Abstract
Recent studies of translational control suggest that translation termination may not be simply the end of synthesizing a protein but rather be involved in modulating both the translation efficiency and stability of a given transcript. Using recombinant eukaryotic release factor 3 (eRF3) and cellular extracts, we have shown for Saccharomyces cerevisiae that yeast eRF3 and Pab1p can interact. This interaction, mediated by the N+M domain of eRF3 and amino acids 473 to 577 of Pab1p, was demonstrated to be direct by the two-hybrid approach. We confirmed that a genetic interaction exists between eRF3 and Pab1p and showed that Pab1p overexpression enhances the efficiency of termination in SUP35 (eRF3) mutant and [PSI+] cells. This effect requires the interaction of Pab1p with eRF3. These data further strengthen the possibility that Pab1p has a role in coupling translation termination events with initiation of translation. Several lines of evidence indicate that Pab1p does not influence [PSI+] propagation. First, “[PSI+]-no-more” mutations do not affect eRF3-Pab1p two-hybrid interaction. Second, overexpression of PAB1 does not cure the [PSI+] phenotype or solubilize detectable amounts of eRF3. Third, prion-curing properties of overexpressed HSP104p, which is required for formation and maintenance of [PSI+], were not modified by excess Pab1p.
In general, termination of protein synthesis occurs when the ribosome elongation machinery encounters an in-frame termination codon on the mRNA. In eukaryotes two release factors have been identified, eukaryotic release factor 1 (eRF1), which recognizes all three stop codons, and eRF3, a GTPase that binds to eRF1 and stimulates its release activity in vitro (35, 109). The eRF1 protein has a structure mimicking that of a tRNA molecule. It recognizes the stop codon in the A site of the ribosome and catalyzes the hydrolysis of the peptidyl-tRNA bond (88).
In Saccharomyces cerevisiae eRF1 and eRF3 termination factors are encoded by the essential genes SUP45 and SUP35, respectively (10, 43, 53, 60, 105). Mutations in either of these genes give the same pleiotropic phenotypes which were selected as omnipotent nonsense suppressors (for a review see reference 49). Moreover, overexpression of both eRF1 and eRF3 is required to enhance the efficiency of termination in yeast (90). In higher eukaryotes, overproduction of eRF1 alone is sufficient to compete with a suppressor tRNA (62). Either Xenopus laevis or human eRF1 alone was also shown previously to have an antisuppressor effect against a suppressor tRNA in the reticulocyte lysate translation system (28). In vitro the eRF1 of higher eukaryotes has a release activity and does not need any other factor (28, 35), and eRF3 by itself binds GTP, but GTPase activity requires the presence of both eRF1 and ribosomes (36). It has been shown previously that eRF1 and eRF3 interact, suggesting that they form a functional complex (70, 90, 99, 109).
Yeast eRF3 (Sup35) protein demonstrates prion-like properties and in aggregated states results in a cytoplasmically inherited suppressor element known as [PSI+] (27, 96, 104). Stop codon suppression is a well-known phenotypic feature of [PSI+] cells (18). Yeast eRF3 consists of an N-terminal prion-forming domain (PrD), a charged M (middle) domain of unknown function, and a C-terminal domain that provides the essential translation termination activity (96, 97, 105). Recently, the minimum length of PrD was defined as amino acids (aa) 1 to 97 (76). It was shown previously that Hsp104 protein is required for formation and maintenance of [PSI+] aggregates of eRF3: its overproduction or inactivation cures cells of [PSI+] (14).
The eRF3 family includes proteins from yeasts, humans, X. laevis, and other species that are strongly conserved in the C-terminal region, which has a significant homology with the translation elongation factor eEF1A (47, 60, 109). In contrast to the C-terminal part, the N-terminal region of eRF3 is not conserved, even though some common fragments can be found (51). It is not known whether eRF3-null mutants are viable in higher eukaryotes, but a decrease of Drosophila melanogaster eRF3 protein level (in testis) prevents male meiosis from occurring properly (5).
Translation termination normally occurs after completion of the full-length polypeptide. The majority of aberrant transcripts containing a premature termination codon are recognized and degraded by the cell via nonsense-mediated mRNA decay (NMD) (reviewed in references 34 and 42). The process whereby mRNAs are monitored to eliminate those that code for potentially deleterious protein fragments is called RNA surveillance (reviewed in reference 19).
The process of NMD has been well studied for yeast. During translation termination a surveillance complex, which consists of the Upf proteins and release factors, is assembled. This complex searches 3′ of the termination codon for specific signals that target the mRNA for rapid degradation (22). If such a signal is encountered, the transcript is rapidly decapped by Dcp1p, followed by 5′-to-3′ degradation by the exonuclease Xrn1p (reviewed in reference 21). Homologues of UPF genes have been identified in different organisms, suggesting that NMD could be an evolutionarily conserved pathway of RNA degradation in eukaryotes (3, 65, 79).
It has been shown previously that Upf1 protein interacts with both of the translation termination factors eRF1 and eRF3 and appears to influence translation termination efficiency (22, 103). Upf2p and Upf3p have been shown previously to interact only with the release factor eRF3 (99). Recently another protein, the product of the MTT1 gene, modulating the efficiency of translation termination and interacting with eRF3, was described (20). Also it has been shown previously that the Sla1 protein, involved in the assembly of cortical actin cytoskeleton, interacts with the prion-forming domain of eRF3 in yeast (4).
More recently, by a two-hybrid approach it was demonstrated that mammalian eRF3 interacts with poly(A)-binding protein (PABP) (46). PABP (called Pab1p for yeast) is a multifunctional RNA binding protein which plays a role in the stabilization of RNA messages (50). PABP also serves to bring the 5′ and 3′ ends of mRNA into proximity by binding the initiation scaffold protein eIF4G (91, 101) and stimulates translation initiation (39). The stabilizing function of PABP appears to be independent of eIF4G binding (16), suggesting that the translation and stabilization functions of PABP are separate (39). Based on in vitro experiments, a role for PABP in translation termination in vertebrates was proposed elsewhere (46).
In this work we show that yeast Pab1p and eRF3 (the product of the SUP35 gene) also interact, and we identify the interaction regions for both proteins. We also examined the significance of that interaction for translation fidelity and termination in the wild-type strain and in strains with the [PSI+] phenotype or the sup35 mutation.
MATERIALS AND METHODS
Yeast strains and media.
The following strains of S. cerevisiae were used: HF7C [MATa ura3-52 his3-200 ade2-101 lys2-801 trp1-901 leu2-3,112 gal4-542 gal80-538 LYS2::GAL1-HIS3 URA3::(GAL4 17-mers)3-CYC1-lacZ] (31), L40 [MATa ade2 his3Δ200 leu2-3,112 trp1-901 LYS::(lexAop)4-HIS3 URA3::(lexAop)8- lacZ gal4 gal80 transformed with PAB1 deletion variants in LexADB] (69) (a gift of Allan Jacobson), 33G-D373 [MATα pheA10 ade2-144,717 his7-1(UAA) lys9-A21(UAA) trp1-289(UAG) ura3-52 leu2-3,112], 2-33G-D373 [MATα pheA10 ade2-144,717 his7-1(UAA) lys9-A21(UAA) trp1-289(UAG) ura3-52 leu2-3,112 sup35-21(ts)] (Peterhoff Genetic Collection of Yeast Strains, St. Petersburg State University, St. Petersburg, Russia), BSC783/4a [PSI+] (MATα ade2-1 his3-11,15 leu2-3,112 ura3-1 SUQ5) (a gift of M. Tuite), GT 81-1C [PSI+] (MATaade1-14 his3Δ200 lys2 ura3-52 leu2-3,112 trp1Δ), and OT55 [PSI+] (MATa ade1-14 his3-Del200 leu2-3,112 trp1-289) (a gift of Y. Chernoff).
Yeast cultures were grown in YPD (1% yeast extract, 2% peptone, 2% glucose) or in synthetic complete minimal medium (SC−; 0.67% yeast nitrogen base, 2% glucose, with appropriate auxotrophic supplements). Transformants were grown in the media selective for plasmid maintenance (SC-Trp, SC-Leu, and SC-Ura). Suppression of nonsense mutations was estimated from growth at 18 and 25°C on synthetic media lacking the corresponding amino acids. Yeast transformations were performed according to the work of Gietz et al. (38). YPD medium containing 5 mM guanidine hydrochloride (GuHCl) was used to eliminate [PSI+]. For phenotypic characterization of transformants YPD media containing 0.05, 0.5, 1.0, and 1.5 mg of paromomycin sulfate (Sigma)/ml were used. 3-AT plates contained different concentrations of 3-amino-1,2,4-triazole (Sigma). The two-hybrid interactions were assessed by the ability to transactivate lacZ (in the plate color assay) and/or HIS3 reporter genes (to confer growth in the absence of histidine and in the presence of different concentrations of 3-AT). The plate color assay with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid) as substrate was performed as described previously (107). For quantitative characterization of nonsense suppression the β-galactosidase reporter system (89) was used. Yeast strains were transformed with UAA, UAG, or UGA plasmids carrying TAA, TAG, or TGA (termination codons), respectively, cloned in frame with lacZ or with control LacZ plasmid containing the lacZ gene without the stop codon (all plasmids were a gift of S. Peltz and W. Wang). β-Galactosidase activity was determined as described previously (63). Values for liquid β-galactosidase assay represent the means of at least three assays for each of three independent transformants. For quantitative analysis of the appearance of Parr colonies in the presence of overproduced Pab1p, individual colonies of transformants bearing plasmid pFL44 or pFL44/PAB1 were grown in liquid synthetic medium selective for plasmids to 2 × 106 to 1 × 107 cells/ml and then plated on solid rich medium with paromomycin (0.3 mg/ml) (to measure the frequency of paromomycin-resistant cells) and on rich medium (to measure the total number of viable cells). Six different transformants were used. Parr colonies were patched on synthetic medium containing 5-fluoroorotate (Sigma) to select Ura− cells that had lost the pFL44 or pFL44/PAB1 plasmid (8). For quantitative analysis of prion curing by overproduced Pab1p and Hsp104, yeast cultures bearing pYX243 or pYX243/PAB1 together with the pYS-GAL104-HSP104 construct were grown in liquid synthetic glucose medium selective for plasmids to 2 × 106 to 1 × 107 cells/ml, washed with H2O, and inoculated into the corresponding synthetic galactose medium. Aliquots were taken and plated onto the solid synthetic medium selective for the plasmid. Colonies were grown and after 4 to 5 days replica plated onto Ade− medium. [PSI+] colonies were identified by growth on Ade− medium after 4 to 6 days of incubation. To assay for [PSI+] curing by overproduced Pab1p alone, transformants bearing plasmid pYX242 or pYX242/PAB1 were tested by the same method, except that only glucose medium was used.
Plasmid constructs.
Plasmid pET21b/ySUP35 was obtained by subcloning an NheI-XhoI PCR-generated fragment containing 2.1 kb of S. cerevisiae SUP35 sequence in the NheI-XhoI sites of pET21b (Novagen) with the primers 5′-CGGGCTAGCTCGGATTCAAAC-3′ and 5′-CAAGACTCGAGCTCGGCAATTTTAAC-3′. Plasmid pET21b/ySUP45 was obtained by subcloning an NheI-XhoI PCR-generated fragment containing 1.3 kb of S. cerevisiae SUP45 sequence in the NheI-XhoI sites of pET21b with the primers 5′-CCCGCTAGCGATAACGAGGTTG-3′ and 5′-CCGCTCGAGAATGAAATC-3′.
Plasmid pGBT9/ySUP35 was constructed by subcloning an EcoRI-XhoI PCR-generated fragment containing 2.1 kb of S. cerevisiae SUP35 sequence (aa 2 to 661) in the EcoRI-SalI sites of pGBT9 (Clontech) with the primers 5′-GCAGAATTCTCGGATTCAAAC-3′ and 5′-CAAGACTCGAGCTCGGCAATTTTAAC-3′. pGADGH/ySUP35 was obtained by subcloning a BamHI-XhoI PCR-generated fragment containing 2.1 kb of yeast SUP35 sequence (aa 3 to 661) into the BamHI-XhoI sites of pGADGH with the primers 5′-CGCGGATCCGGATTCAAACCAAGGC-3′ and 5′-CAAGACTCGAGCTCGGCAATTTTAAC-3′. pSTR7 plasmid (94) was used as the template for PCRs. For construction of pGADGH plasmids with C-terminal deletions of the yeast SUP35 gene, PCR products were generated using forward primer 5′-GGATGATGTATATAACTATCTATTCG-3′ and the reverse primers 5′-GGAATTCTCAAGGCATCAGCACTGG-3′ (for pGADGH/SUP35-Bcl) and 5′-GGAATTCCCTTGAGACTGTGGTTGG-3′ (for pGADGH/SUP35-N123), digested with BamHI and EcoRI, and subcloned in pGADGH (Clontech). For construction of pGADGH plasmids with N-terminal deletions of the yeast SUP35 gene, PCR products were generated using forward primers 5′-CGCGGATCCAGGTATGTCTTTGAA-3′ (for pGADGH/SUP35-2ATG) and 5′-CGCGGATCCGGAAGAAGAAGT-3′ (for pGADGH/SUP35-3ATG) and reverse primer 5′-GAGATGGTGCACGATGC-3′, digested with BamHI and XhoI, and subcloned in pGADGH. For all SUP35 deletion constructions pGADGH/SUP35 was used as the template for PCRs. Plasmid pBTM116/ySUP45 was constructed by subcloning a BglII-PstI PCR-generated fragment (primers 5′-GCGCCCGGGGATAACGAGGTTG-3′ and 5′-GGCTCTGCAGTGAAATCATAGTC-3′) containing 1.3 kb of S. cerevisiae SUP45 sequence in the BamHI-PstI sites of pBTM116. For all SUP45 constructions pYSUP45 plasmid (93) was used as the template for PCRs.
Plasmid pGBT9/hGSPT1 was constructed by subcloning an EcoRI-PstI PCR-generated fragment containing 1.9 kb of Homo sapiens hGSPT1 sequence in EcoRI-PstI sites of pGBT9 with the primers 5′-GGCCCCCCCGGAATTCGATC-3′ and 5′-GTAGCTTCTGCAGTCAATTTTC-3′. pGH5 plasmid (a gift of S. Hoshino [47]) was used as the template for PCR. Plasmid pGBT9/xeRF3 was constructed by subcloning a BamHI-SalI fragment containing Xenopus SUP35 sequence from Bluescript/XSUP35 (109) into BamHI-SalI sites of pGBT9.
Plasmids pACT-SUP35N (N113), pAS-SUP35N (N113), pAS-SUP35N (N113) Δ22-69 (NΔ22-69), pAS/SUP35N-PNM2 (N-PNM2), pAS-eRF3 h-N (N 135), and pACT-eRF3 h-N (N 135) were the gift of Y. Chernoff (4).
Plasmids lexA(DB) PAB1 3-H, 4-H, and P-H were described previously (69). For construction of pGBT9 plasmids with deletions of the yeast PAB1 gene, PCR products were generated using primer pairs 5′-GGAATTCCCACCTCAATTTAGAAATG-3′ and 5′-CGGGATCCAAGCTTGCTCAGTTTG-3′ (for pGBT9/PAB1 C) and 5′-GGAATTCCCACCTCAATTTAGAAATG-3′ and 5′-CGGGATCCGTTCGAACAATTCATCAC-3′ (for pGBT9/PAB1 C Δ24), digested with BamHI and EcoRI, and subcloned in pGBT9. For construction of the pGADGH/PAB1 P+C plasmid, a PCR product was generated using the primers 5′-CGGGATCCTTCTCAATTGGCTC-3′ and 5′-CGCCTCGAGAGCTTGCTCAGTTTGTTG-3′, digested with BamHI-XhoI, and subcloned in pGADGH. For all PAB1 deletion constructions, pACT-PAB1 was used as the template for PCRs. All PCR products were verified by sequencing.
Plasmids pFL44S and pYS-Gal104 were described previously (9, 83). Plasmids pACT-PAB1, pAS2-PAB1, and pFL44/PAB1 were the gift of F. Wyers (2). Plasmid pSK/actin was a gift of M. Vedel. Plasmids containing the complete yeast SUP35 gene (pSTR7-multicopy and pUCH-U2-centromeric) were described previously (24, 95). Plasmid pFL44/PAB1ΔC was constructed by subcloning a 2.4-kb SphI fragment from pFL44/PAB1 containing the PAB1 sequence with the C-terminal region deleted (aa 1 to 472) in the SphI site of pFL44. Plasmid pRS425/PAB1 was constructed by subcloning the 2.8-kb PstI-SpeI fragment from pFL44/PAB1 containing the entire PAB1 sequence in the same sites of pRS425 (86). For construction of plasmids pYX242/PAB1 and pYX243/PAB1, a PCR fragment encoding the entire yeast Pab1 sequence plus an additional 9 aa was generated with the primers 5′-CGGGATCCAACCAATAAAAATAAAATG-3′ and 5′-CGCCTCGAGAGCTTGCTCAGTTTGTTG-3′, digested with BamHI-XhoI, and subcloned in pYX242 or pYX243.
Protein and RNA procedures.
eRF3 and eRF1 proteins were produced as translation fusions with the C-terminal polyhistidine in Escherichia coli BL21(DE3) after 12 h of induction at 21°C with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Proteins were extracted from 1,000 ml of culture and purified on a nickel-agarose column as described by the manufacturer. Fractions containing eRF3 or eRF1 were combined; dialyzed against buffer L containing 50 mM Tris HCl (pH 7.8), 50 mM KCl, 5 mM β-mercaptoethanol, 0.5% IGEPAL (Sigma), and 1 mM phenylmethylsulfonyl fluoride; and stored at −80°C. Purified truncated eRF3 proteins were a gift of L. Frolova. Yeast cell pellets from 0.25 liter of growth were lysed in buffer L. Subsequent to centrifugation at 12,000 × g, the supernatant protein concentration was estimated by the Bradford method. One microgram of His-tagged proteins (eRF3 FL, 2ATG, 3ATG, or eRF1) was incubated with the supernatant (30 μg of total proteins) overnight at 4°C in the presence or absence of 80 μg of RNase A (Sigma)/ml, 50 U of micrococcal nuclease (Pharmacia)/ml, and 1 mM CaCl2. Proteins were then loaded on 40 μl of Talon metal affinity resin (Clontech). After extensive washing, bound proteins were eluted with buffer L containing 0.25 M imidazole. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and revealed by Western blotting with anti-yeast eRF3 (a gift of L. Frolova), anti-Pab1p (a gift of F. Wyers) rabbit polyclonal antibodies, or anti-α-tubulin TAT-1 mouse monoclonal antibodies (a gift of K. Gull [106]). For detection of antibody complexes a chemifluorescence detection system (ECF; Amersham Pharmacia Biotech) was used.
To analyze distribution of eRF3 between the soluble and aggregated forms, yeast cell lysates were prepared and fractionated by centrifugation as described previously (59). The resulting supernatants, pellets, and intermediate fractions were analyzed by Western blotting.
RNA was extracted by a hot-phenol extraction method from 10 ml of yeast culture grown in selective medium to an optical density at 600 nm of 0.6 to 0.7 (7). The abundance of lacZ and actin mRNA was analyzed by Northern blotting as described previously (15). Radioactive signals were directly quantified using the PhosphorImager system (STORM; Amersham Pharmacia Biotech).
RESULTS
Interactions of yeast eRF3 and Pab1p in vitro.
We (unpublished results) and others (46) have shown elsewhere that mammalian PABP and eRF3 could interact, but the biological significance of this association is still unclear. The genetic approach with yeast would favor the interpretation of such interaction if it exists in S. cerevisiae.
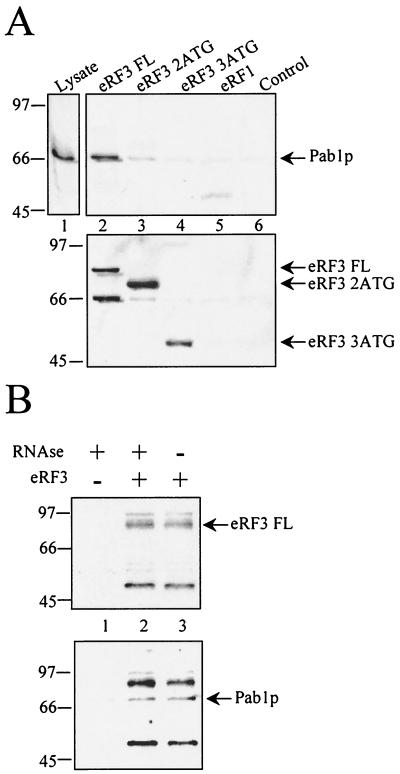
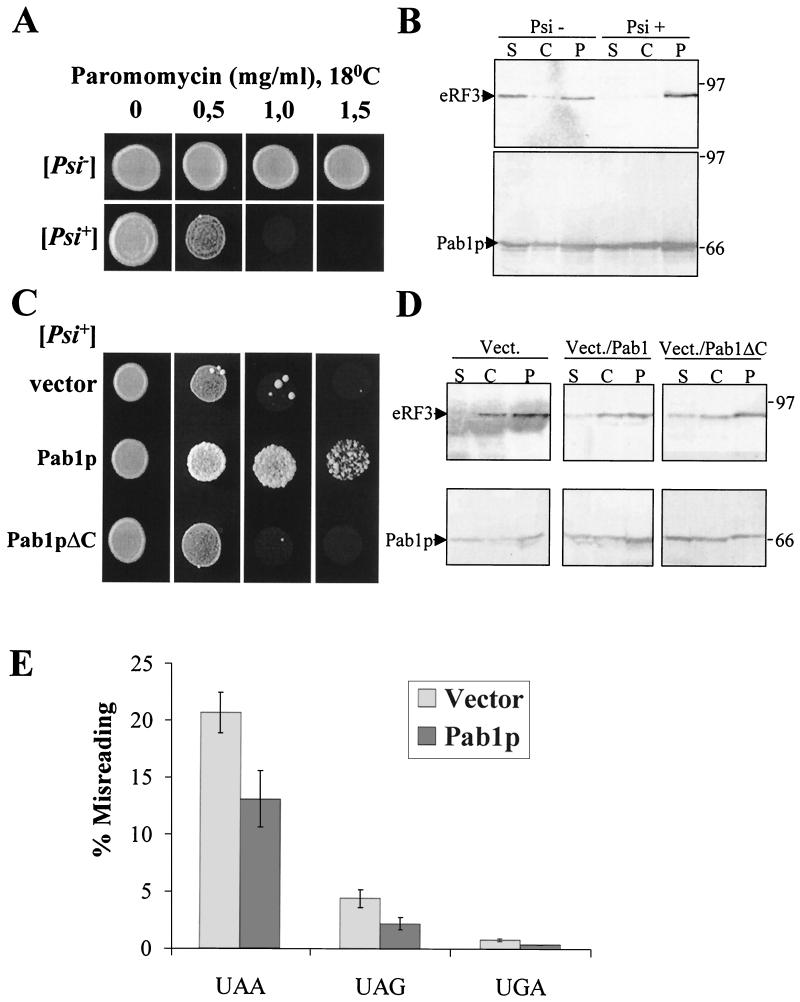
To test eRF3-Pab1p interaction, we first used an in vitro assay mixture made of purified recombinant yeast eRF3 and yeast cellular extract. Three types of His-tagged eRF3 proteins were used: full-length (FL) eRF3; 2ATG, which had the first 123 aa deleted; and 3ATG, which had the first 253 aa deleted. We examined whether the addition of His-tagged eRF3 full-length protein to yeast extract led to the formation of a complex between exogenous eRF3 protein and endogenous yeast Pab1p present in the extract. Recombinant eRF3 was incubated with yeast extracts and then immobilized onto an affinity column. Pab1p was detected by Western blotting in the bound form with the full-length eRF3 (Fig. 1A, lane 2, upper panel). Conversely, deletion of 123 aa (2ATG; Fig. 1A, lane 3, upper panel) or 253 aa (3ATG; Fig. 1A, lane 4, upper panel) in the N-terminal part of the His-tagged eRF3 protein impeded effective binding of endogenous Pab1p, for the same amount of full-length or truncated proteins (Fig. 1A, lower panel). This result demonstrates that eRF3 and Pab1p can be associated in a complex and that the N-terminal domain of yeast eRF3 (aa 1 to 123) is necessary for that association. RNase treatment of yeast lysate does not affect eRF3 binding to Pab1p, suggesting that it is not mediated by the presence of RNA (Fig. 1B).
FIG. 1.
Interaction of endogenous Pab1p with recombinant eRF3. (A) Full-length eRF3 interacts with endogenous Pab1p. The lysate of strain 33G-D373 (lane 1; 1/30 of the extract used per assay was loaded) was incubated with His-tagged eRF3 proteins (full length [aa 1 to 685; lane 2], 2ATG [aa 124 to 685; lane 3], or 3ATG [aa 232 to 685; lane 4]), with eRF1 (lane 5), or with no recombinant protein (lane 6) as a control. Recombinant eRF3 and associated factors were purified by metal affinity chromatography. Bound proteins were eluted and assayed for Pab1p (upper panel) and then eRF3 (lower panel) by immunoblotting. (B) Association between eRF3 and Pab1p is not affected by RNase treatment. Recombinant eRF3 was incubated with yeast lysate in the presence or absence of RNAses. Bound proteins were eluted and assayed for eRF3 (upper panel) and then for Pab1p (lower panel) by immunoblotting. Numbers at left of panels are molecular masses in kilodaltons.
Interactions of yeast eRF3 and Pab1p in the two-hybrid system.
Strain L40 was transformed with Pab1p constructs in which PAB1 fragments were fused in frame with the LexADB domain (a gift of A. Jacobson [69]). The constructs were transformed with pGADGH plasmids in which SUP35 (eRF3) fragments were attached to the GAL4AD domain. As a positive control in all two-hybrid experiments described in this section and below, we used cotransformants with fusion plasmids encoding full-length eRF1 and eRF3 proteins whose interaction has been shown previously (77, 90, 109). The level of eRF3-eRF1 (pGADGH/ySUP35 plus pBTM116/ySUP45) interaction in strain L40 was considered high because cotransformants grow on 100 mM 3-AT. As a negative control, cotransformants bearing empty vector and fusion constructs were used together with transformants bearing a fusion construct alone. All plasmids tested alone could not activate HIS3 and lacZ reporter genes (the corresponding transformants did not grow on SC-His medium and were white on X-Gal plates).
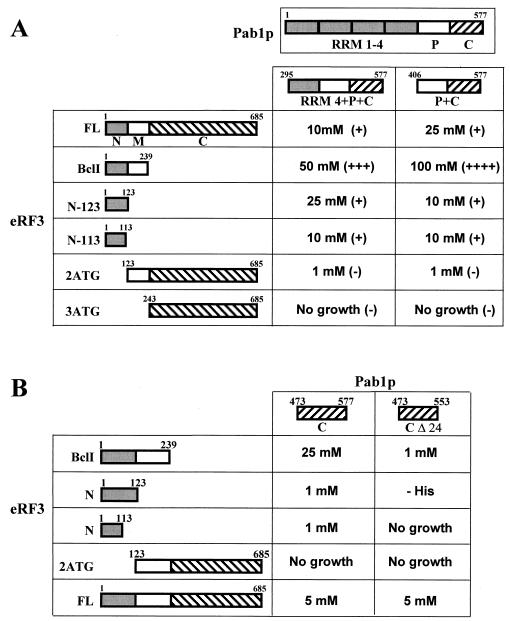
Results with N-terminally truncated Pab1p were positive, whereas full-length Pab1p failed to interact with all tested eRF3 fragments, as previously observed with this set of Pab1p constructs for another Pab1p-interacting protein, Pbp1p (69). Figure 2A shows the main results which demonstrated that aa 406 to 577 for Pab1p and aa 1 to 113 for eRF3 are sufficient to mediate the interaction between Pab1p and eRF3. Interestingly, the minimal interaction region of eRF3 (aa 1 to 113) corresponds to the prion-forming domain of this protein. However, it has to be mentioned that the strongest interaction was observed with fragments corresponding to aa 1 to 239 of eRF3 and aa 406 to 577 of Pab1p.
FIG. 2.
The N+M part of eRF3 interacts with the C part of yeast Pab1 protein. (A) Two-hybrid interactions. Strain L40 carrying different PAB1 deletions in pBTM116 plasmid (69) was transformed with pGADGH/SUP35 (FL), pGADGH/SUP35-Bcl (BclI), pGADGH/SUP35-N123 (N123), pGADGH/SUP35-2ATG (2ATG), pGADGH/SUP35-3ATG (3ATG) (see Materials and Methods), and pACT2/SUP35N (N113) (4). The interactions were tested by the extent of resistance to 3-AT and by an X-Gal filter-lifting assay. Symbols for β-galactosidase assay: ++++, very strong; +++, strong; ++, moderate; +, weak; −, no activity. For the 3-AT assay the highest concentration of 3-AT that still allowed yeast cell growth is noted. Extent of β-galactosidase activity is shown in parentheses. At least five independent transformants were tested in each case. (B) Minimal domain of Pab1p participating in the interaction with yeast eRF3. Strain HF7C was cotransformed with pGADGH/SUP35 (FL), pGADGH/SUP35-Bcl (BclI), pGADGH/SUP35-N123 (N123), pGADGH/SUP35-2ATG (2ATG), pACT2/SUP35N (N113), pGBT9/PAB1 C (C), and pGBT9/PAB1 CΔ24 (ΔC24). The extent of 3-AT resistance is shown; −His corresponds to the growth on His− medium. (C) Minimal domain of eRF3 participating in the interaction with the C-terminal domain of Pab1p. Strain HF7C bearing pGADGH/PAB1 P+C plasmid was transformed with pAS-SUP35N (N113), pAS-SUP35N (N113) Δ22-69 (N-Δ22-69), or pAS/SUP35N-PNM2 (N-PNM2). The interactions were tested in the same way as for panel B. (D) Interactions of yeast Pab1p with human and Xenopus eRF3 in the two-hybrid system. Strain HF7C bearing pGADGH/PAB1 P+C plasmid was transformed with plasmids pGBT9/hGSPT1 (human FL), pAST-eRF3 h-N (human N), and pGBT9/xeRF3 (Xenopus FL), and the resulting cotransformants were assayed for interactions in the same way as for panel B.
Identification of minimal binding domains of Pab1p and yeast eRF3.
Yeast Pab1p consists of four N-terminal RNA recognition motifs (RRMs), which are highly conserved in sequence between species, and a more divergent C-terminal portion in which the conserved C-terminal domain is separated by a proline- and methionine-rich segment of unknown function (1, 57, 82). Recent data showed that the human eRF3 interaction domain is located in the last 90 aa of human PABP (aa 546 to 636), a sequence which is strongly conserved and corresponds to aa 493 to 577 of yeast Pab1p (57).
We next determined if the C-terminal part of the yeast Pab1 protein was also sufficient for the interaction with yeast eRF3. Pab1p C-terminal fragments (C, aa 473 to 577, and C Δ24, aa 473 to 553) were fused in frame with GAL4DB to transform strain HF7C. The results showed that the Pab1p C-terminal fragment (aa 473 to 577) interacts efficiently with eRF3, but not if the last 24 aa are deleted (Fig. 2B), demonstrating that these amino acids of Pab1p are involved in the interaction. The minimal eRF3-interacting domain of Pab1p includes aa 473 to 577.
To identify the region of eRF3 responsible for the interaction with Pab1p, we compared the levels of two-hybrid interaction between different fragments of eRF3 for two strains, L40 (Fig. 2A) and HF7C (Fig. 2B). In both cases, the results showed that aa 1 to 239 of eRF3 have the strongest interaction with Pab1p fragments. Further deletions in eRF3 result in a weak interaction with Pab1p-C, suggesting that part of the interaction domain was lost.
In summary, we defined aa 473 to 577 of Pab1p and aa 1 to 239 of eRF3 as essential for interaction.
Regions of eRF3 interacting with Pab1p and Sla1 are different.
Previously it was shown that the prion-forming domain of eRF3 (Sup35) specifically interacted with Sla1 protein and that so-called [PSI+]-no-more mutations (PNM [27]) abolished this interaction (4). To check whether this was the case for the eRF3-Pab1p interaction, we tested these mutations in a two-hybrid system with the same plasmids (a gift of Y. Chernoff [4]) that were used to study eRF3-Sla1 interactions. The deletion of aa 22 to 69 that has a strong influence on both [PSI+] propagation and formation (25, 27) did not significantly decrease the ability of the amino-terminal domain of eRF3 to interact with Pab1p as well as with the PNM2 mutation, in contrast to the eRF3-Sla1 interaction (4) (Fig. 2C). These data suggest that eRF3 domains involved in Pab1p and Sla1 interactions are different.
Both yeast eRF3 and human eRF3 interact with yeast Pab1p.
Unlike the C-terminal part, the primary structure of the N+M-terminal region of eRF3 is not well conserved between species. However, the size and unusual amino acid composition of the N+M region are the common properties of all described eRF3s (see reference 13 for review). Previously it was shown that the PABP-interacting domain of mouse eRF3 (GSPT2) includes aa 1 to 204 (46). We tested whether the PABP-interacting domain of eRF3 was conserved among eukaryotes by the two-hybrid approach. The results showed that both human and Xenopus full-length eRF3s interact with yeast Pab1p P+C domains and that aa 2 to 135 of human eRF3 (GSPT1) are sufficient for this interaction (Fig. 2D). However, the putative PABP-interacting region is situated in the N+M part of eRF3. The primary structure of those regions in eRF3 proteins (yeast, Xenopus, and human) is only weakly conserved.
Moreover, the deletion of the last 24 aa of Pab1p abolished this interaction (data not shown), as observed also for the yeast eRF3-Pab1p interaction (Fig. 2B). These data suggest that the corresponding interacting domains should be highly conserved in both eRF3 and PABP.
Overexpression of yeast PAB1 in a strain bearing the sup35-21 mutation has an antisuppressor effect.
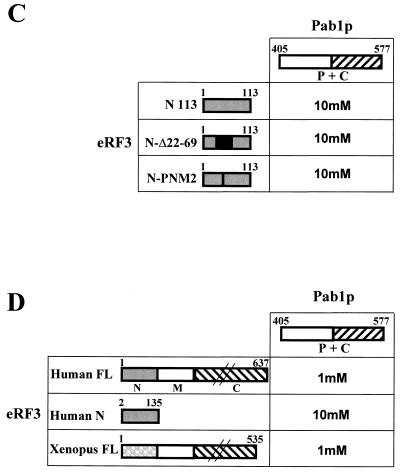
Next, we tested the effects of excessive PAB1 overexpression in a SUP35 mutant. Strain 2-33G-D373 containing the thermosensitive sup35-21 mutation was used. Sequence analysis of the sup35-21 mutation identified the mutated codon as CAA (422Gln)→UAA. This strain was transformed with the following plasmids: pUCH-U2, containing the wild-type SUP35 gene; pFL44 vector; and pFL44/PAB1 or pFL44/PAB1ΔC, encoding Pab1p with the C terminus deleted (containing aa 1 to 472). Ura+ transformants were selected at 25°C and then analyzed for thermosensitivity and efficiency of suppression. The results of these experiments are presented in Fig. 3A. Analysis of the transformants showed that both the vector alone and the plasmid carrying PAB1 or PAB1ΔC could not compensate for temperature sensitivity caused by the sup35 mutation in S. cerevisiae. We concluded that viability was not rescued by overexpression of PAB1.
FIG. 3.
Antisuppressor effect of overexpressed PAB1 in the strain bearing the sup35-21 mutation. (A) The 2-33G-D373 strain was transformed with plasmid pSTR7 bearing the wild-type SUP35 gene (eRF3), the vector pFL44 alone (vector), pFL44/PAB1 bearing the PAB1 gene (Pab1p), and pFL44/PAB1ΔC (Pab1pΔC) encoding the C-terminally deleted Pab1p (containing aa 1 to 472). Growth was assessed under permissive (25°C) and nonpermissive (37°C) conditions on the rich medium (YPD) and on the YPD medium containing 0.5 mg of paromomycin (Par)/ml after 5 days of incubation at 25°C. Drops of yeast suspension of the same density were used. Five independent transformants with each plasmid were tested, and representative results are shown. (B) Antisuppressor effect of overexpressed Pab1p. Strain 2-33G-D373 was cotransformed with plasmids bearing the indicated stop codon in frame with the lacZ coding sequence in combination with pYX242 (vector) or pYX242/PAB1 (Pab1p) plasmid. Percent misreading was quantified as a ratio of β-galactosidase activity in cells harboring lacZ with the stop codon to that in cells harboring lacZ (wt). Results are the means of at least three separate experiments. (C) Basal and overexpressed level of Pab1p. Lysates prepared from the 2-33G-D373 strain transformed with pYX242 (vector) or pYX242/PAB1 (Pab1p) were analyzed by immunoblotting using anti-Pab1p or anti-α-tubulin antibodies. Pab1 protein encoded by pYX242/PAB1 is 8 aa longer than endogenous Pab1p. Numbers at left are molecular masses in kilodaltons. (D) Overexpression of Pab1p does not promote decay of nonsense-containing mRNAs. Total RNA was isolated from 2-33G-D373[pYX242/PAB1] cells harboring the plasmid with lacZ (wt) (WT) or bearing the indicated stop codon. Twenty-five micrograms of RNA was separated on a 1% agarose gel and subjected to quantitative Northern blot analysis. Following hybridization with the lacZ probe, the blot was reprobed for actin to standardize for loading differences. The lacZ/actin ratio was calculated for each sample.
One of the pleiotropic effects of several sup35 mutations is an enhanced sensitivity to paromomycin (for a review see reference 49), an antibiotic that influences translation fidelity and phenotypically suppresses nonsense mutations in yeast (74, 87). To determine whether an excess of Pab1 protein would affect nonsense suppression, we examined transformants of strain 2-33G-D373 for paromomycin sensitivity. Transformants overexpressing wild-type SUP35 or PAB1 showed increased resistance to paromomycin when the same amounts of cells were plated (Fig. 3A). Transformants bearing the vector pFL44 alone or pFL44/PAB1ΔC plasmid exhibited paromomycin sensitivity. The last 105 aa of Pab1p are necessary to restore resistance and for the interaction between eRF3 and Pab1p. Moreover, sensitivity to paromomycin was restored after pUCH-U2 or pFL44/PAB1 was lost (data not shown). Given the known effect of paromomycin on stop codon suppression, these data suggest that overexpression of the full-length PAB1 has an antisuppressor effect on the SUP35 mutant.
To compare the frequencies of paromomycin-resistant (Parr) transformants bearing pFL44 and those bearing pFL44/PAB1, nearly 107 cells of each transformant were plated on paromomycin-containing medium. The frequency of Parr colonies was estimated to be (2.7 ± 0.43) × 10−5 and (1.1 ± 0.19) × 10−3 for 2-33G-D373[pFL44] and 2-33G-D373[pFL44/PAB1], respectively. To confirm that this difference was not due to a mutator effect of overexpressed Pab1p, 133 independent Parr isolates were patched on a medium containing 5-fluoroorotate, which selects against Ura+ cells (8). All Ura− isolates obtained after the loss of the pFL44 plasmid retained their paromomycin resistance phenotype (all 60 colonies tested were Parr). The frequency of Parr in 2-33G-D373[pFL44] is an estimate of spontaneous Parr mutation frequency. Practically all Parr colonies became paromomycin sensitive (Pars) after the loss of pFL44/PAB1 (of 73 colonies tested, 71 were Pars). From these data we conclude that overexpression of Pab1p is responsible for the increased paromomycin resistance of the sup35-21 strain.
Then we directly measured the level of antisuppression activity induced by Pab1p overexpression with a vector-based assay system for quantification of nonsense suppression levels in vivo (89). A control plasmid contained the wild-type lacZ gene [lacZ (wt)]. In the vectors UAA, UAG, and UGA (named by W. Wang and S. Peltz), the lacZ sequence is interrupted by an in-frame UAA, UAG, or UGA stop codon, so active β-galactosidase could be synthesized only in the case of nonsense suppression.
The efficiency of suppression was calculated as the ratio of β-galactosidase activity in cells harboring lacZ with the premature termination codon to that in cells harboring lacZ (wt). The antisuppressor effect of overexpressed Pab1p was detectable only for the UAG and UGA stop codons, not for UAA (Fig. 3B). Western blot analyses were carried out on crude yeast lysates prepared from the wild-type parent strain (33G-D373) and from the sup35-21 strain transformed with pYX242 vector alone (2-33G-D373[pYX242]) or with pYX242/PAB1 (2-33G-D373[pYX242/PAB1]). Probing the immunoblots with anti-Pab1p antibody confirmed that Pab1p was overexpressed in strain 2-33G-D373 (Fig. 3C). The abundance of full-length eRF3 was lower in the sup35-21 mutant than in the wild-type strain (data not shown). Overexpression of PAB1 slightly decreased the level of eRF3, suggesting an antisuppressor effect of Pab1p on mutated eRF3. We could not detect the 422-aa sup35-21 truncated protein, possibly because of its proteolytic sensitivity.
The antisuppressor effect of Pab1p could be explained either by its direct interaction with eRF3 or by its putative role in NMD. In the latter case, the excess of Pab1p would lead to increased degradation of nonsense-containing mRNA, even though previous results demonstrated that the plasmids used in the vector-based β-galactosidase assay were not subjected to NMD (99). We determined by quantitative Northern blot analysis whether the steady-state level of lacZ mRNA would be affected in the presence of overexpressed Pab1p. The RNA was extracted from the transformants used for determination of β-galactosidase activity. The steady-state levels of the lacZ transcript were normalized to those of actin mRNA (Fig. 3D). In the presence of overexpressed Pab1p, the abundance of wild-type lacZ mRNA and that of nonsense-containing lacZ mRNA were nearly identical and were proportional to the amount of actin mRNA. This result demonstrates that Pab1p overexpression affects stop codon suppression and not lacZ mRNA abundance.
The results of all these assays (paromomycin resistance, antisuppressor effect, and lacZ mRNA stability in the presence of an overexpressed PAB1 gene) allow us to conclude that the antisuppressor effect of Pab1p is mediated by its interaction with eRF3 protein.
PAB1 overexpression has an antisuppressor effect on [PSI+]-mediated suppression.
The two-hybrid data and the in vitro binding experiments suggest that prion-forming and Pab1p-interacting domains of eRF3 could overlap. Consequently, Pab1p could play a role in [PSI+]-mediated suppression or in prion propagation. We tested the effects of PAB1 gene overexpression on [PSI+] and [psi−] backgrounds. Previously it was shown that some [PSI+] strains are slightly more sensitive to translation inhibitors such as paromomycin (4, 98). We also found that strain BSC783/4a [PSI+] showed a higher paromomycin sensitivity when grown at 18°C than did its [psi−] derivative obtained after GuHCl treatment (Fig. 4A); no such sensitivity was detected at 25 or 30°C (data not shown).
FIG. 4.
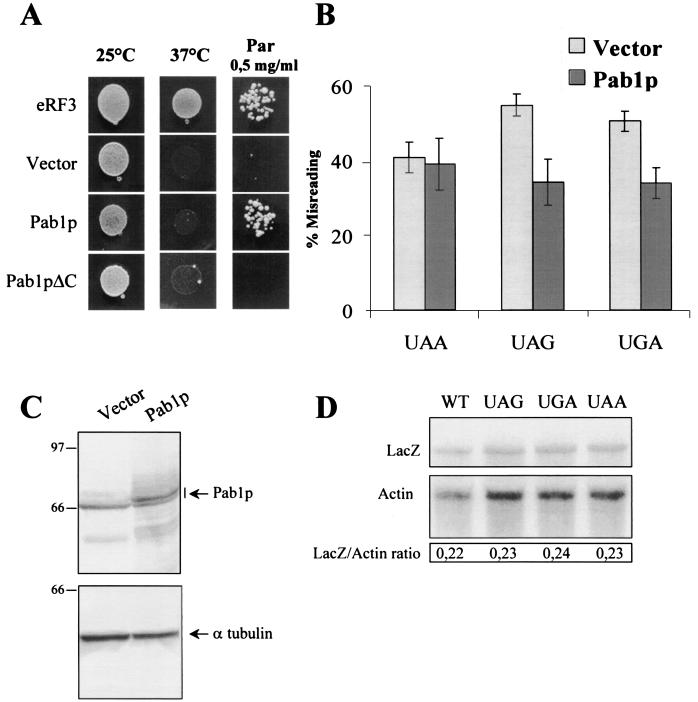
Pab1p has an antisuppressor effect on [PSI+]-mediated suppression. (A) [PSI+] causes sensitivity to paromomycin at 18°C. The sensitivity of the BSC783/4a [PSI+] strain and the resistance of its [psi−] derivative to different concentrations of paromomycin (milligrams per milliliter) at 18°C were assayed. Drops of yeast suspensions with the same cell concentration were placed on YPD medium with paromomycin and incubated at 18°C for 8 days. (B) Distribution of eRF3 and Pab1p between the soluble and aggregated forms. Lysates from BSC783/4a [PSI+] and [psi−] strains were fractionated through a sucrose cushion. Cytosol (C), sucrose fraction (S), and pellet (P) were analyzed by immunoblotting. (C) Overexpression of Pab1p restores the paromomycin resistance of the [PSI+] strain at 18°C. Strain BSC783/4a [PSI+] was transformed with plasmid pSTR7 bearing the wild-type SUP35 gene (eRF3), the vector pFL44 alone (vector), pFL44/PAB1 bearing the PAB1 gene (Pab1p), and pFL44/PAB1ΔC (Pab1pΔC) encoding Pab1p with the C-terminal region deleted (containing aa 1 to 472). The sensitivity to paromomycin was tested in the same way as for panel A. (D) Pab1p overexpression does not lead to eRF3 solubilization from high-molecular-weight aggregates. The eRF3 and Pab1p distribution of transformants described for panel C was performed as described for panel B. (E) Antisuppressor effect of overexpressed PAB1. BSC783/4a [PSI+] was cotransformed with plasmids bearing the wild-type copy of the lacZ gene or with lacZ (UAA), lacZ (UAG), or lacZ (UGA) in combination with pYX242 or pYX242/PAB1 plasmid. Percent misreading was quantified as a ratio of β-galactosidase activity in cells harboring lacZ (UAA) to that in cells harboring lacZ (wt). Results are the means of at least three separate experiments. Numbers at right of panels B and D are molecular masses in kilodaltons.
Growth of various BSC783/4a [PSI+] transformants was tested in the presence of different concentrations of paromomycin (0.5, 1.0, and 1.5 mg/ml) at 18°C. In all cases we could detect increased paromomycin resistance when Pab1p, but not its C-terminally deleted variant, was overexpressed (Fig. 4C). A 2.5-fold increase of the amount of full-length or truncated Pab1p was revealed by Western blot analysis (Fig. 4D).
Overproduction of both eRF3 and Pab1 protein in the BSC783/4a [PSIY+] strain has an additive effect. Cotransformants bearing plasmids pSTR7 (eRF3) and pFL44/PAB1 could grow at 18°C on the medium containing 1.5 mg of paromomycin/ml, but cotransformants with pSTR7 and pFL44 (vector) or pSTR7 and pFL44/PAB1ΔC could not (data not shown). Interestingly, paromomycin sensitivity was restored after plasmid loss, indicating that [PSI+] was not eliminated.
The increase of paromomycin resistance in the presence of the overexpressed PAB1 gene could be explained either by an antisuppressor effect of Pab1p on [PSI+]-mediated suppression or by an inhibitor effect of Pab1p on [PSI+] propagation.
To directly measure whether Pab1 affects [PSI+]-mediated suppression in strain BSC783/4a, the vector-based assay system for quantification of nonsense suppression levels described above (“Overexpression of yeast PAB1 in a strain bearing the sup35-21 mutation has an antisuppressor effect”) was employed. BSC783/4a [PSI+] strains harboring vector pYX242 alone or the pYX242/PAB1 construct were transformed with a plasmid bearing either lacZ (wt) or lacZ containing one of the premature termination codons. The nonsense suppression activity was measured as the ratio of β-galactosidase activity in cells harboring lacZ vectors with different stop codons to that in cells harboring lacZ (wt). The results have shown that cells harboring the multicopy PAB1 plasmid had significantly decreased suppression compared with that for cells containing the control vector (Fig. 4E). Thus, overexpressed Pab1p has an antisuppressor effect on all stop codons. This confirms that the increased resistance to paromomycin produced by excess Pab1p is caused by its antisuppressor effect on [PSI+]-mediated suppression.
PAB1 overexpression does not influence [PSI+] stability.
To test whether overexpression of Pab1p could influence [PSI+] prion propagation, we measured the frequency of spontaneous [PSI+] curing in strain BSC783/4a [PSI+]. The mitotic stability of [PSI+] was assayed by monitoring the appearance of [PSI−] cells on YPD medium. More than 7,000 colonies, with and without an excess of PAB1, were examined. No statistically significant difference in [PSI+] stability was observed: for BSC783/4a [PSI+] bearing pRS425 [PSI+] stability was 99.7% and for BSC783/4a [PSI+] bearing pRS425/PAB1 stability was 99.9%.
As shown earlier, eRF3 forms high-molecular-weight aggregates in [PSI+] cells (78). We examined whether Pab1p overexpression led to solubilization of at least some eRF3 protein. Extracts from [PSI+] and [psi−] variants of strain BSC783/4a were fractionated through a sucrose cushion as described previously (77). Immunoblot analysis using antibodies against eRF3 and Pab1p revealed that, as expected, eRF3 was found in the cytosolic fraction in the [psi−] strain but not in the [PSI+] strain (Fig. 4B) even when Pab1p was overexpressed (Fig. 4D). Furthermore, the [PSI+] status of the strain has no effect on the distribution of Pab1p, which was found in both fractions. We conclude from this experiment that excess Pab1p is not able to solubilize detectable amounts of eRF3 in the [PSI+] strain.
Strain-specific effects on [PSI+] maintenance have been described previously (25). To exclude this possibility, we determined [PSI+] stability in the strain GT81-1C (a gift of Y. Chernoff), which does not contain tRNA suppressor SUQ5. The GT81-1C [PSI+] strain was transformed with pYX242/PAB1 or pYX242 vector alone, and we assayed for the appearance of red colonies. About 7,000 colonies, with and without excess Pab1p, were examined, and no [psi−] colonies were obtained.
To test whether excess Pab1p would influence [PSI+] stability in derivatives containing weak [PSI+] variants, the OT55 [PSI+] strain was transformed with pYX242/PAB1 or pYX242 vector alone, and we assayed for the appearance of red colonies. In all cases the stability of [PSI+] was the same whether Pab1p was overexpressed or not. About 2,000 colonies, with and without excess Pab1p, were examined. No [psi−] colonies were obtained.
One of the characteristics of [PSI+] is that it is cured by either deletion or overexpression of the chaperone protein Hsp104 (14). To test the effects of excess Hsp104 on the propagation of [PSI+] in the presence and absence of overexpressed Pab1p, the mitotic stability of [PSI+] was assayed in the GT81-1C [PSI+] strain. This strain was cotransformed with pYX243/PAB1 plasmid or pYX243 vector alone and pYS-Gal104 encoding Hsp104. We assayed for the presence of red colonies. Both Pab1p overexpression and Hsp104 overexpression were induced by growth on medium containing galactose instead of glucose. The results of these experiments demonstrated that the curing of [PSI+] by excess Hsp104 did not significantly change in the presence of overexpressed Pab1p ([PSI+] stability was 75.3%, compared to 79.6% in the absence of overexpressed Pab1p).
Taken together, all these experiments demonstrate that Pab1p overexpression does not influence [PSI+] stability.
PAB1 overexpression in the wild type has an antisuppressor effect on phenotypic suppression.
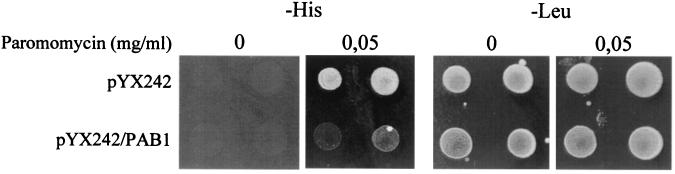
Nonsense mutations in yeast can be phenotypically suppressed by low concentrations of paromomycin (74, 87). To determine whether Pab1p overexpression would affect phenotypic suppression, we used wild-type strain 33G-D373. 33G-D373[pYX242] and 33G-D373[pYX242/PAB1] transformants were grown in the presence of low concentrations of paromomycin (0.05 mg/ml) at 25°C. Phenotypic suppression of the his7-1(UAA) mutation was observed for transformants bearing the vector alone (Fig. 5). In contrast, transformants bearing the pYX242/PAB1 plasmid exhibited an antisuppression phenotype. Low-level phenotypic suppression was also observed for lys9-A21(UAA) and trp1-289(UAG) after 12 days of incubation at 18°C for transformants with vector alone but not in the case of Pab1p overexpression (data not shown). This result suggests that Pab1p has a direct effect on translation termination.
FIG. 5.
Excess Pab1p acts against phenotypic suppression. Strain 33G-D373 was transformed with pYX242 and pYX242/PAB1 plasmids. Drops of yeast suspensions with the same cell concentration were plated on selective medium with paromomycin and incubated at 25°C. The His+ phenotype indicated phenotypic suppression, and the Leu+ phenotype confirmed the plasmid transformation. Ten independent transformants with each plasmid were tested. Representative results are shown.
DISCUSSION
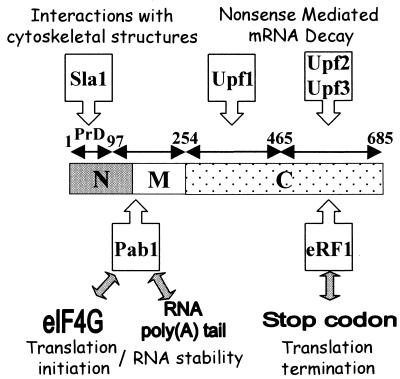
Using recombinant eRF3 and cellular extracts, we have shown that yeast eRF3 and Pab1p can interact. That interaction was demonstrated by the two-hybrid approach to be direct. As shown previously for mammalian eRF3 and PABP (46), we found that yeast eRF3-Pab1p interactions are mediated by the very weakly conserved N-terminal domain of eRF3. At least in yeast, eRF3 plays an essential role in translation termination, interacting with eRF1 via the C-terminal GTPase domain (29, 77, 99). Both factors are said to be essential for translation termination in yeast (90). As shown in Fig. 6, eRF3 also contains domains within its sequence that interact with Upf1, Upf2, and Upf3 proteins, which are the components of the surveillance complex (99), and with Sla1, a protein involved in the assembly of the cortical actin cytoskeleton (4). Although genetic data suggest a functional relationship between eRF3 and Hsp104 (14), a physical interaction between these proteins could not be demonstrated (84). Upf1-like helicase Mtt1 also interacts with eRF3, but the domain of interaction within eRF3 was not precisely localized; two interaction domains were described previously (20).
FIG. 6.
Network of interactions between molecules interacting with eRF3 or involved in RNA stability or degradation and translation (initiation and termination). Most of the interactions shown here were demonstrated between two molecules examined separately. Further analysis must be carried out in vivo to determine whether these interactions occur singly or together. Isolation and analysis of complexes have to be carried out under precise and well-defined conditions for translation and RNA stability or degradation.
Our data suggest that the domain of interaction of eRF3 with Pab1p is situated between those interacting with Sla1 and Upf1 (Fig. 6).
The Pab1p-interacting region in eRF3.
Previously yeast eRF3 was schematically divided into three domains: the N-terminal prion-determining domain (PrD, aa 1 to 123), the middle domain of unknown function (M, aa 124 to 253), and the C-terminal domain, structurally similar to translation elongation factor eEF1A and essential for cell viability (C, aa 254 to 685) (96, 97, 105). Further studies showed that overexpression of only the first 113 aa of eRF3 was sufficient for [PSI+] establishment (55) and that an N-terminal polypeptide consisting of residues 2 to 114 spontaneously aggregated in vitro into amyloid-like filaments (54). Recently, the minimum length of PrD was defined as aa 1 to 97 (76).
aa 1 to 113 of eRF3 are sufficient to mediate the interaction with Pab1p, suggesting that the Pab1p-interacting domain could overlap with PrD. However, several lines of evidence indicate that Pab1p does not influence [PSI+] propagation. Firstly, [PSI+]-no-more mutations do not affect eRF3-Pab1p two-hybrid interaction. Secondly, overexpression of PAB1 does not cure the [PSI+] phenotype or solubilize detectable amounts of eRF3. Finally, prion-curing properties of overexpressed HSP104p, which is required for formation and maintenance of [PSI+] (14), were not modified by excess Pab1p, in contrast to what was obtained with Sla1p. These data also suggest that the eRF3 domains involved in Pab1p and Sla1 interactions are different.
Similar to Pab1p, eRF1, another eRF3-interacting protein, does not influence [PSI+] propagation (23). Possibly, the [PSI+] complex, once formed, is resistant to the putative influence of eRF3-interacting proteins. The only protein influencing [PSI+] propagation identified so far is Hsp104. However, the mechanism of its action remains unclear because no direct interaction with eRF3 could be shown (see reference 85 for a review).
An interaction between Pab1p and the yeast Hsp70 homologue Ssa1 was recently shown (45). Interestingly an excess of Ssa1 could protect [PSI+] from curing by overexpression of Hsp104 (71); this fact suggests that Ssa1 and eRF3 could form a transient complex. Depletion of Ssa1 affects translation initiation, reducing Pab1p-eIF4G interaction (45). Possibly, Ssa1 modulates [PSI+] curing or translation initiation through Pab1p, but this interaction remains to be shown.
Our data show that the interaction of eRF3 with Pab1p is evolutionarily conserved. In particular we have shown that human eRF3 could interact with yeast Pab1p via the same N-terminal domain as with human PABP. However, sequence analysis failed to reveal any significant homology of the primary structures of the identified domains of S. cerevisiae eRF3 and human eRF3. Secondary structure predictions using the SOPM algorithm (37) indicated mostly random coil conformation for PABP-interacting regions of all eRF3 homologues (data not shown). Possibly this region could form a helical structure only when bound to Pab1p-C. This has been shown previously to occur for Paip2 when bound to PABP-C (57). Interestingly the predicted PABC-interacting consensus sequence (LNVNAKPFVP) of Paip2 has also been found in eRF3 proteins from different species (57). In all eRF3 proteins analyzed (hGSPT1, mGSPT2, xSUP35, and yeast eRF3) the PABP-interacting sequence is located near a short helical region (Fig. 7). A comparative secondary structure analysis reveals aa 132 to 140 of yeast eRF3 (qqkqAaPkpk) as a potential Pab1p-interacting region (Fig. 7).
FIG. 7.
Secondary structure prediction for the PABP-interacting region in various eRF3 homologues. Alignment of the N-terminal parts of Xenopus (xeRF3, aa 51 to 87), human (hGSPT1, aa 48 to 85), mouse (mGSPT2, aa 35 to 76), and yeast (yeRF3, aa 109 to 149) eRF3 proteins was performed with the MULTALIN (17) program (http://npsa-pbil.ibcp.fr). The PABP-interacting consensus sequence (LNVNAKPFVP) (57) is shown in boldface. Sec, secondary structure predicted by SOPM algorithm (37); c, random coil; e, extended strand; t, beta turn. The α-helical region (h) is underlined.
The eRF3-interacting region in yeast Pab1p.
PABP is a highly conserved protein that consists of four tandem RNA recognition domains (RRM1 to RRM4) and a carboxy-terminal region which contains a proline- and methionine-rich segment of unknown function (P) and a conserved C-terminal domain (C) (1, 57, 82). RRM1 and RRM2 are required for high-affinity binding to poly(A) in vitro (58, 72). These repeats contain a binding site for eIF4G and Paip1 (39). The C-terminal half of PABP promotes PABP oligomerization (58, 69) and also contains binding sites for Paip1, Pbp1, and some other proteins. The significance of all these interactions remains to be determined (39, 69).
Previously it was shown that interaction of human PABP with mouse eRF3 (GSPT2) appeared to be mediated through the C-terminal domain (aa 369 to 633) of PABP (46). Recently the solution structure of the last 139 residues from human PABP was determined, and it was shown that a domain of 74 aa is sufficient to bind peptides from a number of PABP-interacting proteins, such as eRF3, Paip1, and Paip2 (57). Our data are consistent with this observation. We showed that the deletion of the last 105 aa from the Pab1p C terminus prevents paromomycin resistance in the SUP35 mutant and in the [PSI+] strain BSC4a. Furthermore deletion of the C-terminal 24 aa in Pab1p dramatically decreases interaction with yeast and human eRF3.
A role of PABP in translation termination.
Especially for S. cerevisiae, many data had been accumulated on the PABP, which was initially characterized through its ability to strongly bind to poly(A). Clearly Pab1p plays essential roles in mRNA metabolism and translation. More specifically, in yeast, the protein regulates the poly(A) tail length during the polyadenylation reaction (2). It inhibits the activity of the purified poly(A) polymerase (64) and is required for Pab1p-dependent poly(A) nuclease activity to control mature mRNA tail length (11). Pab1p is able to prevent mRNA decay independently of the presence of a poly(A) tail if tethered to the mRNA (16). Furthermore, PABP prevents access of a 3′-to-5′ poly(A)-specific exoribonuclease activity in mammalian cells (6, 32, 100). It is known that Pab1p participates in translation initiation via its interaction with eIF4G (91). More recently it was shown that the interaction with eIF4G, which also occurs in higher eukaryotes (48, 80), serves to integrate the functions of the 5′ cap and the 3′ poly(A) tail in translation initiation (41, 101). Pab1p also stimulates poly(A)-dependent and cap-dependent translation by different mechanisms in vitro (73). All these activities involve the N-terminal part of Pab1p, which is essential and consists of four RRMs.
In this work, we demonstrate that Pab1p has an antisuppressor effect in vivo. Moreover we show that this effect requires the site of the binding of Pab1p to eRF3, suggesting that Pab1p has an antisuppressor effect on translation termination through its interaction with eRF3. This is a completely new function for Pab1p. Such a role for PABP in higher eukaryotes remains to be demonstrated, but the fact that human PABP and eRF3 also interact suggests that this could be the case.
Nonsense suppression occurs when a near-cognate tRNA successfully competes with the termination factors at a nonsense codon; amino acid incorporation into the peptide chain instead of translation termination occurs at the site of nonsense mutation. Mutations that result in the nonsense suppression phenotype have been identified in several genes. Among them were the SUP45 and SUP35 genes, encoding peptidyl release factors eRF1 and eRF3, and also UPF1, whose disruption promotes suppression of certain nonsense alleles (61, 68, 99, 102). Recently another gene, MTT1, modulating the efficiency of translation termination and interacting with eRF3, was described (20).
Drugs that specifically alter translation termination are not known. For this reason, we looked for different situations where translation termination activity was weak, in order to study the functional significance of Pab1p and eRF3 interaction in this essential step of gene expression.
Paromomycin is an aminoglycoside antibiotic influencing translation fidelity. Recent data clearly show that it interacts with a highly conserved region in the 3′ end of 16S rRNA and that its binding induces a local conformational change in this decoding region (33, 81). Also it has been shown previously that the structures of prokaryotic and eukaryotic decoding region A sites are similar (66, 67) in most details and that paromomycin binds to this decoding site in both prokaryotic and eukaryotic ribosomes. Thus, such binding is highly specific and so paromomycin has a direct influence on translation termination.
It is known that mutations in genes SUP35 and SUP45 cause paromomycin sensitivity (for a review see reference 49). Moreover, a nonsense mutation in yeast can be phenotypically suppressed with paromomycin (74, 87), which also increases the efficiency of [PSI+]-dependent suppression (75). It was found previously that a mutation in a gene encoding a translation initiation factor could also cause paromomycin sensitivity, and it was proposed elsewhere that paromomycin could affect the function of any protein involved in the decoding process (40).
Our data show that PAB1 overexpression in a SUP35 mutant could restore paromomycin resistance and that this effect depends on the presence of the Pab1p C terminus, which is necessary for the interaction with eRF3. With a vector-based assay system we measured the termination activity and confirmed that the overexpressed Pab1p could compensate for the deleterious effects of the eRF3 mutation on translation termination machinery. Quantitative mRNA analysis has not revealed destabilization of nonsense mRNA by Pab1p overexpression. Thus, the implication of our results is that Pab1p overexpression has a an antisuppressor effect which is obtained via its interaction with eRF3 on the termination machinery.
These data are in agreement with the effect of overexpressed Pab1p on [PSI+]-dependent suppression. In [PSI+] cells, most of the eRF3 (Sup35) is converted from a soluble, active state into an insoluble, inactive state that enhances the suppression of nonsense mutations (see reference 85 for a review).
We have shown that together the presence of paromomycin and low temperature (18°C) are lethal for [PSI+] cells. Overexpression of full-length Pab1p but not that of its C-terminally truncated variant restored viability in these conditions. Again, the eRF3 binding domain of Pab1p is necessary, suggesting that overexpressed Pab1p could restore termination of translation via eRF3 interaction. We failed to detect any effect of Pab1p on [PSI+] propagation or eRF3 aggregation, although we could not exclude the possibility that even a limited solubilization of eRF3 could be sufficient to restore termination (see below). All these findings together suggest that overexpressed Pab1p could restore termination of translation via eRF3 interaction.
Overexpression of Pab1p leads to an antisuppressor effect for all stop codons in the [PSI+] strain. Also, it decreases the phenotypic suppression of the his7-1 (UAA) nonsense mutation caused by paromomycin in a wild-type strain. The inability of overexpressed PAB1 to act against suppression of the UAA codon in the sup35-21 strain could be connected with the nature of the sup35-21 mutation, which changes a glutamine codon to a UAA stop codon at aa 422. It has been shown previously that aa 254 to 685 of eRF3 are essential for cell viability and that C-terminally truncated eRF3 (aa 1 to 482) is unable to support viability (97), and so the sup35-21 strain should be viable only in the case of readthrough of the UAA codon; this has likewise been proposed for the sup35-2 (UAG) mutation (108). Indeed, Western blot analysis has shown that the expression of full-length eRF3 in the sup35-21 strain was lower than that in the wild-type strain. Viable but thermosensitive nonsense mutations have been isolated in several essential genes of S. cerevisiae (12, 44, 56) and of Salmonella enterica serovar Typhimurium (52). In the case of the supK584 mutation, which caused an opal (UGA) substitution in the prfB gene of S. enterica serovar Typhimurium, encoding RF2 release factor, it has been shown previously that this mutation reduces the cellular amount of RF2 (52). Such a reduction in RF2 level causes inefficient termination of translation and leads to autosuppression. An analogous mechanism has also been proposed for the sup35-2 mutation (108). Overexpression of PAB1 only slightly decreases the level of eRF3, suggesting that the antisuppressor effect of Pab1p is not mediated by regulating the level of eRF3.
We hypothesize that the mutation CAA (422Gln)→UAA leads to a misincorporation of the amino acid during the readthrough reaction. This change of amino acid would alter the structure of eRF3, leading to the suppression phenotype and thermosensitivity. PAB1 overexpression leads to reversal of this phenotype by increasing termination efficiency. It is unlikely that Pab1p bypasses the eRF3 function in termination, as the overexpression effect requires the eRF3 binding domain. There are several possible ways in which Pab1p overexpression leads to an antisuppressor phenotype.
The first possibility is that eRF3 cannot be recruited to form an active complex with eRF1 because of a conformation problem in the sup35-21 strain and because of aggregation in [PSI+] cells. Pab1p possibly helps to recruit eRF3 and/or restores functional conformation of the altered eRF3 protein during the termination reaction in the ribosome. Results of [PSI+] strain analysis showed that overexpressed Pab1p is unable to solubilize detectable levels of eRF3 protein, although it cannot be excluded that a restricted amount of eRF3 directed to the translation machinery could have an antisuppressor effect. This implies that Pab1p interacts with eRF3 during the termination reaction and promotes its activity.
Another possibility is based on the fact that release factors need to be recycled: the number of eRF1 and eRF3 termination factors is limited compared to that of ribosomes (about one copy of eRF3 per 20 ribosomes [26]). Termination (release of nascent protein from the ribosome) but also posttermination events (release and/or regeneration of the active form of ribosome and associated factors and possibly reinitiation) may determine the kinetics of the termination reaction in a cellular context. Inhibition of recycling may result in reduced translation termination efficiency. During the translation termination a surveillance complex is assembled and searches 3′ of the termination codon for specific signals that target the mRNA for rapid degradation. A dynamic model for the surveillance complex assembly was proposed according to which dissociation of eRF1 allows either Upf2 or Upf3 to bind to the eRF3-Upf1 complex (99). Since eRF3 blocks the ATPase-helicase activity of Upf1p (22), dissociation of eRF3 from the Upf complex—but not necessarily from the ribosome--would be necessary to form the active surveillance complex. In contrast to the general deadenylation-dependent pathway, degradation by the NMD pathway is independent of prior deadenylation of the mRNA and Pab1p was shown previously to be unable to prevent the decay of mRNA subjected to NMD (16). Consequently, it is not likely that Pab1p outcompetes Upf proteins for binding to eRF3, because that would prevent NMD. We do not know the fate of the translation machinery components after peptide release, but eRF3 has previously been found associated with ribosomes and 40S subunits (26, 30), suggesting that eRF3 remains associated with the 40S subunit after termination. As the function of the Pab1p-poly(A) tail complex is to recruit the 40S subunit on the mRNA in order to stimulate its translation (92), we hypothesize that eRF3 could mark a ribosome that has already finished one round of translation and that would be reloaded on the mRNA in order to ensure efficiency of translation. According to this model, Pab1p overexpression would act to stimulate eRF3 in the posttermination recycling step, allowing efficient termination in the next rounds of translation of the same or other mRNAs. This model postulates that mutants in which posttermination events do not occur properly could also be detected by a nonsense suppression phenotype, as observed for Upf-deletion strains. Development of in vitro systems that uncouple posttermination events from peptide release will be needed to demonstrate the role of all these factors. This work will be of major interest, as the 5′ and 3′ ends of the mRNA may interact to form a closed-loop structure.
Acknowledgments
We are very grateful to A. Borchsenius, J. Camonis, M. Tuite, Y. Chernoff, A. Jacobson, D. Mangus, T. Merkulova, L. Frolova, S. Peltz, W. Wang, M. Vedel, K. Gull, and F. Wyers for plasmids, yeast strains, proteins, and antibodies and to O. Jean-Jean, Y. Chernoff, L. Paillard, V. Legagneux, and A. Sachs for very helpful discussions.
B.C. was supported by the Ligue Nationale contre le Cancer and the Fondation pour la Recherche Médicale. This work was supported by a grant to M.P. from the Human Frontier Science Program (RG 0032/1997-M); a common grant to S.I-V., G.Z., and M.P. from INTAS (RFBR 95-1037); a grant to S.I-V., G.Z., S.C., and M.P. from CNRS (PICS 1113); and a grant to S.I-V., G.Z., and S.C. from RFBR (00-04-22001NCNI_a) and was partly supported from CRDF RB1-2037 (to S.C. and G.Z.).
REFERENCES
- 1.Adam, S. A., T. Nakagawa, M. S. Swanson, T. K. Woodruff, and G. Dreyfuss. 1986. mRNA polyadenylate-binding protein: gene isolation and sequencing and identification of a ribonucleoprotein consensus sequence. Mol. Cell. Biol. 6:2932-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amrani, N., M. Minet, M. Le Gouar, F. Lacroute, and F. Wyers. 1997. Yeast Pab1 interacts with Rna15 and participates in the control of the poly(A) tail length in vitro. Mol. Cell. Biol. 17:3694-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Applequist, S. E., M. Selg, C. Raman, and H. M. Jack. 1997. Cloning and characterization of HUPF1, a human homolog of the Saccharomyces cerevisiae nonsense mRNA-reducing UPF1 protein. Nucleic Acids Res. 25:814-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailleul, A. B., G. P. Newnam, J. N. Steenbergen, and Y. O. Chernoff. 1999. Genetic study of interactions between the cytoskeletal assembly protein Sla1 and prion forming domain of the release factor Sup35 (eRF3) in Saccharomyces cerevisiae. Genetics 153:81-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu, J., B. C. Williams, Z. Li, E. V. Williams, and M. L. Goldberg. 1998. Depletion of a Drosophila homolog of yeast Sup35p disrupts spindle assembly, chromosome segregation, and cytokinesis during male meiosis. Cell Motil. Cytoskelet. 39:286-302. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein, P., S. W. Peltz, and J. Ross. 1989. The poly(A)-poly(A)-binding protein complex is a major determinant of mRNA stability in vitro. Mol. Cell. Biol. 9:659-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boeck, R., B. Lapeyre, C. E. Brown, and A. B. Sachs. 1998. Capped mRNA degradation intermediates accumulate in the yeast spb8-2 mutant. Mol. Cell. Biol. 18:5062-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boeke, J. D., F. LaCroute, and G. R. Fink. 1984. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197:345-346. [DOI] [PubMed] [Google Scholar]
- 9.Bonneaud, N., O. Ozier-Kalogeropoulos, G. Y. Li, M. Labouesse, L. Minvielle-Sebastia, and F. Lacroute. 1991. A family of low and high copy replicative, integrative and single-stranded S. cerevisiae/E. coli shuttle vectors. Yeast 7:609-615. [DOI] [PubMed] [Google Scholar]
- 10.Breining, P., and W. Piepersberg. 1986. Yeast omnipotent suppressor SUP1 (SUP45): nucleotide sequence of the wild type and a mutant gene. Nucleic Acids Res. 14:5187-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, C. E., and A. B. Sachs. 1998. Poly(A) tail length control in Saccharomyces cerevisiae occurs by message-specific deadenylation. Mol. Cell. Biol. 18:6548-6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai, T., T. R. Reilly, M. Cerio, and M. E. Schmitt. 1999. Mutagenesis of SNM1, which encodes a protein component of the yeast RNase MRP, reveals a role for this ribonucleoprotein endoribonuclease in plasmid segregation. Mol. Cell. Biol. 19:7857-7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chernoff, Y. O. 2001. Mutation processes at the protein level: is Lamarck back? Mutat. Res. 488:39-64. [DOI] [PubMed] [Google Scholar]
- 14.Chernoff, Y. O., S. L. Lindquist, B. Ono, S. G. Inge-Vechtomov, and S. W. Liebman. 1995. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [PSI+]. Science 268:880-884. [DOI] [PubMed] [Google Scholar]
- 15.Chevalier, S., A. Couturier, I. Chartrain, R. Le Guellec, C. Beckhelling, K. Le Guellec, M. Philippe, and C. C. Ford. 1996. Xenopus cyclin E, a nuclear phosphoprotein, accumulates when oocytes gain the ability to initiate DNA replication. J. Cell Sci. 109:1173-1184. [DOI] [PubMed] [Google Scholar]
- 16.Coller, J. M., N. K. Gray, and M. P. Wickens. 1998. mRNA stabilization by poly(A) binding protein is independent of poly(A) and requires translation. Genes Dev. 12:3226-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox, B. 1994. Cytoplasmic inheritance. Prion-like factors in yeast. Curr. Biol. 4:744-748. [DOI] [PubMed] [Google Scholar]
- 19.Culbertson, M. R. 1999. RNA surveillance. Unforeseen consequences for gene expression, inherited genetic disorders and cancer. Trends Genet. 15:74-80. [DOI] [PubMed] [Google Scholar]
- 20.Czaplinski, K., N. Majlesi, T. Banerjee, and S. W. Peltz. 2000. Mtt1 is a Upf1-like helicase that interacts with the translation termination factors and whose overexpression can modulate termination efficiency. RNA 6:730-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czaplinski, K., M. J. Ruiz-Echevarria, C. I. Gonzalez, and S. W. Peltz. 1999. Should we kill the messenger? The role of the surveillance complex in translation termination and mRNA turnover. Bioessays 21:685-696. [DOI] [PubMed] [Google Scholar]
- 22.Czaplinski, K., M. J. Ruiz-Echevarria, S. V. Paushkin, X. Han, Y. Weng, H. A. Perlick, H. C. Dietz, M. D. Ter-Avanesyan, and S. W. Peltz. 1998. The surveillance complex interacts with the translation release factors to enhance termination and degrade aberrant mRNAs. Genes Dev. 12:1665-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derkatch, I. L., M. E. Bradley, and S. W. Liebman. 1998. Overexpression of the SUP45 gene encoding a Sup35p-binding protein inhibits the induction of the de novo appearance of the [PSI+] prion. Proc. Natl. Acad. Sci. USA 95:2400-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derkatch, I. L., M. E. Bradley, P. Zhou, Y. O. Chernoff, and S. W. Liebman. 1997. Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics 147:507-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derkatch, I. L., M. E. Bradley, P. Zhou, and S. W. Liebman. 1999. The PNM2 mutation in the prion protein domain of SUP35 has distinct effects on different variants of the [PSI+] prion in yeast. Curr. Genet. 35:59-67. [DOI] [PubMed] [Google Scholar]
- 26.Didichenko, S. A., M. D. Ter-Avanesyan, and V. N. Smirnov. 1991. Ribosome-bound EF-1 alpha-like protein of yeast Saccharomyces cerevisiae. Eur. J. Biochem. 198:705-711. [DOI] [PubMed] [Google Scholar]
- 27.Doel, S. M., S. J. McCready, C. R. Nierras, and B. S. Cox. 1994. The dominant PNM2- mutation which eliminates the psi factor of Saccharomyces cerevisiae is the result of a missense mutation in the SUP35 gene. Genetics 137:659-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drugeon, G., O. Jean-Jean, L. Frolova, X. Le Goff, M. Philippe, L. Kisselev, and A. L. Haenni. 1997. Eukaryotic release factor 1 (eRF1) abolishes readthrough and competes with suppressor tRNAs at all three termination codons in messenger RNA. Nucleic Acids Res. 25:2254-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebihara, K., and Y. Nakamura. 1999. C-terminal interaction of translational release factors eRF1 and eRF3 of fission yeast: G-domain uncoupled binding and the role of conserved amino acids. RNA 5:739-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eustice, D. C., L. P. Wakem, J. M. Wilhelm, and F. Sherman. 1986. Altered 40 S ribosomal subunits in omnipotent suppressors of yeast. J. Mol. Biol. 188:207-214. [DOI] [PubMed] [Google Scholar]
- 31.Feilotter, H. E., G. J. Hannon, C. J. Ruddell, and D. Beach. 1994. Construction of an improved host strain for two hybrid screening. Nucleic Acids Res. 22:1502-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford, L. P., P. S. Bagga, and J. Wilusz. 1997. The poly(A) tail inhibits the assembly of a 3′-to-5′ exonuclease in an in vitro RNA stability system. Mol. Cell. Biol. 17:398-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fourmy, D., S. Yoshizawa, and J. D. Puglisi. 1998. Paromomycin binding induces a local conformational change in the A-site of 16 S rRNA. J. Mol. Biol. 277:333-345. [DOI] [PubMed] [Google Scholar]
- 34.Frischmeyer, P. A., and H. C. Dietz. 1999. Nonsense-mediated mRNA decay in health and disease. Hum. Mol. Genet. 8:1893-1900. [DOI] [PubMed] [Google Scholar]
- 35.Frolova, L., X. Le Goff, H. H. Rasmussen, S. Cheperegin, G. Drugeon, M. Kress, I. Arman, A. L. Haenni, J. E. Celis, M. Philippe, et al. 1994. A highly conserved eukaryotic protein family possessing properties of polypeptide chain release factor. Nature 372:701-703. [DOI] [PubMed] [Google Scholar]
- 36.Frolova, L., X. Le Goff, G. Zhouravleva, E. Davydova, M. Philippe, and L. Kisselev. 1996. Eukaryotic polypeptide chain release factor eRF3 is an eRF1- and ribosome-dependent guanosine triphosphatase. RNA 2:334-341. [PMC free article] [PubMed] [Google Scholar]
- 37.Geourjon, C., and G. Deleage. 1994. SOPM: a self-optimized method for protein secondary structure prediction. Protein Eng. 7:157-164. [DOI] [PubMed] [Google Scholar]
- 38.Gietz, R. D., R. H. Schiestl, A. R. Willems, and R. A. Woods. 1995. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11:355-360. [DOI] [PubMed] [Google Scholar]
- 39.Gray, N. K., J. M. Coller, K. S. Dickson, and M. Wickens. 2000. Multiple portions of poly(A)-binding protein stimulate translation in vivo. EMBO J. 19:4723-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenberg, J. R., L. Phan, Z. Gu, A. deSilva, C. Apolito, F. Sherman, A. G. Hinnebusch, and D. S. Goldfarb. 1998. Nip1p associates with 40 S ribosomes and the Prt1p subunit of eukaryotic initiation factor 3 and is required for efficient translation initiation. J. Biol. Chem. 273:23485-23494. [DOI] [PubMed] [Google Scholar]
- 41.Hentze, M. W. 1997. eIF4G: a multipurpose ribosome adaptor? Science 275:500-501. [DOI] [PubMed] [Google Scholar]
- 42.Hentze, M. W., and A. E. Kulozik. 1999. A perfect message: RNA surveillance and nonsense-mediated decay. Cell 96:307-310. [DOI] [PubMed] [Google Scholar]
- 43.Himmelfarb, H. J., E. Maicas, and J. D. Friesen. 1985. Isolation of the SUP45 omnipotent suppressor gene of Saccharomyces cerevisiae and characterization of its gene product. Mol. Cell. Biol. 5:816-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong, B., K. Wu, J. S. Brockenbrough, P. Wu, and J. P. Aris. 2001. Temperature sensitive nop2 alleles defective in synthesis of 25S rRNA and large ribosomal subunits in Saccharomyces cerevisiae. Nucleic Acids Res . 29:2927-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horton, L. E., P. James, E. A. Craig, and J. O. Hensold. 2001. The yeast hsp70 homologue Ssa is required for translation and interacts with Sis1 and Pab1 on translating ribosomes. J. Biol. Chem. 276:14426-14433. [DOI] [PubMed] [Google Scholar]
- 46.Hoshino, S., M. Imai, T. Kobayashi, N. Uchida, and T. Katada. 1999. The eukaryotic polypeptide chain releasing factor (eRF3/GSPT) carrying the translation termination signal to the 3′-poly(A) tail of mRNA. Direct association of erf3/GSPT with polyadenylate-binding protein. J. Biol. Chem. 274:16677-16680. [DOI] [PubMed] [Google Scholar]
- 47.Hoshino, S., H. Miyazawa, T. Enomoto, F. Hanaoka, Y. Kikuchi, A. Kikuchi, and M. Ui. 1989. A human homologue of the yeast GST1 gene codes for a GTP-binding protein and is expressed in a proliferation-dependent manner in mammalian cells. EMBO J. 8:3807-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imataka, H., A. Gradi, and N. Sonenberg. 1998. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J. 17:7480-7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inge-Vechtomov, S. G., L. N. Mironova, and M. D. Ter-Avanesian. 1994. Ambiguity of translation: a eukaryotic version? Genetika 30:1022-1035. (In Russian.) [PubMed] [Google Scholar]
- 50.Jacobson, A., and S. W. Peltz. 1996. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu. Rev. Biochem. 65:693-739. [DOI] [PubMed] [Google Scholar]
- 51.Jean-Jean, O., X. Le Goff, and M. Philippe. 1996. Is there a human [psi]? C. R. Acad. Sci. Ser. III 319:487-492. [PubMed] [Google Scholar]
- 52.Kawakami, K., and Y. Nakamura. 1990. Autogenous suppression of an opal mutation in the gene encoding peptide chain release factor 2. Proc. Natl. Acad. Sci. USA 87:8432-8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kikuchi, Y., H. Shimatake, and A. Kikuchi. 1988. A yeast gene required for the G1-to-S transition encodes a protein containing an A-kinase target site and GTPase domain. EMBO J. 7:1175-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.King, C. Y., P. Tittmann, H. Gross, R. Gebert, M. Aebi, and K. Wuthrich. 1997. Prion-inducing domain 2-114 of yeast Sup35 protein transforms in vitro into amyloid-like filaments. Proc. Natl. Acad. Sci. USA 94:6618-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kochneva-Pervukhova, N. V., A. I. Poznyakovski, V. N. Smirnov, and M. D. Ter-Avanesyan. 1998. C-terminal truncation of the Sup35 protein increases the frequency of de novo generation of a prion-based [PSI+] determinant in Saccharomyces cerevisiae. Curr. Genet. 34:146-151. [DOI] [PubMed] [Google Scholar]
- 56.Kokoska, R. J., L. Stefanovic, J. DeMai, and T. D. Petes. 2000. Increased rates of genomic deletions generated by mutations in the yeast gene encoding DNA polymerase δ or by decreases in the cellular levels of DNA polymerase δ. Mol. Cell. Biol. 20:7490-7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kozlov, G., J. F. Trempe, K. Khaleghpour, A. Kahvejian, I. Ekiel, and K. Gehring. 2001. Structure and function of the C-terminal PABC domain of human poly(A)-binding protein. Proc. Natl. Acad. Sci. USA 98:4409-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuhn, U., and T. Pieler. 1996. Xenopus poly(A) binding protein: functional domains in RNA binding and protein-protein interaction. J. Mol. Biol. 256:20-30. [DOI] [PubMed] [Google Scholar]
- 59.Kushnirov, V. V., N. V. Kochneva-Pervukhova, M. B. Chechenova, N. S. Frolova, and M. D. Ter-Avanesyan. 2000. Prion properties of the Sup35 protein of yeast Pichia methanolica. EMBO J. 19:324-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kushnirov, V. V., M. D. Ter-Avanesyan, M. V. Telckov, A. P. Surguchov, V. N. Smirnov, and S. G. Inge-Vechtomov. 1988. Nucleotide sequence of the SUP2 (SUP35) gene of Saccharomyces cerevisiae. Gene 66:45-54. [DOI] [PubMed] [Google Scholar]
- 61.Leeds, P., S. W. Peltz, A. Jacobson, and M. R. Culbertson. 1991. The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. Genes Dev. 5:2303-2314. [DOI] [PubMed] [Google Scholar]
- 62.Le Goff, X., M. Philippe, and O. Jean-Jean. 1997. Overexpression of human release factor 1 alone has an antisuppressor effect in human cells. Mol. Cell. Biol. 17:3164-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Legrain, P., and M. Rosbash. 1989. Some cis- and trans-acting mutants for splicing target pre-mRNA to the cytoplasm. Cell 57:573-583. [DOI] [PubMed] [Google Scholar]
- 64.Lingner, J., I. Radtke, E. Wahle, and W. Keller. 1991. Purification and characterization of poly(A) polymerase from Saccharomyces cerevisiae. J. Biol. Chem. 266:8741-8746. [PubMed] [Google Scholar]
- 65.Lykke-Andersen, J., M. D. Shu, and J. A. Steitz. 2000. Human Upf proteins target an mRNA for nonsense-mediated decay when bound downstream of a termination codon. Cell 103:1121-1131. [DOI] [PubMed] [Google Scholar]
- 66.Lynch, S. R., and J. D. Puglisi. 2001. Structural origins of aminoglycoside specificity for prokaryotic ribosomes. J. Mol. Biol. 306:1037-1058. [DOI] [PubMed] [Google Scholar]
- 67.Lynch, S. R., and J. D. Puglisi. 2001. Structure of a eukaryotic decoding region A-site RNA. J. Mol. Biol. 306:1023-1035. [DOI] [PubMed] [Google Scholar]
- 68.Maderazo, A. B., F. He, D. A. Mangus, and A. Jacobson. 2000. Upf1p control of nonsense mRNA translation is regulated by Nmd2p and Upf3p. Mol. Cell. Biol. 20:4591-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mangus, D. A., N. Amrani, and A. Jacobson. 1998. Pbp1p, a factor interacting with Saccharomyces cerevisiae poly(A)-binding protein, regulates polyadenylation. Mol. Cell. Biol. 18:7383-7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merkulova, T. I., L. Y. Frolova, M. Lazar, J. Camonis, and L. L. Kisselev. 1999. C-terminal domains of human translation termination factors eRF1 and eRF3 mediate their in vivo interaction. FEBS Lett. 443:41-47. [DOI] [PubMed] [Google Scholar]
- 71.Newnam, G. P., R. D. Wegrzyn, S. L. Lindquist, and Y. O. Chernoff. 1999. Antagonistic interactions between yeast chaperones Hsp104 and Hsp70 in prion curing. Mol. Cell. Biol. 19:1325-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nietfeld, W., H. Mentzel, and T. Pieler. 1990. The Xenopus laevis poly(A) binding protein is composed of multiple functionally independent RNA binding domains. EMBO J. 9:3699-3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Otero, L. J., M. P. Ashe, and A. B. Sachs. 1999. The yeast poly(A)-binding protein pab1p stimulates in vitro poly(A)-dependent and cap-dependent translation by distinct mechanisms. EMBO J. 18:3153-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palmer, E., J. M. Wilhelm, and F. Sherman. 1979. Phenotypic suppression of nonsense mutants in yeast by aminoglycoside antibiotics. Nature 277:148-150. [DOI] [PubMed] [Google Scholar]
- 75.Palmer, E., J. M. Wilhelm, and F. Sherman. 1979. Variation of phenotypic suppression due to the psi+ and psi− extrachromosomal determinants in yeast. J. Mol. Biol. 128:107-110. [DOI] [PubMed] [Google Scholar]
- 76.Parham, S. N., C. G. Resende, and M. F. Tuite. 2001. Oligopeptide repeats in the yeast protein Sup35p stabilize intermolecular prion interactions. EMBO J. 20:2111-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paushkin, S. V., V. V. Kushnirov, V. N. Smirnov, and M. D. Ter-Avanesyan. 1997. Interaction between yeast Sup45p (eRF1) and Sup35p (eRF3) polypeptide chain release factors: implications for prion-dependent regulation. Mol. Cell. Biol. 17:2798-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paushkin, S. V., V. V. Kushnirov, V. N. Smirnov, and M. D. Ter-Avanesyan. 1996. Propagation of the yeast prion-like [PSI+] determinant is mediated by oligomerization of the SUP35-encoded polypeptide chain release factor. EMBO J. 15:3127-3134. [PMC free article] [PubMed] [Google Scholar]
- 79.Perlick, H. A., S. M. Medghalchi, F. A. Spencer, R. J. Kendzior, Jr., and H. C. Dietz. 1996. Mammalian orthologues of a yeast regulator of nonsense transcript stability. Proc. Natl. Acad. Sci. USA 93:10928-10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Piron, M., P. Vende, J. Cohen, and D. Poncet. 1998. Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4F. EMBO J. 17:5811-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Recht, M. I., S. Douthwaite, K. D. Dahlquist, and J. D. Puglisi. 1999. Effect of mutations in the A site of 16 S rRNA on aminoglycoside antibiotic-ribosome interaction. J. Mol. Biol. 286:33-43. [DOI] [PubMed] [Google Scholar]
- 82.Sachs, A. B., M. W. Bond, and R. D. Kornberg. 1986. A single gene from yeast for both nuclear and cytoplasmic polyadenylate-binding proteins: domain structure and expression. Cell 45:827-835. [DOI] [PubMed] [Google Scholar]
- 83.Sanchez, Y., J. Taulien, K. A. Borkovich, and S. Lindquist. 1992. Hsp104 is required for tolerance to many forms of stress. EMBO J. 11:2357-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schirmer, E. C., and S. Lindquist. 1997. Interactions of the chaperone Hsp104 with yeast Sup35 and mammalian PrP. Proc. Natl. Acad. Sci. USA 94:13932-13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Serio, T. R., and S. L. Lindquist. 1999. [PSI+]: an epigenetic modulator of translation termination efficiency. Annu. Rev. Cell Dev. Biol. 15:661-703. [DOI] [PubMed] [Google Scholar]
- 86.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singh, A., D. Ursic, and J. Davies. 1979. Phenotypic suppression and misreading Saccharomyces cerevisiae. Nature 277:146-148. [DOI] [PubMed] [Google Scholar]
- 88.Song, H., P. Mugnier, A. K. Das, H. M. Webb, D. R. Evans, M. F. Tuite, B. A. Hemmings, and D. Barford. 2000. The crystal structure of human eukaryotic release factor eRF1—mechanism of stop codon recognition and peptidyl-tRNA hydrolysis. Cell 100:311-321. [DOI] [PubMed] [Google Scholar]
- 89.Stansfield, I., Akhmaloka, and M. F. Tuite. 1995. A mutant allele of the SUP45 (SAL4) gene of Saccharomyces cerevisiae shows temperature-dependent allosuppressor and omnipotent suppressor phenotypes. Curr. Genet. 27:417-426. [DOI] [PubMed] [Google Scholar]
- 90.Stansfield, I., K. M. Jones, V. V. Kushnirov, A. R. Dagkesamanskaya, A. I. Poznyakovski, S. V. Paushkin, C. R. Nierras, B. S. Cox, M. D. Ter-Avanesyan, and M. F. Tuite. 1995. The products of the SUP45 (eRF1) and SUP35 genes interact to mediate translation termination in Saccharomyces cerevisiae. EMBO J. 14:4365-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tarun, S. Z., Jr., and A. B. Sachs. 1996. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 15:7168-7177. [PMC free article] [PubMed] [Google Scholar]
- 92.Tarun, S. Z., Jr., and A. B. Sachs. 1995. A common function for mRNA 5′ and 3′ ends in translation initiation in yeast. Genes Dev. 9:2997-3007. [DOI] [PubMed] [Google Scholar]
- 93.Tassan, J. P., K. Le Guellec, M. Kress, M. Faure, J. Camonis, M. Jacquet, and M. Philippe. 1993. In Xenopus laevis, the product of a developmentally regulated mRNA is structurally and functionally homologous to a Saccharomyces cerevisiae protein involved in translation fidelity. Mol. Cell. Biol. 13:2815-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Telckov, M. V., A. R. Dagkesamanskaya, V. V. Kushnirov, and V. N. Smirnov. 1986. Isolation of a chromosomal DNA fragment containing SUP2 gene of the yeast Saccharomyces cerevisiae. Genetika 22:17-25. (In Russian.) [Google Scholar]
- 95.Telkov, M. V., and A. P. Surguchev. 1986. Characteristics of transcripts of sup1 and sup2 genes in Saccharomyces yeasts. Dokl. Akad. Nauk SSSR 290:988-990. (In Russian.) [PubMed] [Google Scholar]
- 96.Ter-Avanesyan, M. D., A. R. Dagkesamanskaya, V. V. Kushnirov, and V. N. Smirnov. 1994. The SUP35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [PSI+] in the yeast Saccharomyces cerevisiae. Genetics 137:671-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ter-Avanesyan, M. D., V. V. Kushnirov, A. R. Dagkesamanskaya, S. A. Didichenko, Y. O. Chernoff, S. G. Inge-Vechtomov, and V. N. Smirnov. 1993. Deletion analysis of the SUP35 gene of the yeast Saccharomyces cerevisiae reveals two non-overlapping functional regions in the encoded protein. Mol. Microbiol. 7:683-692.Y [DOI] [PubMed] [Google Scholar]
- 98.True, H. L., and S. L. Lindquist. 2000. A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature 407:477-483. [DOI] [PubMed] [Google Scholar]
- 99.Wang, W., K. Czaplinski, Y. Rao, and S. W. Peltz. 2001. The role of Upf proteins in modulating the translation read-through of nonsense-containing transcripts. EMBO J. 20:880-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang, Z., N. Day, P. Trifillis, and M. Kiledjian. 1999. An mRNA stability complex functions with poly(A)-binding protein to stabilize mRNA in vitro. Mol. Cell. Biol. 19:4552-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wells, S. E., P. E. Hillner, R. D. Vale, and A. B. Sachs. 1998. Circularization of mRNA by eukaryotic translation initiation factors. Mol. Cell 2:135-140. [DOI] [PubMed] [Google Scholar]
- 102.Weng, Y., K. Czaplinski, and S. W. Peltz. 1996. Genetic and biochemical characterization of mutations in the ATPase and helicase regions of the Upf1 protein. Mol. Cell. Biol. 16:5477-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weng, Y., K. Czaplinski, and S. W. Peltz. 1996. Identification and characterization of mutations in the UPF1 gene that affect nonsense suppression and the formation of the Upf protein complex but not mRNA turnover. Mol. Cell. Biol. 16:5491-5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wickner, R. B. 1994. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science 264:566-569. [DOI] [PubMed] [Google Scholar]
- 105.Wilson, P. G., and M. R. Culbertson. 1988. SUF12 suppressor protein of yeast. A fusion protein related to the EF-1 family of elongation factors. J. Mol. Biol. 199:559-573. [DOI] [PubMed] [Google Scholar]
- 106.Woods, A., T. Sherwin, R. Sasse, T. H. MacRae, A. J. Baines, and K. Gull. 1989. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci. 93:491-500. [DOI] [PubMed] [Google Scholar]
- 107.Xie, K., E. J. Lambie, and M. Snyder. 1993. Nuclear dot antigens may specify transcriptional domains in the nucleus. Mol. Cell. Biol. 13:6170-6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou, P., I. L. Derkatch, S. M. Uptain, M. M. Patino, S. Lindquist, and S. W. Liebman. 1999. The yeast non-Mendelian factor [ETA+] is a variant of [PSI+], a prion-like form of release factor eRF3. EMBO J. 18:1182-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhouravleva, G., L. Frolova, X. Le Goff, R. Le Guellec, S. Inge-Vechtomov, L. Kisselev, and M. Philippe. 1995. Termination of translation in eukaryotes is governed by two interacting polypeptide chain release factors, eRF1 and eRF3. EMBO J. 14:4065-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]