Abstract
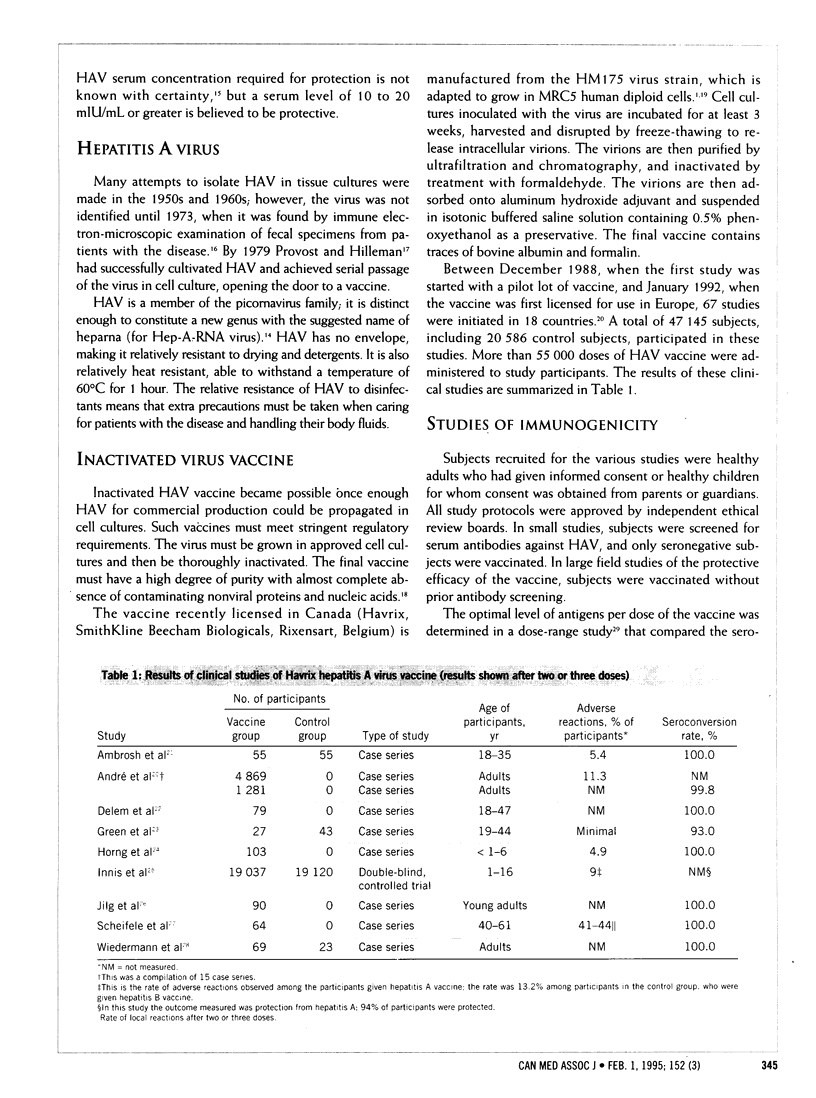
PURPOSE: To examine the evidence concerning the safety and effectiveness of the inactivated hepatitis A virus vaccine recently licensed for use in Canada. DATA SOURCES: The main source of information were papers presented at the International Symposium on Active Immunization against Hepatitis A, held in Vienna, Austria, Jan. 27-29, 1992. The bibliographies of these papers were searched for additional references. Recent articles describing the new vaccine and the epidemiologic aspects of infection with hepatitis A virus (HAV) were also reviewed. STUDY SELECTION: Peer-reviewed reports of trials approved by a government regulatory agency on the safety, immunogenic properties and efficacy of the vaccine. DATA EXTRACTION: The authors assembled key reports on adverse reactions, protection from disease and serologic assessment of immune response in vaccine recipients; data from these reports were tabulated and analysed. RESULTS OF DATA SYNTHESIS: The new vaccine contains the HM175 strain of HAV, which is adapted to grow in tissue culture. The virus is purified, inactivated with the use of formaldehyde and adsorbed onto aluminum hydroxide. The recommended dose for adults is 720 enzyme-linked immunosorbent assay (ELISA) units in a 1.0-mL dose and for children 360 ELISA units in a 0.5-mL dose, injected intramuscularly. The usual schedule is three serial doses, the second given 1 month and the third 6 to 12 months after the initial dose. Reported side effects are infrequent and minor. In healthy persons who have received two doses, the seroconversion rate is almost 100%. Protective efficacy after two doses is estimated to be 94%. However, the persistence of protective antibodies has been studied only over the short term (3 years). CONCLUSIONS: The new HAV vaccine is safe, effective and best suited to pre-exposure prophylaxis in people with an increased risk of infection for an extended period, such as travellers to areas where the disease is endemic. Further studies are needed to determine whether infants respond well to the vaccine and whether the vaccine protects recipients from subclinical infection and associated fecal shedding of HAV. Controlled trials to determine the duration of protection beyond 3 years and the effects of more rapid dosage schedules are also needed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter M. J. Nosocomial hepatitis A infection: can we wash our hands of it? Pediatr Infect Dis. 1984 Jul-Aug;3(4):294–295. [PubMed] [Google Scholar]
- Ambrosch F., André F. E., Delem A., D'Hondt E., Jonas S., Kunz C., Safary A., Wiedermann G. Simultaneous vaccination against hepatitis A and B: results of a controlled study. Vaccine. 1992;10 (Suppl 1):S142–S145. doi: 10.1016/0264-410x(92)90570-a. [DOI] [PubMed] [Google Scholar]
- André F. E., Hepburn A., D'Hondt E. Inactivated candidate vaccines for hepatitis A. Prog Med Virol. 1990;37:72–95. [PubMed] [Google Scholar]
- Berger R., Just M. Vaccination against hepatitis A: control 3 years after the first vaccination. Vaccine. 1992;10(4):295–295. doi: 10.1016/0264-410x(92)90287-t. [DOI] [PubMed] [Google Scholar]
- Corey L., Holmes K. K. Sexual transmission of hepatitis A in homosexual men: incidence and mechanism. N Engl J Med. 1980 Feb 21;302(8):435–438. doi: 10.1056/NEJM198002213020804. [DOI] [PubMed] [Google Scholar]
- Delem A., Safary A., De Namur F., Hauser P., D'Hondt E. Characterization of the immune response of volunteers vaccinated with a killed vaccine against hepatitis A. Vaccine. 1993;11(4):479–484. doi: 10.1016/0264-410x(93)90291-5. [DOI] [PubMed] [Google Scholar]
- Dienstag J. L., Szmuness W., Stevens C. E., Purcell R. H. Hepatitis A virus infection: new insights from seroepidemiologic studies. J Infect Dis. 1978 Mar;137(3):328–340. doi: 10.1093/infdis/137.3.328. [DOI] [PubMed] [Google Scholar]
- Feinstone S. M., Kapikian A. Z., Purceli R. H. Hepatitis A: detection by immune electron microscopy of a viruslike antigen associated with acute illness. Science. 1973 Dec 7;182(4116):1026–1028. doi: 10.1126/science.182.4116.1026. [DOI] [PubMed] [Google Scholar]
- Flehmig B., Heinricy U., Pfisterer M. Simultaneous vaccination for hepatitis A and B. J Infect Dis. 1990 May;161(5):865–868. doi: 10.1093/infdis/161.5.865. [DOI] [PubMed] [Google Scholar]
- Green M. S., Cohen D., Lerman Y., Sjogren M., Binn L. N., Zur S., Slepon R., Robin G., Hoke C., Bancroft W. Depression of the immune response to an inactivated hepatitis A vaccine administered concomitantly with immune globulin. J Infect Dis. 1993 Sep;168(3):740–743. doi: 10.1093/infdis/168.3.740. [DOI] [PubMed] [Google Scholar]
- Hadler S. C., Webster H. M., Erben J. J., Swanson J. E., Maynard J. E. Hepatitis A in day-care centers. A community-wide assessment. N Engl J Med. 1980 May 29;302(22):1222–1227. doi: 10.1056/NEJM198005293022203. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Wehrle G., Berthold H., Köster D. Hepatitis A as an occupational hazard. Vaccine. 1992;10 (Suppl 1):S82–S84. doi: 10.1016/0264-410x(92)90552-u. [DOI] [PubMed] [Google Scholar]
- Horng Y. C., Chang M. H., Lee C. Y., Safary A., Andre F. E., Chen D. S. Safety and immunogenicity of hepatitis A vaccine in healthy children. Pediatr Infect Dis J. 1993 May;12(5):359–362. doi: 10.1097/00006454-199305000-00001. [DOI] [PubMed] [Google Scholar]
- Innis B. L., Snitbhan R., Kunasol P., Laorakpongse T., Poopatanakool W., Kozik C. A., Suntayakorn S., Suknuntapong T., Safary A., Tang D. B. Protection against hepatitis A by an inactivated vaccine. JAMA. 1994 May 4;271(17):1328–1334. [PubMed] [Google Scholar]
- Lange W. R., Frame J. D. High incidence of viral hepatitis among American missionaries in Africa. Am J Trop Med Hyg. 1990 Nov;43(5):527–533. doi: 10.4269/ajtmh.1990.43.527. [DOI] [PubMed] [Google Scholar]
- Lemon S. M. Inactivated hepatitis A vaccines. JAMA. 1994 May 4;271(17):1363–1364. [PubMed] [Google Scholar]
- Lemon S. M. Type A viral hepatitis. New developments in an old disease. N Engl J Med. 1985 Oct 24;313(17):1059–1067. doi: 10.1056/NEJM198510243131706. [DOI] [PubMed] [Google Scholar]
- Margolis H. S., Shapiro C. N. Who should receive hepatitis A vaccine? Considerations for the development of an immunization strategy. Vaccine. 1992;10 (Suppl 1):S85–S87. doi: 10.1016/0264-410x(92)90553-v. [DOI] [PubMed] [Google Scholar]
- Melnick J. L. Properties and classification of hepatitis A virus. Vaccine. 1992;10 (Suppl 1):S24–S26. doi: 10.1016/0264-410x(92)90536-s. [DOI] [PubMed] [Google Scholar]
- Minuk G. Y., Waggoner J. G., Jernigan R., Nicolle L. E., Postl B., Hoofnagle J. H. Prevalence of antibody to hepatitis A virus in a Canadian Inuit community. Can Med Assoc J. 1982 Nov 1;127(9):850–852. [PMC free article] [PubMed] [Google Scholar]
- Provost P. J., Hilleman M. R. Propagation of human hepatitis A virus in cell culture in vitro. Proc Soc Exp Biol Med. 1979 Feb;160(2):213–221. doi: 10.3181/00379727-160-40422. [DOI] [PubMed] [Google Scholar]
- Scheifele D. W., Bjornson G. J. Evaluation of inactivated hepatitis A vaccine in Canadians 40 years of age or more. CMAJ. 1993 Feb 15;148(4):551–555. [PMC free article] [PubMed] [Google Scholar]
- Shapiro C. N., Coleman P. J., McQuillan G. M., Alter M. J., Margolis H. S. Epidemiology of hepatitis A: seroepidemiology and risk groups in the USA. Vaccine. 1992;10 (Suppl 1):S59–S62. doi: 10.1016/0264-410x(92)90545-u. [DOI] [PubMed] [Google Scholar]
- Shouval D., Ashur Y., Adler R., Lewis J. A., Armstrong M. E., Davide J. P., McGuire B., Kuter B., Brown L., Miller W. Single and booster dose responses to an inactivated hepatitis A virus vaccine: comparison with immune serum globulin prophylaxis. Vaccine. 1993;11 (Suppl 1):S9–14. doi: 10.1016/0264-410x(93)90151-m. [DOI] [PubMed] [Google Scholar]
- Steffen R. Risk of hepatitis A in travellers. Vaccine. 1992;10 (Suppl 1):S69–S72. doi: 10.1016/0264-410x(92)90548-x. [DOI] [PubMed] [Google Scholar]
- Tormans G., Van Damme P., Van Doorslaer E. Cost-effectiveness analysis of hepatitis A prevention in travellers. Vaccine. 1992;10 (Suppl 1):S88–S92. doi: 10.1016/0264-410x(92)90554-w. [DOI] [PubMed] [Google Scholar]
- Wagner G., Lavanchy D., Darioli R., Pécoud A., Brulein V., Safary A., Frei P. C. Simultaneous active and passive immunization against hepatitis A studied in a population of travellers. Vaccine. 1993;11(10):1027–1032. doi: 10.1016/0264-410x(93)90128-k. [DOI] [PubMed] [Google Scholar]
- Werzberger A., Mensch B., Kuter B., Brown L., Lewis J., Sitrin R., Miller W., Shouval D., Wiens B., Calandra G. A controlled trial of a formalin-inactivated hepatitis A vaccine in healthy children. N Engl J Med. 1992 Aug 13;327(7):453–457. doi: 10.1056/NEJM199208133270702. [DOI] [PubMed] [Google Scholar]
- Westblom T. U., Gudipati S., DeRousse C., Midkiff B. R., Belshe R. B. Safety and immunogenicity of an inactivated hepatitis A vaccine: effect of dose and vaccination schedule. J Infect Dis. 1994 May;169(5):996–1001. doi: 10.1093/infdis/169.5.996. [DOI] [PubMed] [Google Scholar]
- Wiedermann G., Ambrosch F., André F. E., D'Hondt E., Delem A., Safary A. Persistence of vaccine-induced antibody to hepatitis A virus. Vaccine. 1992;10 (Suppl 1):S129–S131. doi: 10.1016/0264-410x(92)90566-3. [DOI] [PubMed] [Google Scholar]
- Winokur P. L., Stapleton J. T. Immunoglobulin prophylaxis for hepatitis A. Clin Infect Dis. 1992 Feb;14(2):580–586. doi: 10.1093/clinids/14.2.580. [DOI] [PubMed] [Google Scholar]


