Abstract
Oncogenic activation of the mitogen-activated protein (MAP) kinase cascade in murine fibroblasts initiates a senescence-like cell cycle arrest that depends on the ARF/p53 tumor suppressor pathway. To investigate whether p53 is sufficient to induce senescence, we introduced a conditional murine p53 allele (p53val135) into p53-null mouse embryonic fibroblasts and examined cell proliferation and senescence in cells expressing p53, oncogenic Ras, or both gene products. Conditional p53 activation efficiently induced a reversible cell cycle arrest but was unable to induce features of senescence. In contrast, coexpression of oncogenic ras or activated mek1 with p53 enhanced both p53 levels and activity relative to that observed for p53 alone and produced an irreversible cell cycle arrest that displayed features of cellular senescence. p19ARF was required for this effect, since p53−/− ARF−/− double-null cells were unable to undergo senescence following coexpression of oncogenic Ras and p53. Although the levels of exogenous p53 achieved in ARF-null cells were relatively low, the stabilizing effects of p19ARF on p53 could not explain the cooperation between oncogenic Ras and p53 in promoting senescence. Hence, enforced p53 expression without oncogenic ras in p53−/− mdm2−/− double-null cells produced extremely high p53 levels but did not induce senescence. Taken together, our results indicate that oncogenic activation of the MAP kinase pathway in murine fibroblasts converts p53 into a senescence inducer through both quantitative and qualitative mechanisms.
The tumor suppressor p53 coordinates the cellular response to different kinds of stress (11). The stimuli that regulate p53 activity include DNA damage (34, 39), altered ribonucleotide pools (30), hypoxia (14, 15), oncogenes (4, 19, 31, 49, 56, 61), cell adhesion (40), mitotic spindle defects (3), and redox stress (17, 45). These stimuli are believed to activate p53 by increasing its stability and/or inducing posttranslational modifications that enhance the ability of p53 to act as a transcription factor (12, 21, 24). The outcome of p53 activity can be reversible cell cycle arrest (1, 30) or permanent withdrawal from cell proliferation following the induction of apoptosis or cellular senescence (31, 49, 62).
In principle, the various outcomes of p53 activation might be influenced by quantitative or qualitative mechanisms (for a recent review, see reference 52). Some studies suggest that the level of p53 output determines the decision between cell cycle arrest and apoptosis. Consistent with this view, only a subset of the genes induced by high p53 levels are induced by lower p53 levels (65) and introduction of high p53 levels into tumor cell lines induces apoptosis while lower levels only induce a cell cycle arrest (2). However, other studies suggest that the outcome of p53 activation is determined by factors controlled by the tissue origin or cell genotype. For example, whereas fibroblasts typically undergo a p53-dependent cell cycle arrest in response to low doses of gamma radiation, thymocytes undergo p53-dependent apoptosis in response to the same doses (6, 23, 31). Also, in fibroblasts, introduction of E1A promotes p53-dependent apoptosis whereas oncogenic ras promotes a p53-dependent cell cycle arrest (4, 31, 49). In addition, cytokines and growth factors can modify the p53 response, usually favoring a cell cycle arrest over apoptosis (29, 62). Although these diverse factors might also produce quantitative differences in p53 activity, it seems likely that some of their effects modify the quality of the p53 signal. For instance, phosphorylation of p53 at serine 46 in response to severe DNA damage is associated with induction of p53AIP- and p53-dependent apoptosis (41) and phosphorylation at serine 15 correlates with the induction of senescence by oncogenic ras (8). Serine 15 phosphorylation increases the ability of p53 to interact with CBP (25), a protein that colocalizes with p53 in PML-containing nuclear bodies (also called PODs) during the induction of cellular senescence by oncogenic ras (8, 43). Together, these studies suggest that PML and CBP can modulate p53 signaling to favor a permanent cell cycle arrest. Accordingly, ectopic expression of the CBP homologue p300 augments the ability of p53 to induce a cell cycle arrest and prevents its ability to promote apoptosis (26). Other residues of p53 are modified by a variety of phosphorylation, acetylation, and sumoylation reactions (11, 13, 47). This suggests that a particular array of posttranslational modifications in p53 could be associated with different outcomes (41).
Oncogenic activation of the mitogen-activated protein (MAP) kinase pathway in murine fibroblasts initiates a permanent cell cycle arrest that depends on functional p53 and is phenotypically similar to replicative senescence (27, 49). However, whereas replicative senescence of human cells is initiated by telomere malfunction, premature senescence induced by oncogenic ras is provoked by excessive mitogenic signaling (50). Nevertheless, both stimuli produce the same endpoint that, presumably, must be overcome during carcinogenesis. The signaling from aberrant MAP kinase activity to p53 is not fully understood, and the available data support a simple linear model from oncogenic Ras to p53 via induction of p19ARF (28, 42). p19ARF links oncogene activation to the p53 tumor suppressor pathway by inhibiting the Mdm2-dependent degradation of p53 (44, 55, 58, 64). However, activation of the ARF/p53 pathway results in apoptosis or senescence, depending on the type of oncogenic stress (51), implying that additional signals can modulate the outcome of p53 activation. To address these issues, we took advantage of the mouse temperature-sensitive p53 allele (p53val135) (35) that allows conditional and reversible activation of p53. We report that enforced p53 expression in p53−/− knockout mouse embryonic fibroblasts (MEFs) is not sufficient to induce a permanent cell cycle arrest. We also found that oncogenic activation of the MAP kinase pathway by activated alleles of ras or mek1 changes the outcome of p53 activity, promoting a permanent cell cycle arrest with the characteristics of cellular senescence.
MATERIALS AND METHODS
Cell culture.
Primary MEFs from wild-type mice, ARF−/− mice, p53−/− mice, p53−/− ARF−/− double-knockout mice, and p53−/− mdm2−/− double-knockout mice were derived from day 13.5 embryos as described previously (49). Cells expressing murine p53val135 (35) were generated by retrovirus-mediated gene transfer of p53val135 into p53−/− MEFs (TSP cells), p53−/− ARF−/− MEFs (TSPA cells), and p53−/− mdm2−/− MEFs (TSPM cells) (Table 1). Cells were cultured in Dulbecco's modified Eagle medium (GIBCO) supplemented with 10% fetal bovine serum (HyClone, Logan, Utah), 1% penicillin G-streptomycin sulfate (Sigma), and hygromycin (Roche) as indicated in Table 1. For experiments with PD98059 (Calbiochem), cells were treated daily with 50 μM PD98059 diluted in 0.25% dimethyl sulfoxide (DMSO).
TABLE 1.
Summary of the p53val135-expressing cell lines used in this study
| Cell line | Genotype | Phenotype |
|---|---|---|
| TSP | p53−/− MEFs with p53val135 | Growth at 39°C and arrest at 32°C, maintained at 75 μg of hygromycin per ml |
| TSPA | p53−/−ARF−/− MEFs with p53val135 | Growth at 39°C and arrest at 32°C, expression of low levels of p53val135 (Fig. 9A), maintained at 150 μg of hygromycin per ml |
| TSPM | p53−/−mdm2−/− MEFs with p53val135 | Growth at 39°C and arrest at 32°C, expression of high levels of p53val135 (Fig. 9A), maintained at 150 μg of hygromycin per ml |
Retroviral vectors and gene transfer.
The following retroviral vectors were used: p53val135 (35) in pMARXHygro (18), the pBabe vector (38) and its derivatives with oncogenic ras (H-RasV12) (49); mek1Q56P (27) or wild-type p53, the MSCVGFP vector (Clontech) and its derivative with oncogenic ras. Retrovirus-mediated gene transfer was performed as previously described (49). Infected cell populations were selected in puromycin (2.5 μg/ml, 3 days) for pBabe-derived vectors or in hygromycin (Table 1) for MARX-Hygro-based vectors.
Temperature shifts and cell proliferation analysis.
For the temperature shift experiment, 5 × 105 cells were plated in 10-cm-diameter plates. Cells were grown at 39°C (i.e., never incubated at 32°C) or arrested for 1, 2, 4, or 8 days at 32°C; they were then trypsinized, counted with a hemacytometer, and plated for the following growth assays. For the [3H]thymidine incorporation assay 2 × 104 cells were plated in triplicate on 12-well plates and incubated overnight to permit their recovery from plating. The cells were then pulsed for another 24 h with 5 mCi of [methyl-3H]thymidine per ml (2 Ci/mmol; Amersham), washed with phosphate-buffered saline (PBS), trypsinized, treated with ice-cold 10% trichloroacetic acid, and transferred to glass fiber filters (Filtermat; Wallac). The amount of radioactivity incorporated was measured by scintillation counting. To measure bromodeoxyuridine (BrdU) incorporation in situ, subconfluent cultures (5 × 105 cells in 10-cm-diameter plates) were incubated for 3 h in the presence of 10 μM BrdU and fixed and nuclei incorporating BrdU were visualized by immunostaining with a cell proliferation kit (Amersham Pharmacia Biotech). For colony-forming ability assays, cells were plated at 500 or 1,000 cells per 6-cm-diameter plate. After 10 days, colonies were stained with crystal violet and counted.
SA β-Gal activity.
Senescence-associated (SA) β-galactosidase (β-Gal) activity was measured as previously described (49), except that cells were incubated in 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) at pH 5.5 to increase the sensitivity of the assay in MEFs. The percentage of cells expressing SA β-Gal was quantified by inspecting 200 cells per 10-cm-diameter plate three times.
Protein analysis.
Immunoblots were performed with whole-cell lysates obtained by boiling cell pellets solubilized in Laemmli sample buffer as previously described (5). Samples of 20 μg of protein were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon-P membranes (Millipore). The antibodies used were anti-mouse p53 (CM5; 1:2,500; Novocastra), anti PS-15p53 (catalog no. 9284; 1:1000; Cell Signaling Technology), anti-p21, (C-19; 1:500; Santa Cruz); anti-Ras (Op23; 1:500; Oncogene); anti-Mek (αMEK1; 1:500; Calbiochem); anti-p19ARF (1:500; Novus); anti-tubulin (B-5-1-2; 1:2,000; Sigma), and anti-Mdm2 (2A10; 1:250; kindly provided by A. Levine). Western blot assays were performed in accordance with standard procedures by using ECL detection (Amersham) or SuperSignal West Femtomaximum (Pierce).
Northern blot.
Total RNA was extracted by using RNAzolB (Cinna/Biotecx). Samples (30 μg) of total RNA were loaded in formaldehyde-agarose gels and transferred to Hybond membranes (Amersham). Blots were hybridized with 32P-labeled probes specific for exon 1β of the mouse INK4a/ARF locus or the p53 locus. A probe specific for 18S rRNA was used to confirm that the same amount of RNA was present in each lane.
Fluorescence microscopy.
Cells were plated on coverslips and fixed by using 4% paraformaldehyde in PBS for 15 min at room temperature. After washing with PBS, cells were permeabilized for 5 min on ice with 0.2% Triton X-100 in PBS with 3% bovine serum albumin (PBS/BSA). The cells were then washed with PBS/BSA and incubated for 1 h with antibodies against mouse PML (1:200; kindly provided by T. Ley, Washington University) and mouse p53 (Pab246; 1:50; Santa Cruz). After being washed in PBS/BSA, cells were stained with fluorescein isothiocyanate (FITC)- or Texas Red-conjugated secondary antibodies, (1:200) for 45 min at room temperature in a humidified chamber. Finally, cells were washed in PBS, stained with 4,6-diamidino-2-phenylindole (DAPI) at a concentration of 0.1 μg/ml in PBS, and mounted on microscope slides. For fluorescence detection, we used an Axioskop 50 immunofluorescence microscope (Zeiss, Thornwood, N.Y.). For confocal immunofluorescence, we used a Zeiss LSM510 confocal laser scanning microscope with simultaneous scans. Data were collected with eightfold averaging at a resolution of 512 by 512 pixels by using the LSM510 software. Images were prepared with Adobe Photoshop.
RESULTS
Constitutive MAP kinase signaling induces a senescence-like arrest in primary MEFs.
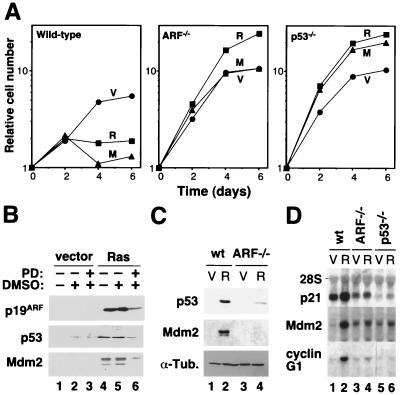
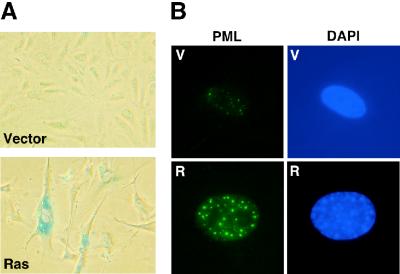
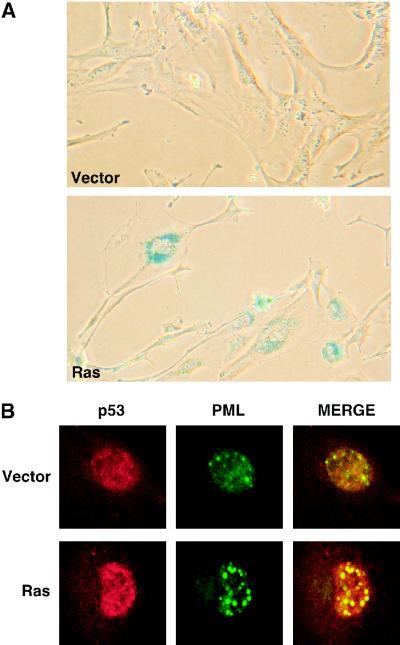
Previous studies have shown that ARF is required for a ras-induced cell cycle arrest in MEFs and that oncogenic Ras activates p53 through the MAP kinase cascade (27, 42). To confirm and extend these results, we compared the abilities of oncogenic ras and activated Mek (mekQ56P) to induce a cell cycle arrest in wild-type, ARF−/−, and p53−/− MEFs. As expected oncogenic ras and mek1 failed to induce a cell cycle arrest in ARF−/− and p53−/− MEFs (Fig. 1A), suggesting that constitutive signaling through the MAP kinase cascade engages the ARF/p53 tumor suppressor pathway. Accordingly, the ability of oncogenic ras to induce p19ARF was severely impaired in the presence of PD98059, a specific Mek inhibitor (Fig. 1B). Consistent with a previous report, we observed that ARF−/− cells failed to induce p53 protein to the extent observed in wild-type cells; however, we consistently observed a modest induction of p53 in ARF-null cells (Fig. 1C). Nevertheless, ARF−/− MEFs displayed a defect in the induction of the p53 transcriptional targets in response to oncogenic ras (Fig. 1D). Note that the modest induction of Mdm2 mRNA in p53−/− MEFs is presumably due to direct Ras signaling to the Mdm2 promoter (46). As has been previously described for senescent cells (49), the cell cycle arrest induced by oncogenic ras was characterized by a typical flat-cell morphology (Fig. 2A), an increase in SA β-Gal activity (7) (Fig. 2A), and an increase in the size and number of PML bodies as measured by immunofluorescence assay with antibodies directed against PML (8, 43) (Fig. 2B). These markers, together with the biochemical evidence of an activated ARF/p53 pathway, distinguished the senescent cell cycle arrest from both proliferating cells or transiently arrested cells (e.g., serum-starved cells).
FIG. 1.
Constitutive activation of the MAP kinase cascade engages the ARF/p53 pathway to promote senescence of primary MEFs. (A) Growth curves of MEFs obtained from wild-type, ARF−/−, and p53−/− knockout mice transduced with an empty retroviral vector (V) or its derivatives expressing oncogenic ras (R) or oncogenic mek1 (M). Each experiment was performed three times with similar results. The data represent the mean (± the standard deviation) of triplicate time points of a representative experiment. (B) p19ARF, p53, and Mdm2 immunoblots of wild-type MEFs expressing an empty vector or oncogenic ras and treated with the MEK inhibitor PD98059 (PD) diluted in DMSO. Untreated cells and cells treated with DMSO alone were used as controls. (C) p53 and Mdm2 immunoblots of cellular lysates from wild-type (wt) and ARF-null (ARF−/−) MEFs containing the empty retroviral vector pBabe (V) or its H-RasV12-expressing derivative (R). (D) Northern blot of total RNA purified from wild-type (wt), ARF-null (ARF−/−) and p53-null (p53−/−) MEFs expressing either the empty retroviral vector pBabe (V) or its derivative expressing oncogenic ras (R). α-Tub., anti-tuberculin antibody.
FIG. 2.
Senescence markers. (A) Wild-type MEFs transduced with pBabe (V) or its derivative expressing oncogenic ras (R) were fixed 8 days after infection and stained for SA β-Gal. Fifty percent of the cells expressing oncogenic Ras were SA β-Gal positive versus 5% of control cells. (B) Cells prepared as described above were stained with an anti-PML antibody and an FITC-conjugated secondary antibody. Images were obtained by immunofluorescence microscopy. Expression of oncogenic ras increased the number of PML bodies from 7 ± 3 in control cells to 32 ± 5.
Ectopic p53 expression induces a reversible cell cycle arrest in MEFs.
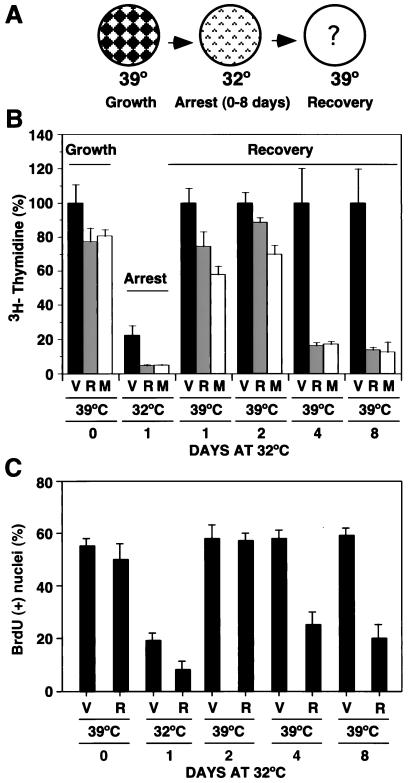
The results presented above, as well as previously published data (28, 42), support a simple linear signaling pathway linking the MAP kinase cascade to p19ARF and p53 during premature senescence induced by oncogenic ras. Although an increase in p53 activity is essential for the establishment of a senescence-like cell cycle arrest, it is not clear whether it is sufficient. To investigate this possibility, we used a retroviral vector that expresses temperature-sensitive p53 (p53val135) (35) to conditionally express p53 in p53−/− MEFs and then examined proliferation at the restrictive (39°C) and permissive (32°C) temperatures for p53 activity. We established a cell line from the infected population and called it the TSP cell line (Table 1). TSP cells, as well as the other p53val135-expressing cell lines described below, were established and maintained at the restrictive temperature to avoid selection against p53 or components of the p53 pathway. As indicated by both a [3H]thymidine incorporation assay and quantification of nuclei incorporating BrdU, TSP cells grew at 39°C and underwent a cell cycle arrest when shifted to 32°C (Fig. 3B and C). In contrast, the parental p53−/− MEFs (lacking p53val135) did not arrest at 32°C, indicating that the cell cycle arrest we observed was not a temperature effect (data not shown). To characterize the cell cycle profile of TSP cells at either 39°C (growing cells) or 32°C (arrested cells), we pulsed the cells with BrdU for 3 h and stained them with FITC-conjugated anti-BrdU antibody and propidium iodide. Analysis of stained cells by flow cytometry revealed that incubation of TSP cells at 32°C for 4 days reduced the percentage of cells in S phase from 37% to less than 0.5%. Most arrested cells were in G1 (77%), while 20% of the cell population was arrested with a G2 DNA content. Importantly, although TSP cells were stably arrested at 32°C, this arrest was readily reversible. Therefore, incubation of these cells for up to 12 days at 32°C did not impair their ability to resume proliferation when they were shifted back to 39°C (data not shown and Fig. 3). These results indicate that, in normal fibroblasts, the outcome of p53 activation is a reversible cell cycle arrest.
FIG. 3.
Oncogenic activation of the MAP kinase pathway modifies the outcome of a p53-dependent cell cycle arrest. (A) Temperature shift assay evaluating the effect of the genetic background on a p53-mediated cell cycle arrest. MEFs expressing p53val135 can be grown at 39°C and arrested at 32°C. The arrested populations are then shifted to 39°C to inactivate p53val135 and investigate their recovery from a cell cycle arrest. (B) [3H]thymidine incorporation assay of TSP-vector (V), TSP-Ras (R), and TSP-Mek cells (M). All values are compared to the value scored by TSP-vector cells (V) growing at 39°C, which is taken as 100%. Shown is the amount of incorporation of cells growing at 39°C (day 0) or arrested at 32°C for 1 day and then the incorporation of the cells shifted to 39°C after being arrested at 32°C for 1, 2, 4, and 8 days (recovery). (C) In situ BrdU incorporation of TSP cells containing the empty vector (V) or a derivative with oncogenic ras (R). The cells were pulsed with 10 μM BrdU for 3 h 24 h after plating. Results are presented as the percentage of BrdU-positive nuclei.
Oncogenic ras or mek1 impairs the ability of TSP cells to recover from a p53-mediated cell cycle arrest.
The reversible cell cycle arrest of TSP cells expressing p53val135 contrasts with the apparently permanent cell cycle arrest of wild-type MEFs in response to oncogenic ras or mek1 (27, 49). We reasoned that oncogenic MAP kinase signaling might change the outcome of p53 activity. To examine this possibility, we made three derivatives of TSP cells by retrovirus-mediated gene transfer of a control vector, oncogenic ras, or mekQ56P into whole populations of TSP cells and again examined proliferation at the restrictive and permissive temperatures. All infected cell populations were initially grown at 39°C, shifted to 32°C for various times, and then returned to 39°C (schematic diagram in Fig. 3A). Cell proliferation was monitored at both 32°C (arrest) and after a return to 39°C (recovery from arrest) by using the [3H]thymidine and BrdU incorporation assays.
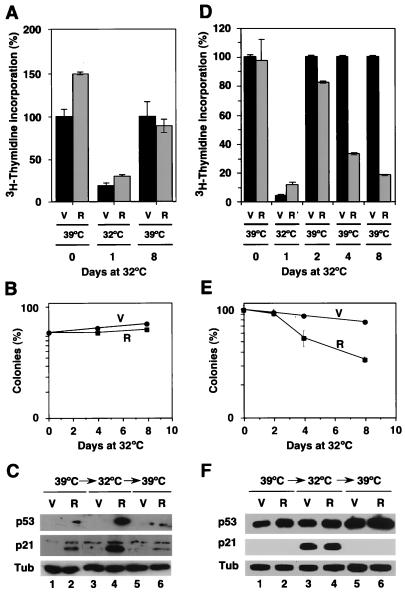
The rates of [3H]thymidine incorporation of cells growing at 39°C and never incubated at 32°C (day 0) were similar (Fig. 3B). Incubation of these cells at 32°C led to a cell cycle arrest and, as a result, a reduction of more than 80% in their rate of [3H]thymidine incorporation (Fig. 3B, black columns). This cell cycle arrest at 32°C was more efficient in the presence of either oncogenic ras or mek1 (Fig. 3B, gray and white columns, respectively). Incubation at 32°C for 1 or 2 days did not impair the ability of any of the cell lines to incorporate [3H]thymidine when they were shifted back to 39°C. However, incubation of TSP-Ras or TSP-Mek cells for 4 or 8 days at 32°C dramatically decreased their ability to incorporate [3H]thymidine after a return to 39°C compared to the TSP-vector control. It is of note that apoptosis was not observed in TSP-Ras or TSP-Mek cell populations, implying that this failure to incorporate BrdU was due to a prolonged cell cycle arrest and not cell death. Consistent with this notion, similar results were obtained with the BrdU incorporation assay, which identifies the ability of individual cells to proliferate after immunochemical detection of incorporated BrdU (Fig. 3C). Again, the presence of oncogenic ras for more than 2 days at 32°C dramatically reduced the percentage of cells capable of reentering the cell cycle upon a return to 39°C.
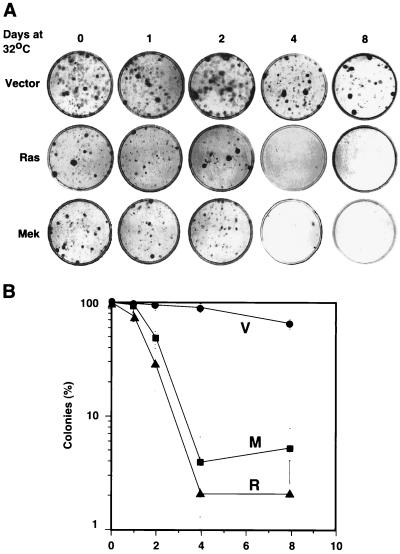
To assess the long-term growth potential of TSP cells upon a shift from 32 to 39°C, we used a clonogenic assay. This assay measures the proliferation capacity of a cell based on its ability to form colonies after plating at low density. Cells were treated in accordance with the scheme in Fig. 3, and then 500 cells were plated in 6-cm-diameter plates and allowed to grow for 10 days. The plating efficiency of TSP cells at 39°C (i.e., never incubated at 32°C) was ∼20% and was not altered by expression of ras or mekQ56P. In contrast to TSP-vector cells, TSP-Ras and TSP-Mek cells lost the ability to form colonies when incubated for more than 2 days at 32°C (Fig. 4A and B). Cells plated at clonogenic density at 32°C never formed colonies, indicating that a cell cycle arrest is incompatible with colony formation (data not shown). Importantly, the differences in clonogenic potential between these cell populations were not due to a differential ability to adhere to plates at low density since we counted similar numbers of attached cells 24 h after plating (data not shown). Identical results were obtained with an independently derived line expressing p53val135 (TSP21; data not shown). We concluded that oncogenic ras and mek1 transmit signals that impair recovery from a p53-mediated cell cycle arrest. This loss of proliferation potential was not an immediate consequence of ras signaling since it was only observed after more than 2 days of incubation at 32°C. A similar delay for senescence induction was observed in primary cells expressing oncogenic Ras (Fig. 1A and references 8 and 27). Together, these data suggest that p53 is required to initiate, but not maintain, a ras-induced cell cycle arrest and demonstrate that constitutive signaling through the MAP kinase cascade can redirect the outcome of p53 activation from a reversible to an irreversible cell cycle arrest.
FIG. 4.
Colony formation assay. (A) Cells growing at 39°C (day 0) or arrested at 32°C for 1, 2, 4, or 8 days were plated at a density of 500 cells per 6-cm-diameter plate and grown at 39°C for 10 days. Colonies were visualized by staining with crystal violet. (B) Same data as above summarizing three independent measures and the standard deviation. The data were normalized to the number of colonies obtained with cells growing at 39°C (day 0), which was taken as 100%.
Oncogenic ras or mek1 increases exogenous p53 activity.
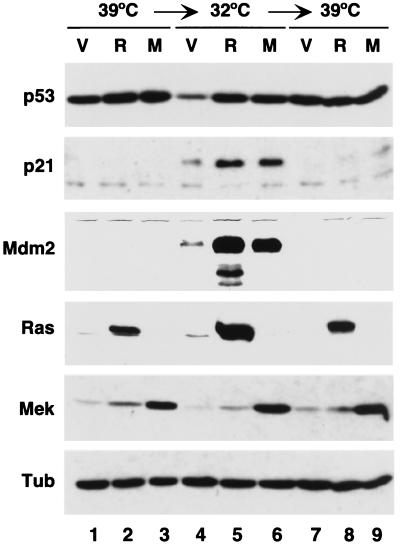
To assess the status of the p53 pathway in TSP cells incubated at different temperatures, we measured the protein levels of p53 and the endogenous p53 target genes p21 and Mdm2 (Fig. 5). Cells were incubated at 39°C, arrested for 4 days at 32°C, and returned for 4 days to 39°C. Under these conditions, TSP-vector cells resumed proliferation whereas the majority of TSP-Ras and TSP-Mek cells remained arrested. The levels of p53 at 39°C in TSP-vector cells were higher than at 32°C, the latter corresponding to cells with p53 in the active conformation (Fig. 5, compare lanes 1 and 4). This is because inactive p53 at 39°C accumulated at high levels in the cytoplasm (data not shown), where it is not recognized by the p53 degradation machinery (10). At 32°C, oncogenic ras and activated mek1 had a stabilizing effect on p53 compared to control (TSP-vector cells) at the same temperature (Fig. 5, compare lane 4 to lanes 5 and 6). The levels of the p53 downstream targets p21 and Mdm2 dramatically increased in TSP-vector cells upon a transfer from 39 to 32°C, and this effect was augmented in cells expressing oncogenic ras or mek1. Importantly, both p21 and Mdm2 levels returned to their basal levels when the cells were shifted back to 39°C, confirming that p53val135 was indeed a temperature-sensitive p53 allele (Fig. 5, compare lanes 4 to 6 to lanes 7 to 9, respectively). These results indicate that the loss of proliferation potential displayed by TSP-Ras and TSP-Mek cells following a p53-mediated arrest does not depend on sustained p53 activity.
FIG. 5.
Oncogenic Ras or Mek1 potentiates the activity of p53val135. Immunoblots of cellular lysates (20 μg of total protein) from TSP-vector (V), TSP-Ras (R), and TSP-Mek (M) cells. Cells were collected after incubation first for 4 days at 32°C and then for 4 days at 39°C.
Oncogenic ras cooperates with p53 in promoting cellular senescence.
Wild-type MEFs expressing oncogenic ras display features of cellular senescence (49) (Fig. 2). To determine whether TSP cells arrested by p53 and oncogenic ras also display senescence markers, we assessed cell morphology, SA β-gal activity, and the accumulation of PML bodies in the different TSP cell populations under the conditions outlined in Fig. 3A. TSP-vector cells incubated at 32°C for 4 or more days did not display any senescence markers (Fig. 6), even though they were clearly arrested and had activated p53 (Fig. 3 and 4). Similarly, TSP-Ras cells maintained in the absence of functional p53 at 39°C did not display senescence markers and, in fact, were morphologically transformed (data not shown). However, TSP-Ras cells incubated at 32°C for more than 4 days displayed an enlarged and flat morphology, a marked increase in SA β-gal activity (50% of the cells versus 5% of control cells with the vector), and an increase in the size and number of PML-containing nuclear bodies from around 5 ± 3 to 28 ± 7 per cell (Fig. 6). This phenotype was indistinguishable from the senescence-like arrest observed in wild-type cells expressing oncogenic ras (Fig. 3). Indeed, as was previously observed in normal fibroblasts arrested in response to oncogenic ras (8, 43), a substantial portion of p53 colocalized with PML in TSP-Ras cells incubated at 32°C.
FIG. 6.
Oncogenic Ras and p53 cooperate to promote senescence. (A) SA β-Gal staining. Cells were grown at 39°C, arrested for 8 days at 32°C, and then fixed for staining. (B) PML bodies. Cells were grown at 39°C and arrested for 4 days at 32°C. They were then fixed on coverslips and incubated with anti-PML and anti-p53 primary antibodies. After staining with FITC- or Texas Red-conjugated secondary antibodies, the cells were imaged by confocal immunofluorescence microscopy.
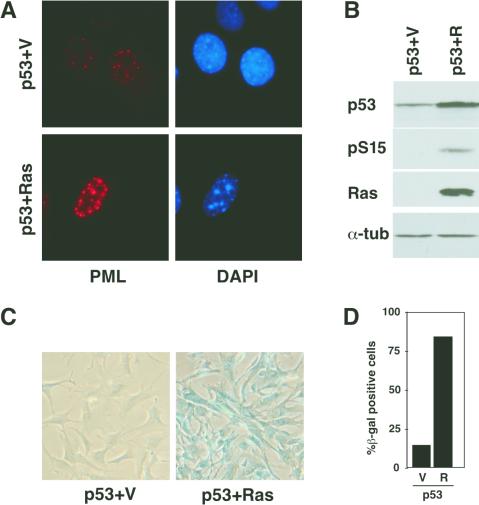
This cooperation between oncogenic Ras and p53 in senescence is not specific for the p53val135 allele or the TSP clone we used. Retrovirus-mediated gene transfer of wild-type p53 alone into p53−/− MEFs failed to induce senescence markers, whereas cotransfer of p53 and ras induced a senescence-like state phenotypically similar to that observed in TSP-Ras cells (Fig. 7). Interestingly, the p53 coexpressed with oncogenic ras was phosphorylated on serine 15. Taken together, these results indicate that oncogenic ras cooperates with p53 to promote cellular senescence.
FIG. 7.
Oncogenic ras cooperates with wild-type (wt) p53 to induce senescence. p53−/− MEFs were coinfected with retroviruses expressing wild-type human p53 (pBabep53) and either a vector control, MSCVGFP (p53 + V), or its derivative expressing oncogenic ras (p53 + R) and analyzed 6 days postinfection. (A) Representative immunofluorescence staining with an anti-PML antibody. Samples were photographed at identical exposures. (B) Western blot analysis showing expression of the indicated proteins. pS15 indicates an anti-human phospho-serine-15 p53 antibody. (C) SA β-Gal staining of representative fields. (D) Quantitation of SA β-Gal activity. See Material and Methods for experimental details. α-tub, anti-tubulin antibody.
p19ARF is required for Ras to cooperate with p53 in promoting senescence. In primary MEFs, p19ARF is required for p53 activation and senescence in response to oncogenic ras (42; also Fig. 1). To determine whether enforced p53 expression, together with oncogenic ras, can eliminate the requirement for ARF in promoting senescence, we generated p53val135-expressing cell lines in MEFs derived from p53−/− ARF−/− double-knockout mice (TSPA cells) and examined their proliferation phenotypes in the presence of a control vector (TSPA-vector) or oncogenic ras (TSPA-Ras). Both cell populations grew at 39°C and arrested at 32°C, as shown with [3H]thymidine incorporation, BrdU incorporation (data not shown), and clonogenic assays (Fig. 8A and B). However, unlike p53−/− MEFs expressing p53val135 (i.e., TSP cells), TSPA cells efficiently recovered from the p53-mediated arrest, even in the presence of oncogenic ras (Fig. 7A and B). Similar results were obtained with three independent clones of TSPA cells (data not shown).
FIG. 8.
Coexpression of p53val135 and oncogenic Ras in p53−/− ARF−/− and p53−/− mdm2−/− MEFs. See Fig. 1 for experimental details. (A and D) Assay of [3H]thymidine incorporation by TSPA (A) and TSPM (D) cells containing the empty pBabe vector (V) or its derivative expressing Ras-V12 (R). (B and E) Summary of the number of colonies formed by TSPA (B) and TSPM (E) cells containing the empty pBabe vector (V) or its derivative expressing Ras-V12 (R) after their arrest at 32°C for the numbers of days indicated. (C and F) Immunoblots for p53 and p21 of TSPA (C) and TSPM (F) cells containing either the empty vector (V) or Ras-expressing retroviruses (R).
The levels of p53val135 achieved in TSPA cells were substantially lower than those expressed in TSP cells (see Fig. 10), presumably reflecting the ability of p19ARF to interfere with Mdm2-directed, ubiquitin-mediated proteolysis of p53 (51, 60). Consistent with this explanation, p53 protein levels in TSPA cells increased upon incubation with the proteasome inhibitor MG132 (data not shown). Surprisingly, oncogenic ras enhanced exogenous p53 activity even in the absence p19ARF, since TSPA-Ras cells expressed higher levels of p21 than did TSPA-vector cells following an arrest at 32°C (Fig. 8C, compare lanes 4 and 3), implying that Ras can signal to p53 independently of p19ARF.
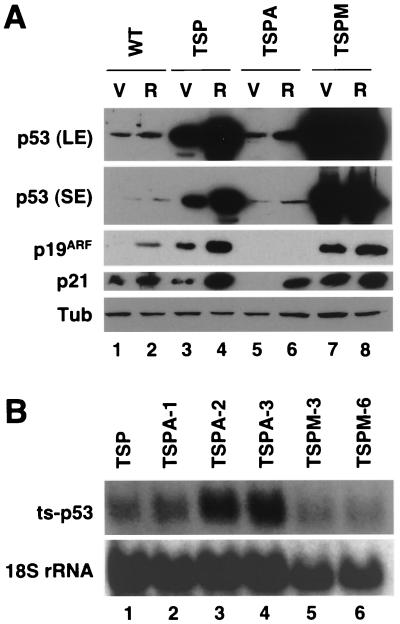
FIG. 10.
p53 levels do not correlate with the senescence phenotype. (A) p53 immunoblots after a long exposure (LE) and a short exposure (SE), p21 and p19ARF immunoblots of cellular lysates (20 μg of total protein) from wild-type (WT) MEFs, TSP cells, TSPA cells, and TSPM cells bearing the empty vector (V) or its derivative with oncogenic ras (R). Cells were collected after 4 days at 32°C. Tub, tubulin. (B) p53 levels in different cell lines expressing p53val135 are not dependent on mRNA levels. Northern blot of p53val135 expressed in TSP cells (p53−/−), three clones of TSPA cells (p53−/− ARF−/−), and two clones of TSPM cells (p53−/− mdm2−/−). ts, temperature sensitive.
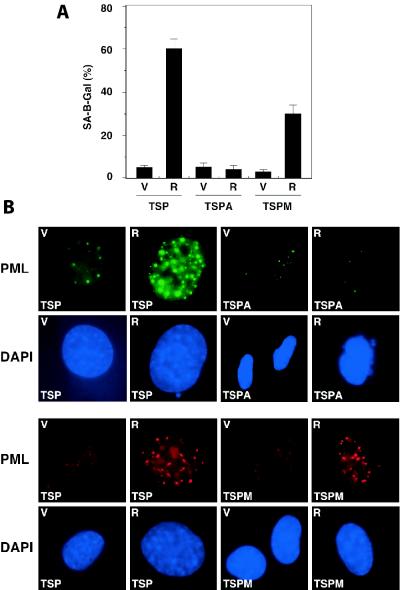
Consistent with the fact that TSPA-Ras cells efficiently recovered from a p53-mediated arrest, TSPA-Ras cells arrested at 32°C did not display senescence markers. Hence, TSPA-Ras cells did not display a senescence morphology (data not shown) nor did they become positive for SA β-gal activity (Fig. 9A) or accumulate PML bodies (Fig. 9B). These results demonstrate that ARF is required for oncogenic ras to convert p53 into a senescence inducer and suggest that this effect is due, in part, to the ability of p19ARF to stabilize p53. However, these results do not rule out qualitative effects of p19ARF on p53 signaling (see below).
FIG. 9.
Analysis of senescence markers in ARF-null and mdm2-null MEFs. (A) Summary of SA β-Gal-positive cells among TSP, TSPA, and TSPM cells expressing the empty vector (V) or its derivative expressing oncogenic ras (R). (B) PML bodies in TSP, TSPA, and TSPM cells. Cells were fixed after 4 days of arrest at 32°C and stained for PML as described in Materials and Methods.
Inhibition of Mdm2 is not sufficient to turn p53 into a senescence inducer.
p19ARF is thought to stabilize p53 through direct inhibition of Mdm2-mediated proteolysis. If Ras signaling converts p53 into a senescence regulator simply by inducing p19ARF, then it follows that mdm2-deficient cells should respond to p53 activation by senescence even in the absence of oncogenic ras. Consistent with this possibility, the proliferation defect of mdm2−/− mouse embryos was rescued by inactivation of p53 (37). To test this directly, we generated several cell lines expressing p53val135 in p53−/− mdm2−/− MEFs (TSPM cells, Table 1) and examined their proliferation phenotype in the absence and presence of oncogenic ras. Because mdm2-deficient cells are particularly sensitive to p53, we were careful to maintain all clones at 39°C to avoid selection for mutations that inactivate the p53 pathway. As demonstrated by both the [3H]thymidine incorporation and clonogenic assays, all of the TSPM clones tested (a total of six) underwent a cell cycle arrest at 32°C and reinitiated proliferation upon a return to 39°C (Fig. 8D and E; data not shown). As in other settings, the arrest at 32°C was mediated by p53, since the parental p53−/− mdm2−/− MEFs continued to proliferate at this temperature (data not shown). Consistent with the inability of mdm2-deficiency to convert the p53 response into a permanent cell cycle arrest, TSPM cells did not accumulate SA β-gal activity or PML bodies at 32°C. Hence, the ability of oncogenic ras to inactivate Mdm2 via p19ARF is not sufficient to convert p53 into a senescence inducer.
To confirm that the mdm2−/− clones retained the ability to undergo senescence, we introduced oncogenic ras into TSPM cells and examined a cell cycle arrest at 32°C and recovery upon a return to 39°C. In contrast to TSPM-vector cells, TSPM-Ras cells were impaired in the ability to reenter the cell cycle following the p53-mediated arrest, although the effect was not as pronounced as the one described for TSP cells (Fig. 8D and E). Interestingly, the levels of exogenous p53 and endogenous p21 expressed in TSPM cells at 32°C were not further increased by oncogenic ras (Fig. 8F, compare lanes 3 and 4), suggesting that Ras signaling cannot further stabilize p53 in the absence of Mdm2. Like TSP cells, TSPM cells displayed an increase in SA β-gal activity and PML bodies when RasV12 and p53 were coexpressed at 32°C (Fig. 9). Hence, although TSPM cells failed to senesce in response to p53 activation alone, they retained the ability to enter senescence in the presence of oncogenic Ras and p53.
The impact of RasV12 on p53 signaling involves both quantitative and qualitative components.
In principle, the cooperation between RasV12 and p53 in promoting senescence could result from quantitative effects of Ras on p53 activity or qualitative effects of Ras on the cell or p53 itself. In order to distinguish between these possibilities, we examined p53 levels and activity (as measured by p21 expression), as well as p19ARF expression, in various cell populations arrested by p53val135 at 32°C. Consistent with the role of p19ARF in stabilizing p53, p19ARF levels correlated with p53 levels in all of the cell lines tested (Fig. 10A). Notably, senescent TSP-Ras cells displayed increased p53 levels and activity compared to nonsenescent TSP-vector controls (Fig. 10A, compare lanes 3 and 4). Moreover, ARF-null TSPA cells, which fail to senesce in the presence of oncogenic ras, express very low levels of p53 compared to TSP cells (Fig. 10A, compare lanes 3 and 4 to 5 and 6, respectively). This effect could result from increased p53 degradation occurring in the absence of p19ARF because the ARF-null TSPA cells expressed comparable or greater levels of p53 mRNA relative to TSP cells (Fig. 10B, compare lane 1 to lanes 2 to 4). Taken together, these observations support a quantitative mechanism in which the outcome of p53 activation is controlled by p53 levels such that oncogenic ras induces senescence by increasing p53 levels beyond a threshold that would permit a reversible cell cycle arrest.
However, a strictly quantitative mechanism is not supported by the fact that, in several instances, p53 levels and activity did not correlate with the ability of MEFs to undergo senescence. Firstly, wild-type MEFs underwent senescence in response to oncogenic ras yet expressed p53 and p21 at levels comparable to those observed in TSPA-Ras cells—and much lower than those observed in TSP-vector cells—that did not senesce (Fig. 10A, compare lane 2 to lanes 3 and 6). Secondly, the levels of enforced p53 expression achieved in mdm2-null cells (TSPM-vector cells) were substantially higher than those produced in TSP cells expressing oncogenic ras (Fig. 10A, compare lanes 4 to 7), yet only the latter cells were impaired in recovery from a p53-mediated arrest and displayed features of senescence. Finally, while oncogenic ras induced p21 in wild-type, TSP, and TSPA cells and to a minor extent in TSPM cells, p21 levels showed no correlation with the ability of p53 to induce cellular senescence. Importantly, although p53 mRNA levels varied slightly between clones, this could not explain the differences in p53 and p21 expression we observed (Fig. 10B). Taken together, these data indicate that the outcome of p53 activity can be influenced by qualitative effects of oncogenic ras on the cell or the p53 output, leading to a transition from a reversible to a senescence-like arrest.
DISCUSSION
Oncogenic ras can activate p53 to promote cellular senescence, which acts to limit the transforming potential of excessive MAP kinase signaling (8, 27, 28, 43, 49). Here we demonstrate that conditional activation of p53 in MEFs produces a reversible cell cycle arrest, whereas activation of p53 in the presence of oncogenic ras leads to a permanent cell cycle arrest with features of cellular senescence. Although oncogenic ras can increase p53 levels, this increase is not sufficient to explain the induction of senescence; therefore, in several settings, p53 levels were a poor predictor of whether senescence ensues (Fig. 10). Perhaps the most compelling illustrations of this discrepancy are that (i) the levels of p53 produced by enforced expression in reversibly arrested TSP-vector cells vastly exceeded endogenous p53 levels in senescent wild-type MEFs expressing oncogenic Ras and (ii) TSPM cells, lacking Mdm2, expressed exceedingly high levels of p53 yet failed to senesce. These results suggest that Ras does not signal to p53 and senescence through a simple linear pathway but propagates a “collateral signal” that modifies the outcome of p53 activation. Hence, oncogenic Ras can convert a p53-dependent reversible cell cycle arrest into senescence.
Senescence as a two-stage process.
The data presented in this study, as well as previous reports, clearly demonstrate that the integrity of the ARF/p53 pathway is essential for oncogenic Ras to engage the senescence machinery in murine fibroblasts. We show that oncogenic Ras and p53 cooperate to induce senescence but that after several days of this arrest, a substantial portion of the cells are unable to reenter the cell cycle even when p53 activity has been removed. This observation suggests that p53 is strictly required to initiate a cell cycle arrest but that the maintenance of the senescent state is less dependent on p53. Similarly, the senescent state induced by conditional Raf (a downstream effector of Ras in the MAP kinase cascade) activation is stable well after Raf removal and the MEK inhibitor PD98059 prevents the initiation of a ras-induced arrest but cannot reverse the arrest after it is engaged (27, 66). The fact that it takes several days before a p53-mediated arrest is converted to a permanent arrest parallels the kinetics of a ras-induced arrest in primary cells and is consistent with a cellular remodeling program similar to terminal differentiation. Correspondingly, conditional expression of MyoD induces a terminal differentiation program in fibroblasts that can be maintained in the absence of MyoD (20). The molecular nature of the senescence-maintenance program is unknown, but it will undoubtedly be an important component of the tumor suppressor network.
Quantitative versus qualitative regulation of the p53 response.
Two different, although not mutually exclusive, models can be proposed to explain the different biological outcomes associated with p53 activation. The quantitative model implies that p53 levels are sufficient to determine outcome. Thus, low p53 levels induce a reversible cell cycle arrest while higher p53 levels induce senescence or apoptosis. This model is supported by studies in which p53 levels can be artificially controlled with appropriate expression systems (2, 65). One potential mechanism that could explain such an effect is based on differential p53 affinity for p53 response elements, such that genes required for a reversible cell cycle arrest have greater affinities than those required for senescence or apoptosis. Although anecdotal evidence of such a mechanism exists, this model has not been directly tested for endogenous p53 and no p53-dependent promoter element has been described that responds only to high p53 levels.
A qualitative model of p53 action implies that nonquantitative factors (collateral signals) controlled by a stimulus, the tissue origin, or the cell genotype influence the outcome of p53 activation. Again, two extreme but not mutually exclusive mechanisms might underlie this biology. Firstly, certain collateral signals might directly modulate p53 activity by changing the conformation of p53 or its association with various coactivators, perhaps leading to the expression of different subsets of p53 target genes. Consistent with this possibility, ionizing radiation and UV light have been shown to induce different subsets of p53-dependent target genes in the same cell type (65). Interestingly, these two stimuli induce different p53 modifications (22, 33, 59), raising the possibility that the activating signal can modulate p53 activity in a qualitative manner by directing p53 to different promoters (41). Similarly, the ability of oncogenes to promote either apoptosis or senescence correlates with different p53 modifications. Hence, oncogenic Ras induces p53 phosphorylation on serine 15 and induces senescence whereas the E1A oncoprotein does not induce serine 15 phosphorylation and promotes apoptosis. The E1A effect is dominant, since cells coexpressing E1A and Ras do not contain p53 phosphorylated on serine 15 and are prone to apoptosis (8, 32). Whether this leads to the expression of different p53 target genes has yet to be determined. Secondly, it is possible that the signal produced by p53 activation is the same in different contexts and the outcome of p53 activation is determined by how this signal is interpreted by the cell. One can envision several mechanisms by which this might occur, but an obvious possibility involves the combinatorial action of p53 and other transcription factors such that the action of p53 on outcome-specific targets is influenced by the presence or absence of these other factors. These other factors, in turn, would be the targets for the hypothetical collateral signal. One precedent for this involves integration of p53 and interferon signaling on the p21 promoter, which contains both p53 and IRF-1 response elements that act to synergistically induce p21 expression during a DNA damage response (54). How different signal transduction pathways integrate to produce new biological outcomes is an important biological problem that may also have an impact on our understanding of p53.
Impact of Ras on p53 signaling.
How does oncogenic ras convert p53 to a senescence inducer? Although it seems likely that a component of this response results from the ability of oncogenic ras to produce quantitative increases in p53 activity via ARF-mediated inhibition of Mdm2, our data provide compelling evidence for collateral signals that modify the outcome of p53 activation leading to senescence. Following the discussion above, it is formally possible that oncogenic ras directly modulates p53 activity or, instead, produces cellular changes that reinterpret the p53 signal. Although our data do not distinguish between these extremes, we provide strong evidence that constitutive signaling through the MAP kinase cascade is responsible for the effect.
One potential mechanism may involve the ability of Ras to induce PML, leading to an increase in the number and size of PML bodies in senescent cells. Although the functions of PML bodies are currently under debate, they may recruit transcription factors to sites of transcription and/or lead to posttranslational modifications that alter their activity (48). Previous work has shown that PML is required for efficient p53 activation and a subsequent cell cycle arrest in ras-expressing MEFs, and enforced PML expression is sufficient to induce senescence in a p53-dependent manner (8, 43). Interestingly, PML can lead to p53 modifications that might alter its activity, including serine 15 phosphorylation (a change also produced by oncogenic ras) and acetylation on lysine 382. Consistent with this idea, a subset of p53 also colocalizes with PML in the PML bodies (8, 43; Fig. 6) and PML can alter p53 transcriptional activity in transient-overexpression/reporter assays (9, 16). Here we noted a precise correlation between the appearance of PML bodies and the impaired ability of cells to reenter the cell cycle following a p53-mediated arrest. In fact, enforced PML expression cooperated with p53 to induce a permanent cell cycle arrest in TSP cells, albeit less efficiently than oncogenic ras (G. Ferbeyre and S. W. Lowe, unpublished observations). Collectively, these data support a causal role for PML in senescence and are consistent with its directly modulating p53 activity.
Together, our results uncover at least three pathways whereby oncogenic Ras signals to p53, leading to the induction of cellular senescence. Firstly, Ras signals to p53 via p19ARF and, correspondingly, ARF−/− cells are defective in the p53 response engaged by oncogenic ras in wild-type MEFs and in TSPA cells. Signaling to p53 through p19ARF is common for both the senescence response to oncogenic ras (42) and the apoptotic response to myc or E1A (5, 67). Thus, p19ARF is not a selective switch with which to modulate p53 activity toward senescence or apoptosis. Consistent with this view, the only known mechanism whereby p19ARF influences p53 activity (Mdm2 inhibition) is not sufficient to convert p53 into a senescence regulator. Secondly, as indicated above, PML is crucial for the induction of senescence by oncogenic ras and apparently acts by directly modifying p53 activity toward a more permanent outcome. Finally, Ras signals to p53 through at least one additional pathway, which would explain the enhanced p53 activity we observed in ARF-null MEFs (TSPA cells; Fig. 8C). This pathway appears independent of PML because oncogenic ras did not up-regulate PML in these MEFs upon enforced expression of p53 (TSPA cells). Importantly, our data do not rule out the possibility that oncogenic Ras also produces cellular changes that reinterpret the p53 signal.
Modulation of p53 activity during tumorigenesis.
Conditional activation of p53 in p53-deficient cells induces an irreversible cell cycle arrest with senescence features in other settings. For example, p53 can induce senescence in EJ bladder carcinoma cells and H1299 lung carcinoma cells (53, 57). Although these tumor-derived cell lines do not express exogenous ras, they both harbor activating ras mutations (36, 63). Consequently, endogenous Ras signaling may contribute to the p53-mediated senescence of these tumor-derived cells much as exogenous ras expression cooperates with p53 to induce senescence in normal cells. This is particularly interesting, since similar mutations present in tumor cells may provide a therapeutic window for exogenous p53 genes or other agents that can mimic the action of p53 to selectively inhibit tumor progression with minimal toxicity to normal cells. In any case, our studies are consistent with the notion that oncogenic ras engages a tumor suppressor network involving p19ARF, PML, and p53, thereby reinforcing a potentially reversible cell cycle arrest program to produce an irreversible outcome (8). Identification of additional components of this network will undoubtedly shed light on the control of the action of p53 during tumor progression.
Acknowledgments
We thank P. Zheng. T. Ley, and A. Levine for reagents; J. Duffy and the Cold Spring Harbor Laboratory Graphic Arts Department for preparing the figures; C. Sherr, M. Roussel, F. Zindy, and the members of the Lowe laboratory for encouragement and comments; and V. Bourdeau and B. Stillman for support.
G.F. is a Tularik Fellow, and E.Q. is supported by a fellowship from the CIHR. E.D.S. is a Human Frontier Science Fellow, and G.H. and S.W.L. are Rita Allen Foundation Scholars. This work was supported by grant AG-16379 from the National Institutes of Health.
REFERENCES
- 1.Agarwal, M. L., A. Agarwal, W. R. Taylor, and G. R. Stark. 1995. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc. Natl. Acad. Sci. USA 92:8493-8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, X., L. J. Ko, L. Jayaraman, and C. Prives. 1996. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 10:2438-2451. [DOI] [PubMed] [Google Scholar]
- 3.Cross, S. M., C. A. Sanchez, C. A. Morgan, M. K. Schimke, S. Ramel, R. L. Idzerda, W. H. Raskind, and B. J. Reid. 1995. A p53-dependent mouse spindle checkpoint. Science 267:1353-1356. [DOI] [PubMed] [Google Scholar]
- 4.Debbas, M., and E. White. 1993. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 7:546-554. [DOI] [PubMed] [Google Scholar]
- 5.de Stanchina, E., M. E. McCurrach, F. Zindy, S. Y. Shieh, G. Ferbeyre, A. V. Samuelson, C. Prives, M. F. Roussel, C. J. Sherr, and S. W. Lowe. 1998. E1A signaling to p53 involves the p19(ARF) tumor suppressor. Genes Dev. 12:2434-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Leonardo, A., S. P. Linke, K. Clarkin, and G. M. Wahl. 1994. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 8:2540-2551. [DOI] [PubMed] [Google Scholar]
- 7.Dimri, G. P., X. Lee, G. Basile, M. Acosta, G. Scott, C. Roskelley, E. E. Medrano, M. Linskens, I. Rubelj, O. Pereira-Smith, M. Peacocke, and J. Campisi. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferbeyre, G., E. de Stanchina, E. Querido, N. Baptiste, C. Prives, and S. W. Lowe. 2000. PML is induced by oncogenic ras and promotes premature senescence. Genes Dev. 14:2015-2027. [PMC free article] [PubMed] [Google Scholar]
- 9.Fogal, V., M. Gostissa, P. Sandy, P. Zacchi, T. Sternsdorf, K. Jensen, P. P. Pandolfi, H. Will, C. Schneider, and G. Del Sal. 2000. Regulation of p53 activity in nuclear bodies by a specific PML isoform. EMBO J. 19:6185-6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gannon, J. V., and D. P. Lane. 1991. Protein synthesis required to anchor a mutant p53 protein which is temperature-sensitive for nuclear transport. Nature 349:802-806. [DOI] [PubMed] [Google Scholar]
- 11.Giaccia, A. J., and M. B. Kastan. 1998. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 12:2973-2983. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg, D., F. Mechta, M. Yaniv, and M. Oren. 1991. Wild-type p53 can down-modulate the activity of various promoters. Proc. Natl. Acad. Sci. USA 88:9979-9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gostissa, M., A. Hengstermann, V. Fogal, P. Sandy, S. E. Schwarz, M. Scheffner, and G. Del Sal. 1999. Activation of p53 by conjugation to the ubiquitin-like protein SUMO-1. EMBO J. 18:6462-6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graeber, T. G., C. Osmanian, T. Jacks, D. E. Housman, C. J. Koch, S. W. Lowe, and A. J. Giaccia. 1996. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 379:88-91. [DOI] [PubMed] [Google Scholar]
- 15.Graeber, T. G., J. F. Peterson, M. Tsai, K. Monica, A. J. J. Fornace, and A. J. Giaccia. 1994. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol. Cell. Biol. 14:6264-6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo, A., P. Salomoni, J. Luo, A. Shih, S. Zhong, W. Gu, and P. Paolo Pandolfi. 2000. The function of PML in p53-dependent apoptosis. Nat. Cell Biol. 2:730-736. [DOI] [PubMed] [Google Scholar]
- 17.Hainaut, P., and J. Milner. 1993. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro. Cancer Res. 53:4469-4473. [PubMed] [Google Scholar]
- 18.Hannon, G. J., P. Sun, A. Carnero, L. Y. Xie, R. Maestro, D. S. Conklin, and D. Beach. 1999. MaRX: an approach to genetics in mammalian cells. Science 283:1129-1130. [DOI] [PubMed] [Google Scholar]
- 19.Hermeking, H., and D. Eick. 1994. Mediation of c-Myc-induced apoptosis by p53. Science 265:2091-2093. [DOI] [PubMed] [Google Scholar]
- 20.Hollenberg, S. M., P. F. Cheng, and H. Weintraub. 1993. Use of a conditional MyoD transcription factor in studies of MyoD trans-activation and muscle determination. Proc. Natl. Acad. Sci. USA 90:8028-8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jimenez, G. S., M. Nister, J. M. Stommel, M. Beeche, E. A. Barcarse, X. Q. Zhang, S. O'Gorman, and G. M. Wahl. 2000. A transactivation-deficient mouse model provides insights into Trp53 regulation and function. Nat. Genet. 26:37-43. [DOI] [PubMed] [Google Scholar]
- 22.Kapoor, M., and G. Lozano. 1998. Functional activation of p53 via phosphorylation following DNA damage by UV but not gamma radiation. Proc. Natl. Acad. Sci. USA 95:2834-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kastan, M. B., Q. Zhan, W. S. el-Deiry, F. Carrier, T. Jacks, W. V. Walsh, B. S. Plunkett, B. Vogelstein, and A. J. Fornace, Jr. 1992. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71:587-597. [DOI] [PubMed] [Google Scholar]
- 24.Kern, S. E., K. W. Kinzler, A. Bruskin, D. Jarosz, P. Friedman, C. Prives, and B. Vogelstein. 1991. Identification of p53 as a sequence-specific DNA-binding protein. Science 252:1708-1711. [DOI] [PubMed] [Google Scholar]
- 25.Lambert, P. F., F. Kashanchi, M. F. Radonovich, R. Shiekhattar, and J. N. Brady. 1998. Phosphorylation of p53 serine 15 increases interaction with CBP. J. Biol. Chem. 273:33048-33053. [DOI] [PubMed] [Google Scholar]
- 26.Lee, C. W., T. S. Sorensen, N. Shikama, and N. B. La Thangue. 1998. Functional interplay between p53 and E2F through co-activator p300. Oncogene 16:2695-2710. [DOI] [PubMed] [Google Scholar]
- 27.Lin, A. W., M. Barradas, J. C. Stone, L. van Aelst, M. Serrano, and S. W. Lowe. 1998. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12:3008-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin, A. W., and S. W. Lowe. 2001. Oncogenic ras activates the ARF-p53 pathway to suppress epithelial cell transformation. Proc. Natl. Acad. Sci. USA 98:5025-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, Y., and S. Benchimol. 1995. Cytokines inhibit p53-mediated apoptosis but not p53-mediated G1 arrest. Mol. Cell. Biol. 15:6045-6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linke, S. P., K. C. Clarkin, A. Di Leonardo, A. Tsou, and G. M. Wahl. 1996. A reversible, p53-dependent G0/G1 cell cycle arrest induced by ribonucleotide depletion in the absence of detectable DNA damage. Genes Dev. 10:934-947. [DOI] [PubMed] [Google Scholar]
- 31.Lowe, S. W., and H. E. Ruley. 1993. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 7:535-545. [DOI] [PubMed] [Google Scholar]
- 32.Lowe, S. W., H. E. Ruley, T. Jacks, and D. E. Housman. 1993. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74:957-967. [DOI] [PubMed] [Google Scholar]
- 33.Lu, H., Y. Taya, M. Ikeda, and A. J. Levine. 1998. Ultraviolet radiation, but not gamma radiation or etoposide-induced DNA damage, results in the phosphorylation of the murine p53 protein at serine-389. Proc. Natl. Acad. Sci. USA 95:6399-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maltzman, W., and L. Czyzyk. 1984. UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol. Cell. Biol. 4:1689-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michalovitz, D., O. Halevy, and M. Oren. 1990. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell 62:671-680. [DOI] [PubMed] [Google Scholar]
- 36.Mitsudomi, T., J. Viallet, J. L. Mulshine, R. I. Linnoila, J. D. Minna, and A. F. Gazdar. 1991. Mutations of ras genes distinguish a subset of non-small-cell lung cancer cell lines from small-cell lung cancer cell lines. Oncogene 6:1353-1362. [PubMed] [Google Scholar]
- 37.Montes de Oca Luna, R., D. S. Wagner, and G. Lozano. 1995. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378:203-206. [DOI] [PubMed] [Google Scholar]
- 38.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson, W. G., and M. B. Kastan. 1994. DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol. Cell. Biol. 14:1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nigro, J. M., K. D. Aldape, S. M. Hess, and T. D. Tlsty. 1997. Cellular adhesion regulates p53 protein levels in primary human keratinocytes. Cancer Res. 57:3635-3639. [PubMed] [Google Scholar]
- 41.Oda, K., H. Arakawa, T. Tanaka, K. Matsuda, C. Tanikawa, T. Mori, H. Nishimori, K. Tamai, T. Tokino, Y. Nakamura, and Y. Taya. 2000. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102:849-862. [DOI] [PubMed] [Google Scholar]
- 42.Palmero, I., C. Pantoja, and M. Serrano. 1998. p19ARF links the tumour suppressor p53 to Ras. Nature 395:125-126. [DOI] [PubMed] [Google Scholar]
- 43.Pearson, M., R. Carbone, C. Sebastiani, M. Cioce, M. Fagioli, S. Saito, Y. Higashimoto, E. Appella, S. Minucci, P. P. Pandolfi, and P. G. Pelicci. 2000. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature 406:207-210. [DOI] [PubMed] [Google Scholar]
- 44.Pomerantz, J., N. Schreiber-Agus, N. J. Liegeois, A. Silverman, L. Alland, L. Chin, J. Potes, K. Chen, I. Orlow, H. W. Lee, C. Cordon-Cardo, and R. A. DePinho. 1998. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell 92:713-723. [DOI] [PubMed] [Google Scholar]
- 45.Rainwater, R., D. Parks, M. E. Anderson, P. Tegtmeyer, and K. Mann. 1995. Role of cysteine residues in regulation of p53 function. Mol. Cell. Biol. 15:3892-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ries, S., C. Biederer, D. Woods, O. Shifman, S. Shirasawa, T. Sasazuki, M. McMahon, M. Oren, and F. McCormick. 2000. Opposing effects of Ras on p53: transcriptional activation of mdm2 and induction of p19ARF. Cell 103:321-330. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez, M. S., J. M. Desterro, S. Lain, C. A. Midgley, D. P. Lane, and R. T. Hay. 1999. SUMO-1 modification activates the transcriptional response of p53. EMBO J. 18:6455-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruggero, D., Z. G. Wang, and P. P. Pandolfi. 2000. The puzzling multiple lives of PML and its role in the genesis of cancer. Bioessays 22:827-835. [DOI] [PubMed] [Google Scholar]
- 49.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 50.Sherr, C. J., and R. A. DePinho. 2000. Cellular senescence: mitotic clock or culture shock? Cell 102:407-410. [DOI] [PubMed] [Google Scholar]
- 51.Sherr, C. J., and J. D. Weber. 2000. The ARF/p53 pathway. Curr. Opin. Genet. Dev. 10:94-99. [DOI] [PubMed] [Google Scholar]
- 52.Sionov, R. V., and Y. Haupt. 1999. The cellular response to p53: the decision between life and death. Oncogene 18:6145-6157. [DOI] [PubMed] [Google Scholar]
- 53.Sugrue, M. M., D. Y. Shin, S. W. Lee, and S. A. Aaronson. 1997. Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc. Natl. Acad. Sci. USA 94:9648-9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka, N., M. Ishihara, M. S. Lamphier, H. Nozawa, T. Matsuyama, T. W. Mak, S. Aizawa, T. Tokino, M. Oren, and T. Taniguchi. 1996. Cooperation of the tumour suppressors IRF-1 and p53 in response to DNA damage. Nature 382:816-818. [DOI] [PubMed] [Google Scholar]
- 55.Tao, W., and A. J. Levine. 1999. P19(ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc. Natl. Acad. Sci. USA 96:6937-6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner, A. J., J. M. Kokontis, and N. Hay. 1994. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21waf1/cip1. Genes Dev. 8:2817-2830. [DOI] [PubMed] [Google Scholar]
- 57.Wang, Y., G. Blandino, M. Oren, and D. Givol. 1998. Induced p53 expression in lung cancer cell line promotes cell senescence and differentially modifies the cytotoxicity of anti-cancer drugs. Oncogene 17:1923-1930. [DOI] [PubMed] [Google Scholar]
- 58.Weber, J. D., L. J. Taylor, M. F. Roussel, C. J. Sherr, and D. Bar-Sagi. 1999. Nucleolar Arf sequesters Mdm2 and activates p53. Nat. Cell Biol. 1:20-26. [DOI] [PubMed] [Google Scholar]
- 59.Webley, K., J. A. Bond, C. J. Jones, J. P. Blaydes, A. Craig, T. Hupp, and D. Wynford-Thomas. 2000. Posttranslational modifications of p53 in replicative senescence overlapping but distinct from those induced by DNA damage. Mol. Cell. Biol. 20:2803-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu, X., J. H. Bayle, D. Olson, and A. J. Levine. 1993. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 7:1126-1132. [DOI] [PubMed] [Google Scholar]
- 61.Wu, X., and A. J. Levine. 1994. p53 and E2F-1 cooperate to mediate apoptosis. Proc. Natl. Acad. Sci. USA 91:3602-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yonish-Rouach, E., D. Resnitzky, J. Lotem, L. Sachs, A. Kimchi, and M. Oren. 1991. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 352:345-347. [DOI] [PubMed] [Google Scholar]
- 63.Yuasa, Y., S. K. Srivastava, C. Y. Dunn, J. S. Rhim, E. P. Reddy, and S. A. Aaronson. 1983. Acquisition of transforming properties by alternative point mutations within c-bas/has human proto-oncogene. Nature 303:775-779. [DOI] [PubMed] [Google Scholar]
- 64.Zhang, Y., Y. Xiong, and W. G. Yarbrough. 1998. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 92:725-734. [DOI] [PubMed] [Google Scholar]
- 65.Zhao, R., K. Gish, M. Murphy, Y. Yin, D. Notterman, W. H. Hoffman, E. Tom, D. H. Mack, and A. J. Levine. 2000. Analysis of p53-regulated gene expression patterns with oligonucleotide arrays. Genes Dev. 14:981-993. [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu, J., D. Woods, M. McMahon, and J. M. Bishop. 1998. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12:2997-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zindy, F., C. M. Eischen, D. H. Randle, T. Kamijo, J. L. Cleveland, C. J. Sherr, and M. F. Roussel. 1998. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 12:2424-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]