. Chem. 273:8691-8698. [DOI] [PubMed] [Google Scholar]
- 5.Chan, A. C., D. M. Desai, and A. Weiss. 1994. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu. Rev. Immunol. 12:555-592. [DOI] [PubMed] [Google Scholar]
- 6.Cochet, C., O. Kashles, E. M. Chambaz, I. Borrello, C. R. King, and J. Schlessinger. 1988. Demonstration of epidermal growth factor-induced receptor dimerization in living cells using a chemical cross-link agent. J. Biol. Chem. 263:3290-3295. [PubMed] [Google Scholar]
- 7.Desai, D. M., J. Sap, J. Schlessinger, and A. Weiss. 1993. Ligand-mediated negative regulation of a chimeric transmembrane receptor tyrosine phosphatase. Cell 73:541-554. [DOI] [PubMed] [Google Scholar]
- 8.Desai, D. M., J. Sap, O. Silvennoinen, J. Schlessinger, and A. Weiss. 1994. The catalytic activity of the CD45 membrane-proximal phosphatase domain is required for TCR signaling and regulation. EMBO J. 13:4002-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flint, A. J., T. Tiganis, D. Barford, and N. K. Tonks. 1997. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 94:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frearson, J. A., and D. R. Alexander. 1997. The role of phosphotyrosine phosphatases in haematopoietic cell signal transduction. Bioessays 19:417-427. [DOI] [PubMed] [Google Scholar]
- 11.Guan, K. L., and J. E. Dixon. 1991. Evidence for protein-tyrosine-phosphatase catalysis proceeding via a cysteine-phosphate intermediate. J. Biol. Chem. 266:17026-17030. [PubMed] [Google Scholar]
- 12.Huyer, G., S. Liu, J. Kelly, J. Moffat, P. Payette, B. Kennedy, G. Tsaprailis, M. J. Gresser, and C. Ramachandran. 1997. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J. Biol. Chem. 272:843-851. [DOI] [PubMed] [Google Scholar]
- 13.Kang, H., C. Freund, J. S. Duke-Cohan, A. Musacchio, G. Wagner, and C. E. Rudd. 2000. SH3 domain recognition of a proline-independent tyrosine-based RKxxYxxY motif in immune cell adaptor SKAP55. EMBO J. 19:2889-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keegan, K., and J. A. Cooper. 1996. Use of the two hybrid system to detect the association of the protein-tyrosine-phosphatase, SHPTP2, with another SH2-containing protein, Grb7. Oncogene 4:1537-1544. [PubMed] [Google Scholar]
- 15.Koretzky, G. A., J. Picus, T. Schultz, and A. Weiss. 1991. Tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc. Natl. Acad. Sci. USA 88:2037-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koretzky, G. A. 1993. Role of the CD45 tyrosine phosphatase in signal transduction in the immune system. FASEB J. 7:420-426. [DOI] [PubMed] [Google Scholar]
- 17.Liu, J., H. Kang, M. Raab, A. J. da Silva, S. K. Kraeft, and C. E. Rudd. 1998. FYB serves as a binding partner for lymphoid protein and FYN kinase substrate SKAP55 and a SKAP55-related protein in T cells. Proc. Natl. Acad. Sci. USA 95:8779-8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marie-Cardine, A., A. M. Verhagen, C. Eckerskorn, and B. Schraven. 1998. SKAP-HOM, a novel adaptor protein homologous to the FYN-associated protein SKAP55. FEBS Lett. 435:55-60. [DOI] [PubMed] [Google Scholar]
- 19.Marie-Cardine, A., and B. Schraven. 1999. Coupling the TCR to downstream signaling pathways: the role of cytoplasmic and transmembrane adaptor proteins. Cell Signal. 11:705-712. [DOI] [PubMed] [Google Scholar]
- 20.Marie-Cardine, A., E. Bruyns, A. M. Verhagen, C. Eckerskorn, H. Kirchgessner, S. C. Meuer, and B. Schraven. 1997. Molecular cloning of SKAP55, a novel protein that associates with the protein tyrosine kinase p59fyn in human T-lymphocytes. J. Biol. Chem. 272:16077-16080. [DOI] [PubMed] [Google Scholar]
- 21.Marie-Cardine, A., H. Kirchgessner, and B. Schraven. 1999. Molecular alterations of the Fyn-complex occur as late events of human T cell activation. Eur. J. Immunol. 29:1175-1187. [DOI] [PubMed] [Google Scholar]
- 22.McFarland, E. D., T. R. Hurley, J. T. Pingel, B. M. Sefton, A. Shaw, and M. L. Thomas. 1993. Correlation between Src family member regulation by the protein-tyrosine-phosphatase CD45 and transmembrane signaling through the T-cell receptor. Proc. Natl. Acad. Sci. USA 90:1402-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudd, C. E. 1999. Adaptors and molecular scaffolds in immune cell signaling. Cell 96:5-8. [DOI] [PubMed] [Google Scholar]
- 24.Schraven, B., H. Kirchgessner, B. Gaber, Y. Samstag, and S. Meuer. 1991. A functional complex is formed in human T lymphocytes between the protein tyrosine phosphatase CD45, the protein tyrosine kinase p56lck and pp32, a possible common substrate. Eur. J. Immunol. 21:2469-2477. [DOI] [PubMed] [Google Scholar]
- 25.Streuli, M., L. R. Hall, Y. Saga, S. F. Schlossman, and H. Saito. 1987. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J. Exp. Med. 166:1548-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su, L., Z. Zhao, P. Bouchard, D. Banville, E. H. Fischer, E. G. Krebs, and S. H. Shen. 1996. Positive effect of overexpressed protein-tyrosine phosphatase PTP1C on mitogen-activated signaling in 293 cells. J. Biol. Chem. 271:10385-10390. [DOI] [PubMed] [Google Scholar]
- 27.Thomas, M. L. 1994. The regulation of B- and T-lymphocyte activation by the transmembrane protein tyrosine phosphatase CD45. Curr. Opin. Cell Biol. 6:247-252. [DOI] [PubMed] [Google Scholar]
- 28.Thomas, M. L. 1999. The regulation of antigen-receptor signaling by protein tyrosine phosphatases: a hole in the story. Curr. Opin. Immunol. 11:270-276. [DOI] [PubMed] [Google Scholar]
- 29.Tiganis, T., A. M. Bennett, K. S. Ravichandran, and N. K. Tonks. 1998. Epidermal growth factor receptor and the adaptor protein p52 Shc are specific substrates of T-cell protein tyrosine phosphatase. Mol. Cell. Biol. 18:1622-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trowbridge, I. S., and M. L. Thomas. 1994. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu. Rev. Immunol. 12:85-116. [DOI] [PubMed] [Google Scholar]
- 31.Wang, Y., L. Liang, and W. J. Esselman. 2000. Regulation of the Calcium/NF-AT T cell activation pathway by the D2 domain of CD45. J. Immunol. 164:2557-2564. [DOI] [PubMed] [Google Scholar]
- 32.Weiss, A., and D. R. Littman. 1994. Signal transduction by lymphocyte antigen receptors. Cell 76:263-274. [DOI] [PubMed] [Google Scholar]