Abstract
Using mouse knockouts for mitogen- and stress-activated protein kinase 1 (MSK1) and MSK2 and a double knockout of both MSK1 and MSK2, we show that these protein kinases are required for the stress-induced phosphorylation of transcription factors CREB and ATF1 in primary embryonic fibroblasts. In contrast mitogen-induced phosphorylation of CREB and ATF1 is greatly reduced but not totally abolished. The mitogen- and stress-induced phosphorylation of CREB at Ser133 has been linked to the transcription of several immediate early genes, including c-fos, junB, and egr1. The knockout of both MSK1 and MSK2 resulted in a 50% reduction in c-fos and junB gene transcription in response to anisomycin or UV-C radiation but only a small reduction in response to tetradecanoyl phorbol acetate or epidermal growth factor in fibroblasts. The transcription of egr1 in response to both mitogenic and stress stimuli, as well as stress-induced apoptosis, was unaffected in the MSK1/MSK2 double knockout.
Mitogen-activated protein kinase (MAPK) cascades are involved in the transduction of signals from mitogens and cellular stresses into appropriate cellular responses and are required for many functions including cell proliferation, differentiation, and survival (10). One of the ways in which MAPKs produce cellular responses is by the phosphorylation and activation of transcription factors, either directly or indirectly by other protein kinases that they activate. One such transcription factor is the cyclic AMP response element-binding protein, CREB, which requires phosphorylation at Ser133 to become active. Ser133 phosphorylation is induced by stimulating cells with cyclic AMP-elevating agents or mitogens or by exposure to cellular stresses. The cyclic AMP-induced phosphorylation of CREB is catalyzed by cyclic AMP-dependent protein kinase (PKA), but mitogen-induced phosphorylation is prevented by compounds PD-98059, U0126, and PD-184352 (4, 14, 41), which prevent the activation of MAPK kinase 1 (MKK1) and hence block the classical MAPK cascade relatively specifically. At higher concentrations they also inhibit the activation of MKK5 and its substrate extracellular signal-regulated kinase 5 (ERK5) (24, 29). In contrast, the stress-induced phosphorylation of CREB is prevented by SB-203580 (14), an inhibitor of another MAPK family member, stress-activated protein kinase 2 (SAPK2), or p38, which is a component of a distinct signal transduction pathway.
The phosphorylation of CREB at Ser133 is not catalyzed by MAPK family members directly but by other protein kinases that they activate. Protein kinases that are activated by mitogenic stimuli and that phosphorylate CREB at Ser133 in vitro include the isoforms of MAPK-activated protein kinase 1 (MAPKAP-K1, also called RSK) and mitogen and stress-activated protein kinase (MSK), which are activated by ERK1 and ERK2 of the classical MAPK cascade. However, whether both of these protein kinases or just one of them mediates the phosphorylation of CREB in vivo under different conditions and in different cells and tissues is unclear. MAPKAP-K1b (also called RSK2) was reported to be the major CREB kinase in extracts from nerve growth factor (NGF)-stimulated PC12 cells, and the NGF-induced phosphorylation of CREB was inhibited by the overexpression of a dominant-negative mutant (44, 45). Moreover, fibroblast cell lines derived from human patients with Coffin-Lowry syndrome, which carry an inactivating mutation in the MAPKAP-K1b/RSK2 gene, were reported to be unable to phosphorylate CREB after stimulation with EGF (15). However, more recently, insulin-like growth factor 1 and platelet-derived growth factor were found to induce a normal phosphorylation of CREB at Ser133 in fibroblasts isolated from a knockout of MAPKAP-K1b/RSK2 in mice (8). On the other hand, MSK1, which is localized to the cell nucleus, was found to phosphorylate CREB at Ser133 in vitro with a far lower Km value than those of MAPKAP-K1/RSK isoforms (14). Moreover, mitogen-induced phosphorylation of CREB at Ser133 was greatly reduced in embryonic stem (ES) cells unable to express MSK1 (4). The mitogen-induced activation of MAPKAP-K1a/RSK1, MAPKAP-K1b/RSK2, and MAPKAP-K1c/RSK3 is normal in these cells.
MSK1 and the closely related MSK2 are attractive candidates to mediate the stress-induced phosphorylation of CREB, because they are also activated in cells by SAPK2/p38 (14). In addition, the overexpression of MSK2 stimulates CREB-dependent reporter gene transcription in transfected cells (32). Several other protein kinases are activated by SAPK2/p38, such as MAPKAP-K2, MAPKAP-K3, PRAK, MNK1, and MNK2; the first two of these have been shown to phosphorylate CREB at Ser133 (27, 39). However, the activity of all five of these enzymes is unaffected by Ro-318220 or H-89, two compounds that block the stress-induced phosphorylation of CREB (and that inhibit MSK1 and MSK2 [13, 14]), excluding their involvement in the stress-induced phosphorylation of CREB at Ser133. However, Ro-31-8220 and H-89 inhibit several protein kinases besides MSK1 and MSK2 (13). For this reason, the possibility that these compounds exert their effects by inhibiting another protein kinase(s) that is also activated by SAPK2/p38 is not ruled out.
The transcription of a number of immediate-early genes, such as c-fos and egr1, is dependent on the activation of MAPK cascades. Their mitogen-stimulated transcription is suppressed by inhibition of the classical MAPK cascade (22, 41), and their stress-induced transcription is suppressed by inhibition of SAPK2/p38 (18, 20, 21). The serum response element (SRE) and the cyclic AMP response element (CRE) in the promoters of these genes are reported to be important for mitogen- and stress-induced transcription of these genes and are the elements targeted by MAPK cascades. A ternary complex factor (TCF) and the serum response factor bind to the SRE, and transcription is stimulated by the direct phosphorylation of a TCF by a MAPK family member (17, 23, 28, 33, 46). CREB binds to the CRE, and transcription is increased by the phosphorylation of CREB at Ser133 (16, 19, 37).
To determine the roles of MSK1 and MSK2 in the activation of CREB and the induction of immediate-early genes, we produced knockout mice that do not express MSK1 or MSK2 and double knockouts unable to produce both protein kinases. Using embryonic fibroblasts derived from these mice we were able to demonstrate an important role for these enzymes in the activation of CREB and the closely related transcription factor ATF1. We also studied the mitogen- and stress-induced transcription of the c-fos, egr1, and junB genes in the knockout animals.
MATERIALS AND METHODS
Materials.
PD-184352 was from Upstate Biotechnology (Park Leys, Buckingham, United Kingdom) and SB-203580, U0126, H-89, and Ro-31-8220 were from Calbiochem (Nottingham, United Kingdom). Tetradecanoyl phorbol acetate (TPA) and anisomycin were from Sigma-Aldrich (Poole, United Kingdom), and epidermal growth factor (EGF) was from Life Technologies (Paisley, United Kingdom).
Generation of MSK1 and MSK2 knockouts.
The production of ES cells containing a targeted MSK1 gene has been described previously (4). The mouse MSK2 gene was obtained by screening a 129SvJ mouse BAC genomic library (Genome Systems) with an expressed sequence tag corresponding to the 3′ region of the mouse MSK2 cDNA. The MSK2 gene was then subcloned from a positive BAC clone and fully sequenced. The gene was found to comprise 17 exons and spanned 15 kb.
A targeting vector was designed to delete exons 5 to 7 of the MSK2 gene and replace them with a neomycin resistance gene. Exons 5 to 7 encode amino acids 155 to 251 of MSK2, which constitute the middle of the N-terminal kinase domain of MSK2. The neomycin resistance gene contains polyadenylation sequences which are expected to block transcription of the 3′ region of the gene. If readthrough did occur, splicing from exons 4 to 8 would result in a frameshift mutation (Fig. 1A). The targeting vector comprised a NotI site followed by a 5′ arm of homology of 1 kb, which was generated by PCR. This was followed by the neomycin resistance gene and a 3′ arm of homology of 6.3 kb and finally a thymidine kinase cassette for negative selection. The 3′ arm of homology was generated by a combination of a 620-bp PCR fragment joined to a 5.7-kb XbaI fragment subcloned from the MSK2 BAC clone. All PCR fragments were checked by DNA sequencing to ensure that there were no PCR-generated mutations. The vector was linearized using NotI before transfection of the ES cells.
FIG. 1.
Generation of ES cells with a targeted MSK2 gene. (A) A targeting vector was made to delete exons 5 to 7 of the murine MSK2 gene through the addition of a neomycin selection cassette and polyadenylation sequences. A thymidine kinase cassette acts as a negative selection marker. Positions of the 3′ and 5′ probes used to screen for the correct incorporation of the targeting vector by NcoI digestion are shown. (B and C) Genomic DNA from wild-type and MSK2 heterozygous ES cells was digested with NcoI, run on an 0.8% agarose gel, and Southern blotted with either the 3′ (B) or 5′ (C) probe. An 11.8-kb band indicating a wild-type locus and an 8.4-kb band indicating a targeted locus (due to an additional NcoI site within the neomycin selection cassette) are shown for blots with the 3′ probe. For blots with the 5′ probe, an 11.8-kb band for the wild-type locus and a 3.4-kb band for a targeted locus are shown.
For the ES cell culture, ES cells were grown on fibroblasts whose growth was arrested with mitomycin C and which carried an inserted neomycin resistance gene. ES cells were cultured and transfected as described previously (5, 43). Colonies resistant to G418 and ganciclovir after transfection of the MSK2 gene-targeting vector were expanded and screened for homologous recombination by Southern analysis of NcoI digests using 3′ and 5′ probes external to the targeting vector (Fig. 1B and C).
Heterozygous ES cell clones for MSK1 or MSK2 were injected into C57BL/6 × BALB/c mouse blastocysts, which were then reimplanted into recipient female mice. Chimeric male mice showing a high percentage of ES cell contribution were identified by coat color and crossed to BALB/c females. Animals were maintained under specific-pathogen-free conditions, and all procedures were carried out in accordance with University of Dundee and United Kingdom Home Office regulations. Germ line transmission was identified by a characteristic grey coat color, and the genotypes of these mice were confirmed by Southern analysis on tail biopsy samples. Subsequent genotyping was carried out by PCR using, for MSK1 knockout mice, primers CACTTCGCCCAATAGCAGCCAGTCCCTTCC (targeted), TCCGCAGCTCGTGCTTGACAGTAAGGAGC (wild type), and AATAGCGCTGGTGGCTCAGGGCTGT (targeted or wild type), which give a fragment of 870 bp from a targeted gene and 350 bp from a wild-type gene. Genotyping for MSK2 knockout mice was carried out with primers CGTTGGCTACCCGTAATATTGCTGAAGAGC (targeted), AAGATCTTCAGGGCATCTCTTTATCCTACG (wild type), and TTGTGCTCCCCATGCTGCAGCCCGGCCTTC (targeted or wild type), which give a fragment of 1,030 bp for a wild-type gene and 600 bp for a targeted gene. The PCR program consisted of a hot start at 80°C; this was followed by 35 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 2.5 min.
Cell culture and lysis.
Primary mouse fibroblasts were isolated from day 13.5 mouse embryos. The heads and internal organs were removed from the embryos. The remaining tissues were then cut into small pieces, and single cells were obtained by incubation in trypsin. Cells were then cultured in Dulbecco modified Eagle medium (DMEM) containing 10% serum (Sigma), 2 mM l-glutamine, 50 U of penicillin G/ml, and 50 μg of streptomycin (Life Technologies)/ml and used at passages 3 to 5. Cells were serum starved in DMEM with l-glutamine, penicillin, and streptomycin for 16 h before stimulation. Cells were stimulated with TPA (400 ng/ml), EGF (100 ng/ml), anisomycin (10 μg/ml), or UV-C (25 J/m2 or 200 J/m2) for various times, as indicated in the figure legends. Cells were then lysed in a solution containing 50 mM Tris-HCl, pH 7.5, 1 mM EGTA, 1 mM EDTA, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 1 mM sodium pyrophosphate, 0.27 M sucrose, 1 μM microcystin-LR, 1% (vol/vol) Triton X-100, 0.1% (vol/vol) 2-mercaptoethanol, and Complete proteinase inhibitor cocktail (Roche, East Sussex, United Kingdom). The lysates were centrifuged at 18,000 × g for 5 min at 4°C and the supernatants were removed, quick-frozen in liquid nitrogen, and stored at −80°C until use. To study the phosphorylation of CREB, cells were lysed directly into the same buffer supplemented with 1% (wt/vol) sodium dodecyl sulfate (SDS) and 5% (vol/vol) glycerol and heated for 10 min at 95°C. For measurement of kinase activities by immunoprecipitation assays, preliminary time course experiments were carried out with wild-type fibroblasts and subsequent stimulations were carried out at the time point which gave maximal activation of MSK1, MSK2, MAPKAP-K1, and MAPKAP-K2.
Immunoprecipitation and assay of protein kinases.
Antipeptide antibodies that recognize MSK1 (residues 384 to 402), MSK2 (residues 753 to 772), MAPKAP-K1b/RSK2 (residues 712 to 734), and MAPKAP-K2 (residues 343 to 358) (2, 9, 12, 14) were used to immunoprecipitate these protein kinases from cell lysates as described previously (42). MSK1 and MSK2 were assayed after immunoprecipitation from 0.5 mg of cell lysate that had first been precleared with protein G-Sepharose, and MAPKAP-K1/RSK and MAPKAP-K2 were assayed after immunoprecipitation from 0.05 mg of cell lysate. Crosstide (the peptide GRPRTSSFAEG) was used for the assays of MSK1, MSK2, and MAPKAP-K1/RSK, and peptide KKLNRTLSVA was used for assays of MAPKAP-K2. One unit of activity was that amount of enzyme that incorporates 1 nmol of phosphate into the peptide substrate in 1 min.
Immunoblotting.
Samples were run on 4 to 12% polyacrylamide gels (Novex; Invitrogen) and transferred onto nitrocellulose membranes. Antibodies that recognize ERK, phospho-ERK, SAPK2, phospho-SAPK2/p38, and CREB were from New England Biolabs (Hitchin, United Kingdom). Antibodies against phospho-CREB were from Upstate Biotechnology. Horseradish peroxidase-conjugated secondary antibodies were from Pierce (Cheshire, United Kingdom), and detection was performed using the enhanced chemiluminescence reagent from Amersham (Buckinghamshire, United Kingdom).
Analysis of the expression of immediate-early genes.
Total RNA was isolated from primary fibroblasts with the RNeasy Mini-Kit (Qiagen, Crawley, United Kingdom). Reverse transcription-PCR was carried out with Access RT-PCR (Promega, Southampton, United Kingdom) for both reverse transcription and amplification. Total RNA (50 ng) was amplified using 28 cycles of 94°C for 30 s, 63°C for 1 min, and 68°C for 2 min. Primers for c-fos and 18S RNA were from Ambion (Cambridgeshire, United Kingdom), and primers for egr1 were GACCCGTTCGGCTCCTTTCCTCAC and GCTGTCGTTTGGACGGCACGGCACA. Relative RNA levels for c-fos, egr1, and cyclophilin were quantified by RNase protection with the RPA II kit (Ambion) using the pTRI templates for c-fos, egr1, junB, and cyclophilin (Ambion). RNA levels were determined with a phosphorimager and standardized to the cyclophilin control.
Apoptosis assays.
Apoptotic cell death was measured with the cell death detection ELISA PLUS kit (Roche). Primary embryonic fibroblasts at passage 3 were plated onto 96-well plates at 3,000 cells/well. After 36 h the cells were treated with either UV at 25 J/m2 or 200 J/m2 or anisomycin at 10 μg/ml and incubated in serum-free medium for a further 24 h. Cells were then lysed, and the enzyme-linked immunosorbent assay was carried out in accordance with the manufacturer's protocol. The level of cell death was quantified as fold stimulation over that for serum-deprived untreated cells, after correcting for cell number.
RESULTS
Generation of MSK1 and MSK2 knockout mice.
The targeting construct for MSK1 has been described previously (4), and that for MSK2 is shown in Fig. 1A. The heterozygous ES cells containing the targeted genes were identified by the appearance of 8.4- and 3.4-kb bands on Southern blots when using a 3′ (Fig. 1B) or 5′ (Fig. 1C) probe, respectively, in addition to the 11.8-kb wild-type band. Full details of how the mouse knockouts were generated are given in Materials and Methods. For both the MSK1 and MSK2 knockouts, germ line transmission was obtained for multiple ES cell clones. Both the MSK1 and MSK2 knockouts were viable and fertile and of normal appearance and had no obvious health problems when kept under specific-pathogen-free conditions. To confirm that a double knockout of both MSK1 and MSK2 could be produced by intercrossing, the chromosomal localization of MSK1 and MSK2 was determined by fluorescent in situ hybridization analysis. MSK1 localized to chromosome 12F, while MSK2 localized to chromosome 19B (data not shown). MSK1/MSK2 double knockouts were therefore generated by crossing mice with single knockouts of each protein kinase to give mice heterozygous for both the MSK1 and MSK2 knockout. The double-knockout mice were then obtained by intercrossing, and genotypes were confirmed by PCR (Fig. 2A). Like the single knockouts, the double knockout was viable and fertile and had no obvious health problems.
FIG. 2.
Genotyping and expression levels of MSK1 and MSK2 in the knockout mice. (A) Genotyping for MSK1 (top) and MSK2 (bottom) knockout mice from a tail biopsy sample. DNA samples were subjected to PCR as described in Materials and Methods, electrophoresed on 1.5% (wt/vol) agarose gels, and examined by ethidium bromide staining. (B) Lysates from wild-type, MSK1, MSK2, and MSK1/MSK2 knockout fibroblasts (30 μg of protein) were immunoblotted as described in Materials and Methods with an antibody raised against the MSK1 protein. Alternatively, MSK2 was immunoprecipitated from 1 mg of fibroblast cell lysate protein using an antipeptide antibody raised against residues 753 to 772 of MSK2 in sheep. The immunoprecipitates were resuspended in SDS, electrophoresed, and immunoblotted as described in Materials and Methods with an antibody against the MSK2 protein raised in rabbits. Lysates from two different dishes of cells were used for each condition.
To determine the effects of the knockouts, embryonic fibroblasts were derived from day 13.5 embryos. Immunoblotting using an antibody raised against the whole MSK1 protein showed that it was expressed at similar levels in the wild-type and MSK2 knockout fibroblasts but was not detectable in the MSK1 or double-knockout fibroblasts (Fig. 2B). To detect the MSK2 protein, it was first immunoprecipitated with an anti-MSK2 peptide antibody and then immunoblotted with an anti-MSK2 protein antibody. This showed similar levels of MSK2 in wild-type and MSK1 knockout fibroblasts but no MSK2 in the MSK2 knockout and double-knockout fibroblasts (Fig. 2B). The levels of expression of ERK1, ERK2, SAPK2/p38, MAPKAP-K1b/RSK2, and MAPKAP-K1c/RSK3 in either the single or double knockouts were similar to those for wild-type mice, as judged by immunoblotting with specific antibodies. MAPKAP-K1a/RSK1 did not appear to be present in the fibroblasts from either the wild-type or knockout cells (data not shown).
Activation of the classical MAPK cascade and SAPK2/p38 pathways in primary fibroblasts.
To identify the times at which the activation of MSK1, MSK2, MAPKAP-K1b/RSK2, and MAPKAP-K2 and the phosphorylation of CREB were maximal, we examined the effects of TPA, EGF, UV-C radiation, and protein synthesis inhibitor anisomycin at a variety of times ranging from 5 to 90 min. These experiments showed that the activation of MSK1 and MSK2 and MAPKAP-K1b/RSK2 and the phosphorylation of CREB were all maximal at the same time after mitogenic stimulation. Similarly, the activation of MSK1 and MSK2 and MAPKAP-K2 and the phosphorylation of CREB peaked at the same times after exposure to the stress stimuli (data not shown). All subsequent experiments were therefore carried at the time when activation in response a particular stimulus was maximal (Fig. 3).
FIG.3.
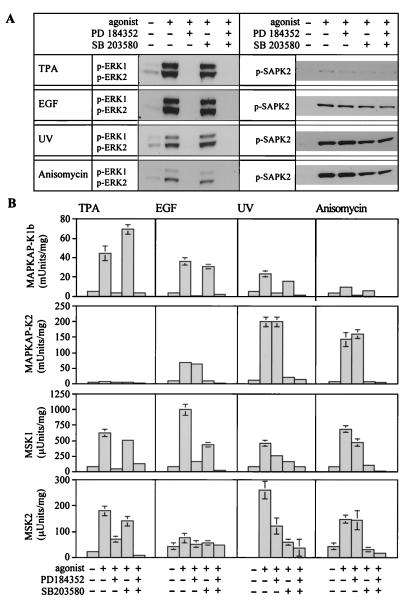
Inhibition of MAPK cascades by PD-184352 and SB-203580 in wild-type fibroblasts. Fibroblasts were serum starved overnight, preincubated for 1 h with or without PD-184352 (5 μM), SB-203580 (10 μM), or both PD-184352 and SB-203580 and then incubated with or without TPA (400 ng/ml, 10 min) or EGF (100 ng/ml, 5 min) or exposed to UV-C radiation (200 J/m2, followed by a 15-min incubation at 37°C) or anisomycin (10 μg/ml, 30 min). The cells were then lysed. (A) Cell lysates were immunoblotted with antibodies that recognize ERK1 and ERK2 or SAPK2/p38 only when they are phosphorylated at the Thr-X-Tyr motif. (B) MAPKAP-K1b/RSK2, MAPKAP-K2, MSK1, and MSK2 were immunoprecipitated from fibroblast lysates and assayed. Further details are given in Materials and Methods. One unit of activity was that amount of enzyme that incorporates 1 nmol of phosphate into the peptide substrate in 1 min. Error bars represent the standard errors of the means of duplicate immunoprecipitations from three different dishes of cells.
The activation of ERK1 and ERK2 (Fig. 3A) and MAPKAP-K1b/RSK2 and MSK1 (Fig. 3B) in response to TPA was blocked by PD-184352 but not SB-203580, confirming that, as in other cells, these kinases are activated by ERK1 or ERK2. Significant phosphorylation of SAPK2/p38 (Fig. 3A) or activation of MAPKAP-K2 (Fig. 3B) in response to TPA was not detected (Fig. 3A).
Stimulation with EGF also resulted in the phosphorylation of ERK1 and ERK2 (Fig. 3A) and activation of MAPKAP-K1b/RSK2 (Fig. 3B), and this was prevented by PD-184352 but not by SB-203580. However, in contrast to TPA, EGF also induced some phosphorylation of SAPK2/p38 (Fig. 3A) and activation of its substrate MAPKAP-K2 (Fig. 3B). The EGF-induced activation of MAPKAP-K2 was blocked by SB-203580 but not by PD-184352. The EGF-induced activation of MSK1 was inhibited considerably by PD-184352, but a combination of PD-184352 and SB-203580 was needed to achieve a complete block (Fig. 3B), consistent with EGF activating both pathways.
UV-C radiation and anisomycin both caused a strong phosphorylation of SAPK2/p38, together with a weaker phosphorylation of ERK1 and ERK2 (Fig. 3A). MSK1 and MAPKAP-K2 were both strongly activated by UV-C or anisomycin (Fig. 3B). The activation of MAPKAP-K2 was blocked by SB-203580 but was unaffected by PD-184352. The activation of MSK1 was partially blocked by SB-203580 or PD-184352 and was completely blocked by a combination of these compounds. Consistent with the weak activation of ERK1 and ERK2, relatively weak activation of MAPKAP-K1b/RSK2 was also seen in response to UV-C radiation and anisomycin; this activation was blocked by PD-184352 and was unaffected by SB-203580 (Fig. 3B).
The activity of the endogenous MSK2 in cell lysates was lower than that of MSK1 and was difficult to quantitate with the same accuracy. Nevertheless, the results obtained with MSK2 and MSK1 were similar (Fig. 3B). Taken together, these results establish that MSK1 and MSK2 are activated by either ERK1 or ERK2 or SAPK2/p38 in primary fibroblasts, while MAPKAP-K1b/RSK2 is activated only by ERK1 and ERK2 and MAPKAP-K2 is activated only by SAPK2/p38.
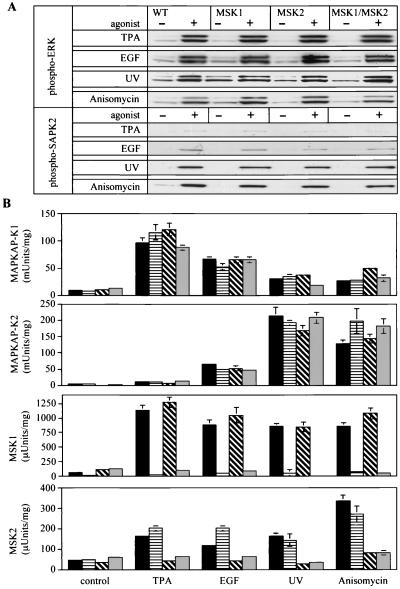
To determine if the knockout of MSK1 and/or MSK2 affected the activation of the classical MAPK cascade or the SAPK2/p38 pathway, the effects of TPA, EGF, UV-C radiation, and anisomycin in fibroblasts from the knockout mice were examined. The phosphorylation of ERK1, ERK2, and SAPK2/p38 in response to these stimuli was not affected significantly by the single knockout of MSK1 and MSK2 or by the double knockout of both MSK1 and MSK2 (Fig. 4A). The knockout of MSK2 or MSK1 plus MSK2 appeared to result in a slight decrease in the phosphorylation of SAPK2/p38 in response to EGF (Fig. 4A), but not to a level sufficient to reduce the level of activation of MAPKAP-K2 or MSK1 and MSK2 (Fig. 4B). The activation of MAPKAP-K1b/RSK2 and MAPKAP-K2 in the different knockout cells after stimulation with TPA, EGF, UV-C, or anisomycin was normal (Fig. 4B).
FIG. 4.
Activation of components of MAPK cascades from different MSK knockout fibroblasts. (A) Confluent fibroblasts from wild-type (WT), MSK1 knockout, MSK2 knockout, and MSK1/MSK2 double-knockout embryos were serum starved overnight and then left unstimulated (−) or stimulated (+) with TPA (400 ng/ml, 10 min), EGF (100 ng/ml, 5 min), anisomycin (10 μg/ml, 30 min), or UV-C (200 J/m2, followed by a 15-min incubation at 37°C). Cells were then lysed, and 20 μg of lysate protein was denatured in SDS, electrophoresed on 4 to 12% Novex polyacrylamide gels, transferred to nitrocellulose membranes, and immunoblotted for phosphorylated ERK1 and ERK2 or for phosphorylated SAPK2/p38. The levels of ERK1 and ERK2 and SAPK2/p38 were measured with an antibody that recognizes the phosphorylated and dephosphorylated enzymes equally well and were identical in the different cell lines as judged by immunoblotting (not shown). (B) Same as for panel A, except that MAPKAP-K1/RSK, MAPKAP-K2, MSK1, and MSK2 activities were determined after their immunoprecipitation from the lysates (see Materials and Methods). Activities are shown as follows: black bars, wild-type cells; horizontally hatched bars, MSK1 knockout cells; diagonally hatched bars, MSK2 knockout cells; grey bars, MSK1/MSK2 double-knockout cells. One unit of activity was that amount of enzyme that incorporates 1 nmol of phosphate into the peptide substrate in 1 min. Error bars represent the standard errors of the means of duplicate immunoprecipitations from three different dishes of cells.
As expected, the activation of MSK1 was not detected in the MSK1 knockout cells, while the activation of MSK2 was normal. Similarly in MSK2 knockout cells there was no MSK2 activation, while the activation of MSK1 was normal. These observations demonstrated that there was no compensatory up-regulation of the activation of one isoform when the other was knocked out. In MSK1/MSK2 double knockout fibroblasts, no MSK1 or MSK2 activity could be detected (Fig. 4B).
CREB and ATF1 phosphorylation in primary mouse fibroblasts.
The amino acid sequences surrounding Ser133 of CREB and Ser63 of ATF1 are identical, allowing the phosphorylation of both proteins to be monitored with the same phospho-specific antibody. Transcription factors CREB and ATF1 were phosphorylated in response to mitogenic or stress stimuli in primary fibroblasts. The anisomycin-induced phosphorylation of CREB and ATF1 was blocked by SB-203580 but not by PD-184352, indicating that phosphorylation occurs via the the SAPK2/p38 pathway but not via the classical MAPK cascade (Fig. 5A). In contrast, CREB and ATF1 phosphorylation in response to TPA was blocked by PD-184352 but not by SB-203580, indicating that phosphorylation occurs via the classical MAPK cascade and not via the SAPK2/p38 pathway (Fig. 5B). However, the blockade of both signal transduction pathways was required to suppress the EGF- or UV-induced phosphorylation of CREB and ATF1 (Fig. 5C and D). The effects of inhibitors on the phosphorylation of CREB are similar to their effects on the activation of MSK1 and MSK2 (Fig. 4B).
FIG. 5.
Inhibition of CREB and ATF1 phosphorylation in wild-type fibroblasts. Confluent wild-type fibroblasts were serum starved overnight and then left untreated or incubated with 5 μM PD184352, 10 μM SB-203580, or both PD-184352 and SB-203580. After 1 h, cells were left unstimulated or stimulated for 30 min with 10 μg of anisomycin/ml (A), for 10 min with 400 ng of TPA/ml (B), or for 5 min with 100 ng of EGF/ml (C) or were exposed to UV-C at 200 J/m2 followed by incubation for 15 min at 37°C (D). Cells were then lysed in 1% SDS, and 20 μg of lysate protein was electrophoresed on 4 to 12% Novex polyacrylamide gels, transferred to nitrocellulose and immunoblotted with an antibody that recognizes CREB phosphorylated at Ser133 and ATF1 phosphorylated at Ser63.
The anisomycin-induced phosphorylation of CREB and ATF1 was partially suppressed in the MSK1 knockout and MSK2 knockout fibroblasts and essentially abolished in the MSK1/MSK2 double-knockout cells (Fig. 6A). Similar results were obtained after exposure to UV-C radiation at 200 (Fig. 6B) or 25 J/m2 (not shown). TPA or EGF induced the phosphorylation of CREB and ATF1 to similar levels in wild-type and MSK2 knockout cells. However, MSK1 knockout fibroblasts had slightly reduced levels of CREB and ATF1 phosphorylation. In contrast, TPA stimulation produced only a weak phosphorylation of CREB in the MSK1/MSK2 double-knockout cells (Fig. 7A). The results obtained after EGF stimulation resembled the results obtained in response to TPA. However, EGF-induced phosphorylation of both CREB and ATF1 was reduced in the MSK2 knockout animals (Fig. 7B).
FIG. 6.
CREB and ATF1 phosphorylation in wild-type and knockout fibroblasts after stimulation with anisomycin or UV-C. Fibroblasts from wild-type (WT), MSK1 knockout, MSK2 knockout, and MSK1/MSK2 double-knockout mice were serum starved overnight and then stimulated with 10 μg of anisomycin/ml (A) or UV-C at 200 J/m2 followed by incubation at 37°C (B) for the times indicated. Lysates were then immunoblotted for phosphorylated CREB and ATF1 or with an antibody that recognizes the phosphorylated and dephosphorylated forms of CREB equally well (total CREB). The intensity of the phospho-CREB band with respect to the intensity of total-CREB band was quantified. Error bars represent the standard errors of the means of three separate experiments.
FIG. 7.
CREB and ATF1 phosphorylation in wild-type and knockout fibroblasts after stimulation with TPA or EGF. Fibroblasts from wild-type (WT), MSK1 knockout, MSK2 knockout, and MSK1/MSK2 double-knockout mice were serum starved overnight and then stimulated with 400 ng of TPA/ml (A) or 100 ng of EGF/ml (B) for the times indicated. Lysates were then immunoblotted for phosphorylated CREB and ATF1 or with an antibody that recognizes the phosphorylated and dephosphorylated forms of CREB equally well (total CREB). The intensity of the phospho-CREB band with respect to the intensity of the total-CREB band was quantified. Error bars represent the standard errors of the means of three separate experiments.
The residual TPA-stimulated CREB phosphorylation in the double-knockout cells was blocked by PD-184352 (Fig. 8), suggesting that phosphorylation is catalyzed by another protein kinase that is activated by ERK1 and ERK2. Similar results were obtained with U0126, a structurally unrelated inhibitor of the classical MAPK cascade (data not shown). The residual phosphorylation was also blocked by Ro-31-8220 but not by H-89 or SB-203580 (Fig. 8). These results are considered further in Discussion.
FIG. 8.
Inhibition of residual TPA-stimulated CREB and ATF1 phosphorylation in fibroblasts from MSK1/MSK2 double-knockout fibroblasts. The fibroblasts were serum starved overnight and then incubated for 1 h with or without PD-184352 (5 μM), SB-203580 (10 μM), PD-184352 plus SB-203580, Ro-31-8220 (5 μM), or H-89 (10 or 25 μM). Cells were then left unstimulated or stimulated with TPA (400 ng/ml, 10 min) as indicated. Lysates were immunoblotted for phosphorylated CREB and ATF1. The intensity of the phospho-CREB band with respect to the intensity of the total-CREB band was quantified. Error bars represent the standard errors of the means of three separate experiments.
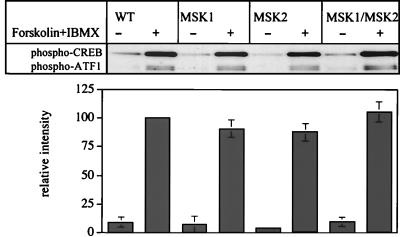
CREB and ATF1 become phosphorylated in response to agents that elevate intracellular cyclic AMP, such as a combination of forskolin and 3-isobutyl 1-methyl xanthine. The phosphorylation of CREB and ATF1 induced by these agonists was unaffected by the knockout of MSK1, MSK2, or both protein kinases in primary fibroblasts (Fig. 9) and is consistent with the similar levels of the CREB protein in the wild-type and knockout cells (Fig. 6). The effects of forskolin and IBMX are presumably mediated by the direct PKA-mediated phosphorylation of CREB and ATF1 and not indirectly by the activation of MSK1 or MSK2, because the presence of PD-184352 plus SB-203580 has no effect (data not shown). The results also demonstrate that the double knockout of MSK1 and MSK2 does not result in a modification of the CREB and ATF1 proteins that might render them inaccessible to other protein kinases, such as PKA.
FIG. 9.
Phosphorylation of CREB by PKA is normal in the MSK1/MSK2 double-knockout fibroblasts. Fibroblasts from wild-type, MSK1 knockout, MSK2 knockout, and MSK1/MSK2 double-knockout mice were serum starved overnight and then stimulated with a combination of forskolin (20 μM) and IBMX (10 μM). Lysates were immunoblotted for phosphorylated CREB and ATF1. The intensity of the phospho-CREB band with respect to the intensity of the total-CREB band was quantified. Error bars represent the standard errors of the means of three separate experiments.
Agonist-induced transcription of immediate-early genes.
We next studied the effects of TPA, EGF, UV, and anisomycin on the induction of c-fos, egr1, and junB, immediate-early genes whose transcription has been linked to CREB. To determine if the levels of mRNA in the knockout cells were affected, the levels relative to those of cyclophilin were determined by RNase protection assays. Exposure of wild-type and MSK1/MSK2 double-knockout fibroblasts to anisomycin resulted in an induction of c-fos, egr1, and junB which was maximal after 1 h and which was sustained for at least 2 h (data not shown). The anisomycin-induced transcription of c-fos and junB was reduced by 50% in the double-knockout animals after 60 (Fig. 10A) or 30 min (data not shown). The reduction in the fibroblasts from the single-knockout animals was slight.
FIG. 10.
Transcription of the c-fos, egr1, and junB genes in MSK knockout cells. Fibroblasts were serum starved overnight and then stimulated with anisomycin (A; 10 μg/ml, 60 min), UV-C (25 J/m2) followed by incubation for 60 min (B), TPA (C; 400 ng/ml, 30 min), or EGF (D; 100 ng/ml, 15 min), and total RNA was isolated using the Qiagen RNeasy Mini-Kit. RNase protection assays for c-fos, egr1, and junB were carried out and quantified as described in Materials and Methods. Relative mRNA levels for c-fos, egr1, and junB for wild-type cells (black bars), MSK1 knockout cells (horizontally hatched bars), MSK2 knockout cells (diagonally hatched bars), and MSK1/MSK2 double-knockout cells (grey bars) are shown. Error bars represent the standard errors of the means of four points.
There was no significant induction of c-fos, egr1, or junB after exposure to UV-C radiation at 200 J/m2 in either the wild-type or knockout cells, as judged by reverse transcription-PCR or RNase protection assays (data not shown). However induction of c-fos, junB, and egr1 was observed at 25 J/m2. Induction of both c-fos and junB was decreased in the MSK1/MSK2 double-knockout cells (Fig. 10B), similar to the reduction observed after exposure to anisomycin. In contrast, the reduction in TPA- or EGF-induced transcription of c-fos and junB was small, even in the double-knockout animals (Fig. 10C and D). The induction of c-fos mRNA over a range of EGF concentrations from 0.01 to 100 ng/ml was studied with similar results (data not shown). The induction of egr1 in the fibroblasts from single or double knockout animals was unaffected under any conditions tested (Fig. 10).
The induction of egr1 by anisomycin is partially inhibited by SB-203580, and this has been reported to be dependent on the CRE in the promoter, suggesting that it may be explained by the inhibition of CREB phosphorylation by SB-203580 (34). In the present study, the induction of egr1 mRNA by SB-203580 in the wild-type and double-knockout cells was found to be inhibited similarly (Fig. 11).
FIG. 11.
Inhibition of the transcription of egr1 by SB-203580. Wild-type and double-knockout fibroblasts were serum starved overnight, incubated for 1 h with or without SB-203580 (10 μM), and then stimulated with anisomycin for the times indicated. egr1 RNA levels were determined as described in Materials and Methods. Solid circles, wild-type cells without SB-203580; open circles, wild-type cells with SB-203580; solid triangles, double-knockout cells without SB-203580; open triangles, double-knockout cells with SB-203580.
Effect of the double knockout of MSK1 and MSK2 on apoptosis and proliferation in fibroblasts.
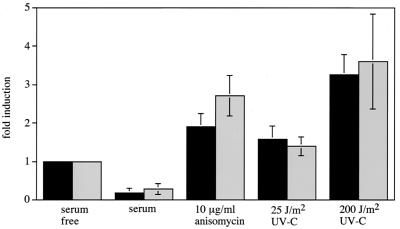
Exposure of fibroblasts from control mice to UV-C radiation or anisomycin induced cell death, as judged by the production of free nucleosomes after 24 h. Cell death induced by these stress stimuli was unaffected in the MSK1/MSK2 double-knockout mice under the conditions tested (Fig. 12).
FIG. 12.
UV-C and anisomycin induced cell death. Fibroblasts from wild-type (black bars) and double-knockout (grey bars) mice were grown for 36 h in serum. Cells were then either serum starved for 24 h in the presence or absence of anisomycin (10 μg/ml) or exposed to UV-C at 25 or 200 J/m2 or grown in serum for 24 h. Cells were then analyzed for cell death by an enzyme-linked immunosorbent assay for free nucleosomes as described in Materials and Methods. Cell death was calculated as fold induction relative to the serum-starved control after correcting for cell number. Error bars represent the standard errors of the means of four separate experiments.
The primary embryonic fibroblasts from the double-knockout animals and those from control mice proliferated at similar rates when incubated in serum. The addition of 10 or 100 ng of EGF/ml to serum-starved fibroblasts did not stimulate the proliferation of the fibroblasts from control mice or MSK1/MSK2 double-knockout animals for up to 3 days (data not shown).
DISCUSSION
MSK1 and MSK2 have been shown previously to be activated via both the classical MAPK cascade and the SAPK2/p38 pathway in several cell lines (9, 14, 32), and we have confirmed that this is also the case in primary murine fibroblasts by the use of specific inhibitors. Although PD-184352 blocks the activation of MKK5 and its substrate ERK5, as well as the classical MAPK cascade, higher concentrations of this compound are required to block the MKK5/ERK5 pathway in fibroblasts (29). In particular, incubation of cells with 2 μM PD-184352 completely blocks the classical MAPK cascade, at which concentration the MKK5/ERK5 pathway is unaffected. Although most of the experiments presented in this paper were carried out at 5 μM PD-184352, we have also established that the mitogenic activation of MSK1 and MSK2 and the phosphorylation of CREB are completely blocked at 2 μM PD-184352 (36), excluding an involvement of the MKK5/ERK5 pathway.
In primary murine fibroblasts, TPA activates MSK1 and MSK2 via the classical MAPK cascade while anisomycin activates them largely via the SAPK2/p38 pathway. Both signaling pathways contribute to the activation of MSK1 and MSK2 by EGF and UV-C radiation (Fig. 3). We have also shown that, as in other cells (9, 14), the inhibition by PD-184352 and/or SB-203580 of the activation of MSK1 and MSK2 by TPA, EGF, anisomycin, or UV-C radiation correlates with inhibition of the phosphorylation of CREB and ATF1 in response to the same stimuli. In the present study we used gene targeting to produce knockout mice that lack MSK1, MSK2, or both of these protein kinases in order to evaluate whether these or other protein kinases mediate mitogen- and stress-induced activation of CREB.
Primary embryonic fibroblasts from these mice were used to determine the effects of the knockouts on MAPK signaling cascades. The single knockout of MSK1 or MSK2 and the double knockout of both did not affect other components of the classical MAPK cascade since the activation of ERK1, ERK2, and MAPKAP-K1b/RSK2 in the knockout cells was similar to that in wild-type cells. Similarly, the single and double knockouts did not affect the activation of SAPK2/p38 or its substrate MAPKAP-K2 (Fig. 4). The knockout of MSK1 did not lead to a compensatory increase in the level of MSK2 protein or activity, and vice versa.
Our results clearly demonstrate that MSK1 and MSK2 are the major, if not the only, protein kinases that mediate the phosphorylation of CREB at Ser133 and of ATF1 at Ser63 in fibroblasts by agonists that activate SAPK2/p38 in fibroblasts, because the phosphorylation of these transcription factors in response to anisomycin or UV-C radiation was virtually abolished in the MSK1/MSK2 double knockouts. A partial reduction of CREB and ATF1 phosphorylation was observed in the single knockouts, indicating that MSK1 and MSK2 both contribute to the stress-induced phosphorylation of CREB and ATF1 (Fig. 6). The absence of CREB phosphorylation in the double-knockout cells was not due to a decrease in the expression of the CREB protein, and, in addition, CREB was phosphorylated normally by PKA in response to cyclic AMP-elevating agents (Fig. 9).
Consistent with our earlier report that MSK1 knockout ES cells show a considerable reduction in CREB and ATF1 phosphorylation in response to TPA and EGF, the phosphorylation of CREB and ATF1 was also reduced in MSK1 knockout fibroblasts (Fig. 7). However, the reduction was not as great as in ES cells, and this may be due to the higher levels of MSK2 in fibroblasts than in ES cells, where MSK2 activity could not be detected. The double knockout of MSK1 and MSK2 caused a further reduction in CREB and ATF1 phosphorylation compared to that in the MSK1 knockout fibroblasts, indicating that MSK2 contributes to CREB and ATF1 phosphorylation in response to TPA and EGF.
Some residual phosphorylation of CREB and ATF1 was seen in fibroblasts from the double knockout of MSK1 and MSK2 in response to TPA and EGF (Fig. 8), suggesting that another protein kinase can phosphorylate CREB in the absence of MSK1 and MSK2. Moreover, this residual phosphorylation was blocked by prior incubation with PD-184352 but was unaffected by SB-203580, indicating that it is likely to be catalyzed by another protein kinase activated by ERK1 or ERK2. No additional MSK isoform appears to be present in the human genome. Moreover, the residual phosphorylation was unaffected by H-89 at concentrations that inhibit MSK1 and MSK2 (9) and PKA (11, 26) in cells. The effects of the inhibitors on the residual CREB phosphorylation are consistent with it being catalyzed by an MAPKAP-K1/RSK isoform (26, 36). However, this appears not to be the case, because a potent cell-permeable inhibitor of MAPKAP-K1b/RSK2 that has recently been developed and that does not inhibit MSK1 and many other protein kinases tested has no effect on the residual TPA-induced phosphorylation of CREB in the MSK1/MSK2 double-knockout cells under conditions where the phosphorylation of other MAPKAP-K1/RSK substrates is blocked completely (G. Sapkota, D. R. Alessi, and G. Wiggin, unpublished experiments).
The evidence presented in this paper indicates that MAPKAP-K1b/RSK2 is not rate limiting for the phosphorylation of CREB in fibroblasts. Instead, our data indicate that MSK1 and MSK2 are the major mediators of CREB and ATF1 phosphorylation after mitogenic stimulation. This is consistent with CREB phosphorylation in fibroblasts being unaffected by the knockout of the MAPKAP-K1b/RSK2 gene (8). However it conflicts with the report that immortalized fibroblasts from human patients with Coffin-Lowry syndrome, which possess an inactivating mutation in the MAPKAP-K1b/RSK2 gene (40), do not phosphorylate CREB in response to EGF (15). These findings led to the conclusion that MAPKAP-K1b/RSK2 is the major, if not the only, protein kinase that mediates the mitogen-induced phosphorylation of CREB in fibroblasts. We have therefore repeated the experiments with the same immortalized fibroblasts from Coffin-Lowry syndrome patients (a generous gift from A. Hanauer). In our hands, CREB phosphorylation was readily detectable in response to either EGF or TPA in the Coffin-Lowry cells (A. Kieloch, S. Arthur, and D. R. Alessi, unpublished work). While it remains possible that MAPKAP-K1b/RSK2 mediates the phosphorylation of CREB in cells that we have not yet studied, the finding that a dominant-negative MAPKAP-K1b/RSK2 mutant inhibits the mitogen-induced phosphorylation of CREB could be explained by the binding of this mutant to ERK1 or ERK2, which may prevent the mitogenic activation of MSK1 and MSK2.
Mice with knockouts of MSK1, MSK2, or both MSK1 and MSK2 were viable and fertile and showed no obvious health defects. This is in contrast to knockout or transgenic CREB mutants (7, 35, 38), which have severe phenotypes. This difference may reflect the wider role of CREB in mediating the effects of many agonists that act via different signal transduction cascades. In particular, agonists that elevate the level of cyclic AMP and that activate PKA also induce the phosphorylation of CREB, and this is unimpaired in the MSK1/MSK2 knockout cells (Fig. 9).
The phosphorylation of CREB has been implicated in the induction of certain immediate-early genes, such as c-fos and egr1, as CREB phosphorylation appears to be sufficient for induction of these genes via PKA. In contrast, CREB phosphorylation induced by activation of different MAPK cascades is not sufficient for gene induction, and phosphorylation of additional transcription factors, such as TCFs, is also required (1, 6, 22). It has been shown previously that approximately 50% of c-fos induction by UV-C radiation in a variety of cell types, including fibroblasts, can be blocked by a dominant-negative form of CREB that cannot be phosphorylated. Mutation of the CRE site in the c-fos promoter also caused a 50% reduction in c-fos, and the remaining induction was unaffected by dominant-negative CREB, indicating that the c-fos promoter can be transcribed independently of the CRE site and CREB (1). Similar results were also obtained from PC12 cells with a dominant-negative form of CREB that blocked CREB binding to DNA (21). This was found to reduce c-fos induction by NGF but completely blocked c-fos induction by PKA. Thus the lack of CREB phosphorylation in the MSK1/MSK2 double knockout would not be expected to completely block c-fos induction. Consistent with these previous observations, a 50% reduction in anisomycin-stimulated c-fos induction in the MSK1/MSK2 double-knockout cells (Fig. 10), where there is almost no CREB phosphorylation (Fig. 6), was observed. The induction of c-fos in the MSK1/MSK2 double-knockout cells is likely to result from the direct SAPK2a/p38- and ERK-catalyzed activation of the TCF that binds to the SRE in the c-fos promoter. The much smaller reduction in c-fos gene transcription observed in the double-knockout cells after stimulation with TPA or EGF (Fig. 10) may be explained by the residual phosphorylation of CREB after stimulation with TPA or EGF (Fig. 7). If this is the case, then relatively small increases in CREB phosphorylation may be sufficient to produce maximal effects on the transcription of immediate-early genes.
The induction of junB in the double-knockout fibroblasts was also reduced. The transcription of junB is less well studied; however it has been reported to be controlled by SRE- and CRE-like sequences, which are located both 3′ and 5′ to the gene (3, 25, 30, 31). The reduction of anisomycin-stimulated induction of junB in the double knockouts may be due to the reduction of CREB phosphorylation in these cells.
The egr1 gene promoter contains a CRE and multiple SRE sequences. It has been reported that the mutation of the CRE reduces the induction from the egr1 promoter in response to stress and that inhibition of the egr1 promoter by SB-203580 is lost when the CRE site is mutated in 293 cells (34). However, there was no significant reduction in the transcription of egr1 in response to anisomycin in the fibroblasts from the MSK1/MSK2 double-knockout mice, even though the phosphorylation of CREB was abolished (Fig. 6 and 10). The induction of egr1 in response to anisomycin was also inhibited by SB-203580 to the same extent in wild-type and double-knockout fibroblasts (Fig. 11).
The TPA- and EGF-stimulated induction of egr1 in fibroblasts from the double knockout animals was also unaffected. It therefore appears that the phosphorylation of CREB is not required for the transcription of egr1 in fibroblasts. It is possible that, in the absence of CREB phosphorylation, another SB-203580-sensitive transcription factor that occupies the CRE is able to stimulate transcription of egr1 in fibroblasts. Alternatively, the results may reflect differences in the relative importance of the CRE and SRE in the egr1 promoter between the primary fibroblasts used in the present study and the 293 cells used previously (34).
In summary, we have identified MSK1 and MSK2 as the major protein kinases that phosphorylate CREB and ATF1 in fibroblasts in response to mitogens and cellular stress and have shown that CREB phosphorylation is required for the full induction of c-fos and junB in response to anisomycin and UV-C radiation. We are currently studying whether CREB phosphorylation is deficient in other cells and tissues in the MSK1/MSK2 double-knockout mice. Since CREB has been implicated in learning and memory (7), it will clearly also be of great interest to examine whether this process is impaired in the MSK1 and MSK2 knockout mice. These animals should facilitate the identification of additional physiological substrates and roles of MSK1 and MSK2.
Acknowledgments
We thank Valeria Poli for help and advice on the generation of the mouse knockouts and Chris Armstrong and Jane Leitch for the production and purification of the antibodies used in this work. We thank The Sequencing Service (School of Life Sciences, University of Dundee, Scotland) for DNA sequencing.
G.R.W. is the recipient of a postgraduate studentship from the United Kingdom Medical Research Council. This research was supported by grants from the Medical Research Council, Diabetes UK, The Royal Society, Astra-Zeneca, Boehringer-Ingelheim, GlaxoSmithKline, NovoNordisk, and Pfizer.
REFERENCES
- 1.Ahn, S., M. Olive, S. Aggarwal, D. Krylov, D. D. Ginty, and C. Vinson. 1998. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol. Cell. Biol. 18:967-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi, D. R., A. Cuenda, P. Cohen, D. T. Dudley, and A. R. Saltiel. 1995. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270:27489-27494. [DOI] [PubMed] [Google Scholar]
- 3.Amato, S. F., K. Nakajima, T. Hirano, and T. C. Chiles. 1996. Transcriptional regulation of the junB promoter in mature B lymphocytes. Activation through a cyclic adenosine 3′,5′-monophosphate-like binding site. J. Immunol. 157:146-155. [PubMed] [Google Scholar]
- 4.Arthur, J. S. C., and P. Cohen. 2000. MSK1 is required for CREB phosphorylation in response to mitogens in mouse embryonic stem cells. FEBS Lett. 482:44-48. [DOI] [PubMed] [Google Scholar]
- 5.Arthur, J. S. C., J. S. Elce, C. Hegadorn, K. Williams, and P. A. Greer. 2000. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol. Cell. Biol. 20:4474-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonni, A., D. D. Ginty, H. Dudek, and M. E. Greenberg. 1995. Serine 133-phosphorylated CREB induces transcription via a cooperative mechanism that may confer specificity to neurotrophin signals. Mol. Cell. Neurosci. 6:168-183. [DOI] [PubMed] [Google Scholar]
- 7.Bourtchuladze, R., B. Frenguelli, J. Blendy, D. Cioffi, G. Schutz, and A. J. Silva. 1994. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79:59-68. [DOI] [PubMed] [Google Scholar]
- 8.Bruning, J. C., J. A. Gillette, Y. Zhao, C. Bjorbaeck, J. Kotzka, B. Knebel, H. Avci, B. Hanstein, P. Lingohr, D. E. Moller, W. Krone, C. R. Kahn, and D. Muller-Wieland. 2000. Ribosomal subunit kinase-2 is required for growth factor-stimulated transcription of the c-fos gene. Proc. Natl. Acad. Sci. USA 97:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caivano, M., and P. Cohen. 2000. Role of mitogen-activated protein kinase cascades in mediating lipopolysaccharide-stimulated induction of cyclooxygenase-2 and IL-1 beta in RAW264 macrophages. J. Immunol. 164:3018-3025. [DOI] [PubMed] [Google Scholar]
- 10.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 11.Chijiwa, T., A. Mishima, M. Hagiwara, M. Sano, K. Hayashi, T. Inoue, K. Naito, T. Toshioka, and H. Hidaka. 1990. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-p-bromocinnamylaminoethyl]-5-isoquinolinesulfonamide H-89, of PC12D pheochromocytoma cells. J. Biol. Chem. 265:5267-5272. [PubMed] [Google Scholar]
- 12.Clifton, A. D., P. R. Young, and P. Cohen. 1996. A comparison of the substrate specificity of MAPKAP kinase 2 and MAPKAP kinase 3 and their activation by cytokines and cellular stress. FEBS Lett. 392:209-214. [DOI] [PubMed] [Google Scholar]
- 13.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deak, M., A. D. Clifton, J. M. Lucocq, and D. R. Alessi. 1998. Mitogen- and stress-activated protein kinase-1 MSK1 is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17:4426-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Cesare, D., S. Jacquot, A. Hanauer, and P. Sassone-Corsi. 1998. Rsk-2 activity is necessary for epidermal growth factor-induced phosphorylation of CREB protein and transcription of c-fos gene. Proc. Natl. Acad. Sci. USA 95:12202-12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Cesare, D., and P. Sassone-Corsi. 2000. Transcriptional regulation by cyclic AMP-responsive factors. Prog. Nucleic Acid Res. Mol. Biol. 64:343-369. [DOI] [PubMed] [Google Scholar]
- 17.Gille, H., M. Kortenjann, O. Thomae, C. Moomaw, C. Slaughter, M. H. Cobb, and P. E. Shaw. 1995. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 14:951-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gineitis, D., and R. Treisman. 2001. Differential usage of signal transduction pathways defines two types of serum response factor target gene. J. Biol. Chem. 276:24531-24539. [DOI] [PubMed] [Google Scholar]
- 19.Ginty, D. D., A. Bonni, and M. E. Greenberg. 1994. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell 77:713-725. [DOI] [PubMed] [Google Scholar]
- 20.Hazzalin, C. A., A. Cuenda, E. Cano, P. Cohen, and L. C. Mahadevan. 1997. Effects of the inhibition of p38/RK MAP kinase on the induction of five fos and jun genes by diverse stimuli. Oncogene 15:2321-2331. [DOI] [PubMed] [Google Scholar]
- 21.Iordanov, M., K. Bender, T. Ade, W. Schmid, C. Sachsenmaier, K. Engel, H. J. Rahmsdorf, and P. Herrlich. 1997. CREB is activated by UVC through a p38/HOG-1-dependent protein kinase. EMBO J. 16:1009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janknecht, R., M. A. Cahill, and A. Nordheim. 1995. Signal integration at the c-fos promoter. Carcinogenesis 16:443-450. [DOI] [PubMed] [Google Scholar]
- 23.Janknect, R., and T. Hunter. 1997. Convergence of MAP kinase pathways on the ternary complex factor Sap-1a. EMBO J. 16:1620-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamakura, S., T. Moriguchi, and E. Nishida. 1999. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification of a signaling pathway to the nucleus. J. Biol. Chem. 274:26563-26571. [DOI] [PubMed] [Google Scholar]
- 25.Kitabayashi, I., Z. Kawakami, T. Matsuoka, R. Chiu, G. Gachelin, and K. Yokoyama. 1993. Two cis-regulatory elements that mediate different signaling pathways for serum-dependent activation of the junB gene. J. Biol. Chem. 268:14482-14489. [PubMed] [Google Scholar]
- 26.Lizcano, J. M., N. Morrice, and P. Cohen. 2000. Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem. J. 349:547-557. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Maizels, E. T., A. Mukherjee, G. Sithanandam, C. A. Peters, J. Cottom, K. E. Mayo, and M. Hunzicker-Dunn. 2001. Developmental regulation of mitogen-activated protein kinase-activated kinases-2 and -3 (MAPKAPK-2/-3) in vivo during corpus luteum formation in the rat. Mol. Endocrinol. 15:716-733. [DOI] [PubMed] [Google Scholar]
- 28.Marais, R., J. Wynne, and R. Treisman. 1993. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional domain. Cell 73:381-393. [DOI] [PubMed] [Google Scholar]
- 29.Mody, N., J. Leitch, C. G. Armstrong, J. Dixon, and P. Cohen. 2001. Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett. 172:227-238. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima, K., T. Kusafuka, T. Takeda, Y. Fujitani, K. Nakae, and T. Hirano. 1993. Identification of a novel interleukin-6 response element containing an Ets-binding site and a CRE-like site in the junB promoter. Mol. Cell. Biol. 13:3027-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Albuerne, E. D., G. Schatteman, L. K. Sanders, and D. Nathans. 1993. Transcriptional regulatory elements downstream of the JunB gene. Proc. Natl. Acad. Sci. USA 90:11960-11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierrat, B., J. S. Correia, J. L. Mary, M. Tomas-Zuber, and W. Lesslauer. 1998. RSK-B, a novel ribosomal S6 kinase family member, is a CREB kinase under dominant control of p38alpha mitogen-activated protein kinase (p38αMAPK). J. Biol. Chem. 273:29661-29671. [DOI] [PubMed] [Google Scholar]
- 33.Price, M. A., F. H. Cruzalegui, and R. Treisman. 1996. The p38 and ERK MAP kinase pathways cooperate to activate ternary complex factors and c-fos transcription in response to UV light. EMBO J. 15:6552-6563. [PMC free article] [PubMed] [Google Scholar]
- 34.Roli, M., A. Kotlyarov, K. M. Sakamoto, M. Gaestel, and A. Neininger. 1999. Stress-induced stimulation of early growth response gene-1 by a p38/stress activated protein kinase 2 is mediated by a cAMP-responsive promoter element in a MAPKAP kinase 2 independent manner. J. Biol. Chem. 274:19559-19564. [DOI] [PubMed] [Google Scholar]
- 35.Rudolph, R., A. Tafuri, P. Gass, G. L. Hammerling, and G. Schutz. 1998. Impaired fetal T cell development and perinatal lethality in mice lacking the cAMP response element binding protein. Proc. Natl. Acad. Sci. USA 95:4481-4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sapkota, G. P., A. Kieloch, J. M. Lizcano, S. Lain, J. S. C. Arthur, M. R. Williams, N. Morrice, M. Deak, and D. R. Alessi. 2001. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90RSK and cAMP-dependent protein kinase, but not its farnesylation at Cys433, is essential for LKB1 to suppress cell growth. J. Biol. Chem. 276:19469-19482. [DOI] [PubMed] [Google Scholar]
- 37.Shaywitz, A. J., and M. E. Greenberg. 1999. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu. Rev. Biochem. 68:821-861. [DOI] [PubMed] [Google Scholar]
- 38.Struthers, R. S., W. W. Vale, C. Arias, P. E. Sawchenko, and M. R. Montminy. 1991. Somatrophe hypoplasia and dwarfism in transgenic mice expressing a non-phosphorylatable CREB mutant. Nature 350:622-624. [DOI] [PubMed] [Google Scholar]
- 39.Tan, Y., J. Rouse, A. Zhang, S. Cariati, P. Cohen, and M. J. Comb. 1996. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 15:4629-4642. [PMC free article] [PubMed] [Google Scholar]
- 40.Trivier, E., D. De Cesare, S. Jacquot, S. Pannetier, E. Zackai, I. Young, J. L. Mandel, P. Sassone-Corsi, and A. Hanauer. 1996. Mutations in the kinase Rsk-2 associated with Coffin-Lowry syndrome. Nature 384:567-570. [DOI] [PubMed] [Google Scholar]
- 41.Vanhoutte, P., J. V. Barnier, B. Guibert, C. Pages, M. J. Besson, R. A. Hipskind, and J. Caboche. 1999. Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol. Cell. Biol. 19:136-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams, M. R., J. C. Arthur, A. Balendran, J. van der Kaay, V. Poli, P. Cohen, and D. R. Alessi. 2000. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr. Biol. 10:439-448. [DOI] [PubMed] [Google Scholar]
- 43.Wurst, W., and A. L. Joyner. 1993. Production of targeted embryonic stem cell clones, p. 101-131. In L. Joyner (ed.), Gene targeting. ILR Press, Oxford, United Kingdom.
- 44.Xing, J., D. D. Ginty, and M. E. Greenberg. 1996. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273:959-963. [DOI] [PubMed] [Google Scholar]
- 45.Xing, J., J. M. Kornhauser, Z. Xia, E. A. Thiele, and M. E. Greenberg. 1998. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol. Cell. Biol. 18:1946-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zinck, R., R. A. Hipskind, V. Pingoud, and A. Nordheim. 1993. c-fos transcriptional activation and repression correlate temporally with the phosphorylation status of TCF. EMBO J. 12:2377-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]