Abstract
APS is a Cbl-binding protein that is tyrosine phosphorylated by the insulin receptor kinase. Insulin-stimulated phosphorylation of tyrosine 618 in APS is necessary for its association with c-Cbl and the subsequent tyrosine phosphorylation of Cbl by the insulin receptor in both 3T3-L1 adipocytes and CHO-IR cells. When overexpressed in these cells, wild-type APS but not an APS/Y618F mutant facilitated the tyrosine phosphorylation of coexpressed Cbl and its association with Crk upon insulin stimulation. APS-facilitated phosphorylation occurred on tyrosines 371, 700, and 774 in the Cbl protein. APS also interacted directly with the c-Cbl-associated protein (CAP) and colocalized with the protein in cells. The association was dependent on the SH3 domains of CAP and was independent of insulin treatment. Overexpression of the APS/Y618F mutant in 3T3-L1 adipocytes blocked the insulin-stimulated tyrosine phosphorylation of endogenous Cbl and binding to Crk. Moreover, the translocation of GLUT4 from intracellular vesicles to the plasma membrane was also inhibited by overexpression of the APS/Y618F mutant. These data suggest that APS serves as an adapter protein linking the CAP/Cbl pathway to the insulin receptor and, further, that APS-facilitated Cbl tyrosine phosphorylation catalyzed by the insulin receptor is a crucial event in the stimulation of glucose transport by insulin.
c-Cbl is the cellular homolog of the transforming v-Cbl oncogene (4, 17). Cbl contains numerous tyrosine residues, which could serve as docking sites for multiple SH2-containing signaling molecules upon phosphorylation. Cbl consists of an N-terminal variant SH2 domain, a Ring finger domain, multiple proline-rich stretches, several potential tyrosine phosphorylation sites, and a conserved ubiquitin-associated domain. Cbl is tyrosine phosphorylated in response to epidermal growth factor (EGF), platelet-derived growth factor (PDGF), various antigens, integrins, and cytokines (21, 41). Src family kinases may catalyze c-Cbl tyrosine phosphorylation in the antigen and integrin signaling pathways (26, 30, 40), and the EGF and PDGF receptors appear to phosphorylate c-Cbl directly upon ligand binding (5, 9). Tyrosine-phosphorylated Cbl can bind the SH2 domains of the non-receptor tyrosine kinases Fyn and Syk, the p85 subunit of phosphatidylinositol (PI) 3-kinase, and the adapter protein Crk (2, 7, 8, 22, 36). c-Cbl contains an E2-dependent ubiquitin ligase activity and can function as a negative regulator of tyrosine kinase signaling (13, 14, 18, 24, 43). However, there is increasing evidence that c-Cbl can also serve a positive role. For example, the protein enhances proliferation and survival through PI 3-kinase-dependent pathways after cytokine simulation (11, 42) and enhances mitogen-activated protein (MAP) kinase activation in response to stimulation of the Met receptor (10).
We previously showed that insulin stimulates the tyrosine phosphorylation of Cbl in 3T3-L1 adipocytes, inducing its association with Crk (36). The phosphorylation of Cbl in response to insulin correlates with expression of the Cbl-associated protein (CAP). CAP contains three SH3 domains in its C terminus and a region of homology to the gut peptide sorbin (SoHo domain) in its N terminus. CAP interacts with Cbl via its C-terminal SH3 domain (15, 35). Upon Cbl phosphorylation, the CAP/Cbl complex migrates to caveolin-enriched lipid rafts, due to the interaction of the SoHo domain on CAP with the lipid raft-associated protein flotillin (3, 15). This leads to the recruitment of the Crk/C3G complex to this microdomain of the plasma membrane, where C3G, a guanyl nucleotide exchange factor, activates the small G protein TC10 (6). Both translocation of the CAP/Cbl complex and activation of TC10 have been shown to occur independently of the PI 3-kinase pathway and, more importantly, to be crucial to insulin-stimulated GLUT4 translocation (3, 6).
In 3T3-L1 adipocytes, overexpression of a CAP mutant in which either the SH3 domains or the SoHo domain have been deleted inhibited insulin-stimulated recruitment of Cbl to lipid rafts and subsequently blocked the translocation of GLUT4 in response to insulin (3, 15). However, it remained unclear whether CAP directly targeted Cbl to insulin receptor for phosphorylation or if a separate protein existed to serve this function. One such candidate is an adapter protein containing PH and SH2 domains, called APS. APS is a member of the Lnk family of adapter proteins that is highly expressed in insulin-responsive tissues such as fat, skeletal muscle, and heart (28). Recently APS was identified as a substrate for the PDGF and insulin receptors, both of which catalyze the tyrosine phosphorylation of APS at a C-terminal phosphorylation site (28, 45). Ectopic expression of APS in Chinese hamster ovary (CHO) cells overexpressing insulin receptor led to concomitant ubiquitination of the insulin receptor, possibly through recruitment of Cbl (1). However, the function of APS in cells metabolically responsive to insulin has not been evaluated.
We report here that APS is necessary for the insulin-stimulated tyrosine phosphorylation of Cbl and its subsequent association with Crk in differentiated adipocytes. We also demonstrate that APS specifically interacts and colocalizes with CAP in an insulin-independent manner. Moreover, APS-facilitated Cbl phosphorylation is crucial for the translocation of GLUT4 in response to insulin. Thus, APS is an essential component of the CAP/Cbl pathway in the control of insulin-stimulated glucose transport.
MATERIALS AND METHODS
Antibodies.
The antibodies to hemagglutinin (HA) (F-7), Myc (9E10), c-Cbl (C-15), phospho-ERK, and p85 subunit of PI 3-kinase were purchased from Santa Cruz, Inc. The FLAG (M2) monoclonal antibody was obtained from Stratagene. The antiphosphotyrosine monoclonal antibody (4G10) was from Upstate Biotechnology, Inc. The Crk monoclonal antibody was purchased from Transduction Laboratories. Horseradish peroxidase-linked secondary antibodies were from Pierce Chemical Co. The Alexa Fluor secondary antibodies were from Molecular Probes.
Plasmids and mutagenesis.
The Myc-APS construct was kindly provided by David Ginty (32). c-Cbl full-length cDNA was derived from pSX-HA-Cbl by PCR. Triple-HA-tagged Cbl was constructed by placing c-Cbl cDNA in frame in the BamHI site of pKH3 vector. FLAG- and glutathione S-transferase (GST)-tagged CAP constructs were made as described previously (3, 15, 35). All mutated forms of APS and Cbl were generated by using the Stratagene Quick Change mutagenesis kit, according to the manufacturer's protocol. The mutations were confirmed by automated DNA sequencing.
Cell culture and transfection.
CHO-IR cells were maintained in α-minimal essential medium containing 10% fetal bovine serum. COS-1 cells were grown in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum. 3T3-L1 fibroblasts were maintained in DMEM supplemented with 10% calf serum, 100 U of penicillin G sodium per ml, and 100 μg of streptomycin sulfate per ml. Differentiation to adipocytes was induced as previously described (37). The cells were then cultured in DMEM containing 10% fetal bovine serum. Before insulin treatment, CHO-IR cells were serum deprived for 3 h in F-12 Ham's medium. 3T3-L1 adipocytes were routinely serum starved for 3 h in low-glucose DMEM. Both CHO-IR cells and 3T3-L1 adipocytes were transfected by electroporation as described previously (3, 27). COS-1 cells in 60-mm-diameter dishes were transfected by using FuGene 6 reagent (Roche Diagnostics) as described previously (20).
Immunoprecipitation and immunoblotting.
Cells in 60-mm-diameter dishes were washed twice with ice-cold phosphate-buffered saline and were lysed for 30 min at 4°C with buffer containing 50 mM Tris-HCl (pH 8.0), 135 mM NaCl, 1% Triton X-100, 1.0 mM EDTA, 1.0 mM sodium pyrophosphate, 1.0 mM sodium orthovanadate, 10 mM NaF, and protease inhibitors (one tablet per 7 ml of buffer) (Roche Diagnostics). The clarified lysates were incubated with the indicated antibodies for 1 h at 4°C. The immune complexes were precipitated with protein A/G agarose (Santa Cruz, Inc.) for 1 h at 4°C and were washed extensively with lysis buffer before solubilization in sodium dodecyl sulfate (SDS) sample buffer. For anti-Cbl immunoprecipitation, Cbl antibody conjugated on agarose beads was incubated with lysates for 2 h to overnight at 4°C. Bound proteins were resolved by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Individual proteins were detected with the specific antibodies and visualized by blotting with horseradish peroxidase-conjugated secondary antibodies.
In vitro pull-down assays.
GST fusion proteins containing all or individual SH3 domains of CAP were expressed in Escherichia coli strain BL21 and purified as described previously (20). COS-1 cells were transfected with Myc-APS construct, and whole-cell lysates were prepared as described above for immunoprecipitation. Cell lysates were incubated either with GST alone or with GST-CAP variants immobilized on glutathione-Sepharose beads (Amersham Pharmacia) for 1.5 h at 4°C. The beads were washed extensively with lysis buffer, and the bound proteins were analyzed by SDS-polyacrylamide gel electrophoresis prior to Coomassie blue staining and immunoblotting.
Fluorescence microscopy.
Electroporated 3T3-L1 adipocytes were grown on glass coverslips in six-well dishes. After insulin treatment, cells were fixed with 10% formalin for 15 min, permeabilized with 0.5% Triton X-100 for 5 min, and then blocked with 1% bovine serum albumin and 1% ovalbumin for 1 h. Primary and Alexa Fluor secondary antibodies were used at 2 μg/μl in blocking solution, and samples were mounted on glass slides with Vectashield (Vector Laboratories). Cells were imaged using confocal fluorescence microscopy. Fluorescence intensities were quantitated by using the Olympus Fluoview software. Images were then imported into Photoshop (Adobe Systems, Inc.) for processing.
RESULTS
Phosphorylation of APS by insulin is necessary for its association with Cbl in differentiated adipocytes.
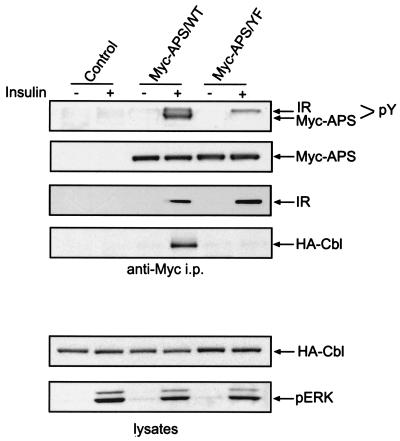
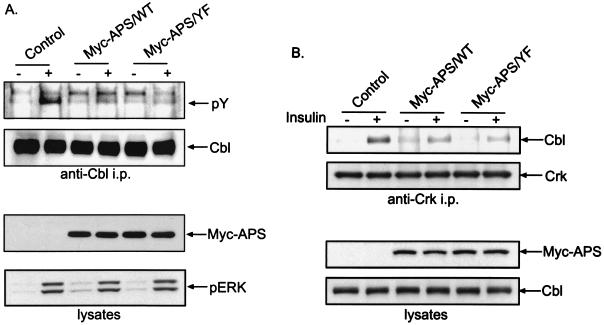
APS is an SH2 and PH domain-containing protein closely related to Lnk and SH2-B. It was recently reported that APS couples c-Cbl to the insulin receptor upon insulin binding (1). To evaluate the role of the tyrosine phosphorylation of APS in its association with Cbl, we transiently expressed HA-tagged c-Cbl in 3T3-L1 adipocytes together with a Myc-tagged wild-type APS or mutant form of APS in which tyrosine 618 was mutated to phenylalanine (APS/Y618F). Lysates were prepared from cells treated with or without insulin, and immunoprecipitation was performed with anti-Myc antibodies (Fig. 1). Anti-Myc immunoblotting of the immunoprecipitates indicated that the same amount of Myc-APS was precipitated under all conditions. Insulin stimulated the rapid tyrosine phosphorylation of Myc-tagged wild-type APS, as demonstrated by antiphosphotyrosine immunoblotting. Mutation of Y618 to F completely abolished the tyrosine phosphorylation of Myc-APS in response to insulin (Fig. 1). This result is in agreement with the previously reported finding that Y618 is the only site in APS that is tyrosine phosphorylated in response to insulin (28). Insulin stimulated the association of its receptor with both forms of Myc-APS. However, HA-Cbl associated only with wild-type APS and not with the Y618F mutant (Fig. 1). Immunoblotting of cell lysates showed that an equivalent amount of HA-Cbl was precipitated in all samples (Fig. 1). Moreover, overexpression of either form of APS did not affect the activation of the MAP kinase pathway in response to insulin, as revealed by anti-phosphorylated-ERK immunoblotting. Therefore, insulin-stimulated phosphorylation of Y618 in APS is responsible for the binding of Cbl.
FIG. 1.
Phosphorylation of tyrosine 618 in APS is required for its association with Cbl in response to insulin. 3T3-L1 adipocytes were cotransfected with HA-Cbl and a Myc-tagged wild-type APS (APS/WT) or Y618F mutant APS (APS/YF). After treatment with or without 100 nM insulin for 2 min, cells were lysed and immunoprecipitation (i.p.) was performed using an anti-Myc antibody. Myc-APS and insulin receptor (IR) were detected in the immunoprecipitates by immunoblotting with antiphosphotyrosine, anti-Myc, and anti-insulin receptor β chain antibodies (upper panels). HA-Cbl was detected by anti-HA immunoblotting in both immunoprecipitates and cell lysates. Phosphorylated ERK was detected in cell lysates by immunoblotting with an anti-phospho-ERK antibody (lower panels).
Association with APS facilitates insulin-stimulated tyrosine phosphorylation of Cbl and subsequent recruitment of Crk.
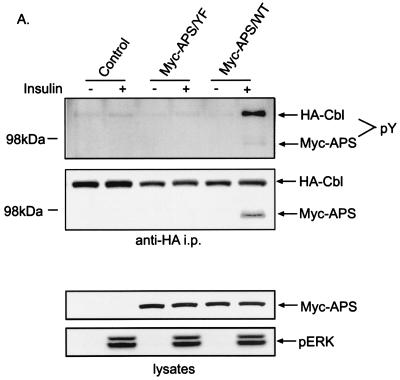
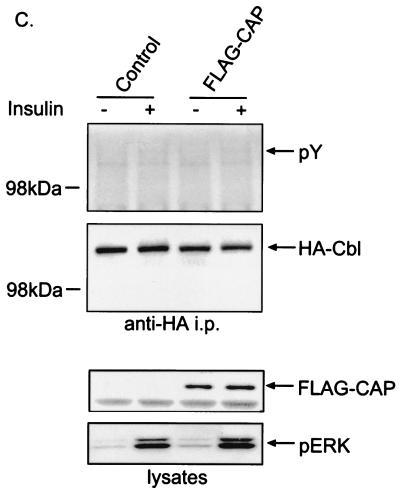
To determine whether the association with APS is essential for the tyrosine phosphorylation of Cbl in response to insulin, both CHO-IR cells and 3T3-L1 adipocytes were transfected with HA-Cbl plus Myc-tagged wild-type APS or APS/Y618F. Following treatment of cells with or without insulin, comparable amounts of HA-Cbl were isolated by anti-HA immunoprecipitation (Fig. 2A and B). When wild-type but not mutant APS was coexpressed, insulin stimulated the tyrosine phosphorylation of two proteins in the anti-HA immunoprecipitates, as detected by antiphosphotyrosine immunoblotting. The 120-kDa protein corresponds to HA-Cbl, and the 95-kDa band corresponds to Myc-APS (Fig. 2A). In addition, anti-Myc immunoblotting indicated that only wild-type APS and not APS/Y618F bound to HA-Cbl upon insulin stimulation (Fig. 2A and B). Taken together, these results suggest that direct association of APS with Cbl facilitates the tyrosine phosphorylation of Cbl in response to insulin. In comparison, the same experiment was performed by cotransfection of 3T3-L1 cells with FLAG-CAP and HA-Cbl (Fig. 2C). Anti-HA immunoprecipitation followed by antiphosphotyrosine immunoblotting showed that overexpression of FLAG-CAP did not restore the tyrosine phosphorylation of HA-Cbl in response to insulin. Therefore, APS, but not CAP, is the adapter protein that targets Cbl to insulin receptor.
FIG. 2.
Association with APS is necessary for the tyrosine phosphorylation of Cbl by the insulin receptor. CHO-IR cells (A) or 3T3-L1 adipocytes (B) were cotransfected with HA-Cbl and vector DNA, Myc-tagged wild-type APS (Myc-APS/WT) or APS/Y618F. Separately, CHO-IR cells were also cotransfected with HA-Cbl and empty vector or FLAG-CAP (C). After treatment with or without 100 nM insulin for 2 min, cells were lysed and immunoprecipitation (i.p.) was performed using an anti-HA antibody. Immunoprecipitates were analyzed by antiphosphotyrosine, anti-HA, anti-Myc, and anti-Crk immunoblotting (upper panels). Cell lysates were immunoblotted with anti-Myc, anti-FLAG, anti-HA, and anti-phospho-ERK antibodies (lower panels).
Because tyrosine-phosphorylated Cbl can potentially bind to the SH2 domains of Crk, Fyn, and the p85 subunit of PI 3-kinase (2, 7, 8, 22, 36), we evaluated whether the insulin-stimulated tyrosine phosphorylation of Cbl facilitated by APS might result in a similar function. As shown in Fig. 2B, treatment of transfected 3T3-L1 adipocytes with insulin induced the association of HA-Cbl with endogenous Crk when wild-type but not mutant APS was coexpressed. Expression of Crk remained the same in all lysates, as detected by anti-Crk immunoblotting (Fig. 2B). Interestingly, there was no detectable binding of Cbl to the p85 regulatory subunit of PI 3-kinase or Fyn under all conditions (data not shown). This result suggests that Crk is the major SH2-containing signaling molecule that associates with Cbl upon APS-facilitated Cbl phosphorylation.
Insulin receptor phosphorylates Cbl on tyrosines 371, 700, and 774 in the presence of APS.
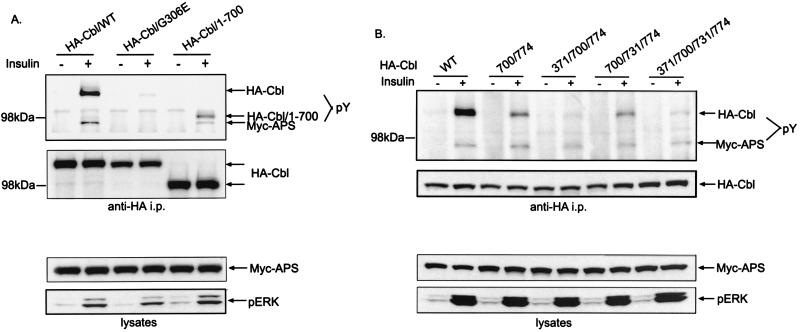
In order to characterize the relative importance of the variant SH2 domain and different tyrosine residues in the tyrosine phosphorylation of Cbl facilitated by APS, a series of HA-tagged Cbl mutants were transfected into CHO-IR cells together with Myc-APS. We compared the phosphorylation of wild-type Cbl, a C-terminally truncated Cbl (Cbl/1-700), or a full-length Cbl containing point mutations within either the SH2 domain (G306E) or potential tyrosine phosphorylation sites (Fig. 3A and B). The cells were treated with or without insulin, and lysates prepared from the cells were immunoprecipitated with anti-HA antibodies. The resultant immunoprecipitates were analyzed by antiphosphotyrosine and anti-HA immunoblotting. The transfected wild-type Cbl protein was tyrosine phosphorylated upon the addition of insulin. Mutation of G306 in the SH2 domain to E eliminated the ability of Cbl to become tyrosine phosphorylated in response to insulin (Fig. 3A). The result shows that a functional SH2 domain is critical for APS-facilitated tyrosine phosphorylation of Cbl in response to insulin.
FIG. 3.
APS facilitates the phosphorylation of tyrosines 371, 700, and 774 in Cbl by the insulin receptor. CHO-IR cells were cotransfected with Myc-tagged wild-type APS (Myc-APS/WT) plus HA-tagged Cbl/WT, Cbl/G306E, or Cbl/1-700 (A) or Cbl/WT, Cbl/Y700F/Y774F, Cbl/Y371F/Y700F/Y774F, Cbl/Y700F/Y731F/Y774F, or Cbl/Y371F/Y700F/Y731F/Y774F (B). After treatment with or without 100 nM insulin for 2 min, cells were lysed and immunoprecipitation (i.p.) was performed using an anti-HA antibody. Immunoprecipitates were analyzed by antiphosphotyrosine and anti-HA immunoblotting (upper panels). Cell lysates were immunoblotted with anti-Myc and anti-phospho-ERK antibodies (lower panels).
There are four principal tyrosine phosphorylation sites in Cbl, with one (Y371) located within the linker region between the SH2 domain and the Ring finger domain and the other three (Y700, Y731, and Y774) located at the C-terminal end of the proline-rich region. Previous studies have indicated that phosphorylation of Y371 plays an important role in the suppression of EGF receptor signaling by Cbl (18). While phosphorylation of Y731 results in association with the p85 subunit of PI 3-kinase, phosphorylation of Y700 and Y774 has been shown to provide docking sites for the SH2 domain of Crk (2, 7, 8, 22). Truncation of the C-terminal 206 amino acid residues of Cbl (Cbl/1-700) greatly reduced but did not abolish its tyrosine phosphorylation (Fig. 3A), suggesting that phosphorylation occurred on tyrosines at both N-terminal and C-terminal portions of the protein.
Since none of the Y-to-F single mutations, Y371F, Y700F, Y731F, and Y774F, resulted in a great decrease in the tyrosine phosphorylation of the protein (data not shown), we generated constructs with different combinations of Y-to-F mutations in Cbl. As shown in Fig. 3B, double mutations of Y700F and Y774F dramatically diminished the tyrosine phosphorylation of Cbl compared to wild-type Cbl, and an additional mutation at Y371 resulted in a further significant decrease in the extent of phosphorylation. Meanwhile, the inclusion of a Y-to-F mutation at site 731 in either the double mutant Y700F/Y774F or the triple mutant Y371F/Y700F/Y774F did not appear to have an additional effect on the phosphorylation state of Cbl. We therefore concluded that tyrosines 371, 700, and 774, but not tyrosine 731, are the major phosphorylation sites utilized by the insulin receptor in the presence of APS. This is consistent with our finding that APS-facilitated Cbl phosphorylation increases Crk binding (Fig. 2B) but has no effect on the association of PI 3-kinase with Cbl.
APS is specifically associated with CAP in vivo and in vitro.
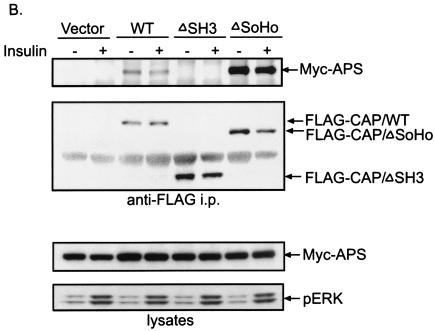
CAP is a bifunctional adapter protein with three SH3 domains and a region of similarity to the peptide sorbin (SoHo domain). Like APS, CAP is expressed in differentiated adipocytes but not in precursor fibroblasts (35). Overexpression of a CAPΔSoHo mutant in 3T3-L1 adipocytes blocks the insulin-stimulated tyrosine phosphorylation of Cbl (15), implying a critical role of CAP in the regulation of Cbl tyrosine phosphorylation by insulin in these metabolically active cells. To address the relative roles of APS and CAP in the control of Cbl phosphorylation, we performed cotransfection experiments to determine whether these two proteins, one containing three SH3 domains and the other containing two putative proline-rich regions, specifically interact with each other. We overexpressed FLAG-tagged CAP, CAPΔSH3, or CAPΔSoHo in COS-1 cells along with Myc-APS and evaluated the coimmunoprecipitation of Myc-APS with these variants of FLAG-CAP. Immunoblotting of cell lysates indicated that Myc-APS and different FLAG-CAP proteins were expressed at comparable levels. Myc-APS was found to interact specifically with the wild-type CAP. However, anti-FLAG immunoprecipitation showed that deletion of the three SH3 domains in CAP completely abolished the coprecipitation of Myc-APS (Fig. 4A). Similar results were also obtained by using 3T3-L1 adipocytes for transfection (Fig. 4B). Treatment of cells with insulin had no effect on the association of FLAG-CAP with Myc-APS (Fig. 4B). These data indicate that CAP and APS are capable of forming complexes via the interaction between the SH3 domain(s) of CAP and the proline-rich region(s) of APS.
FIG.4.
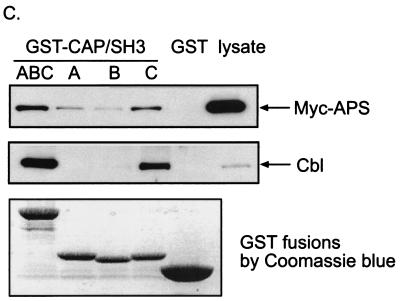
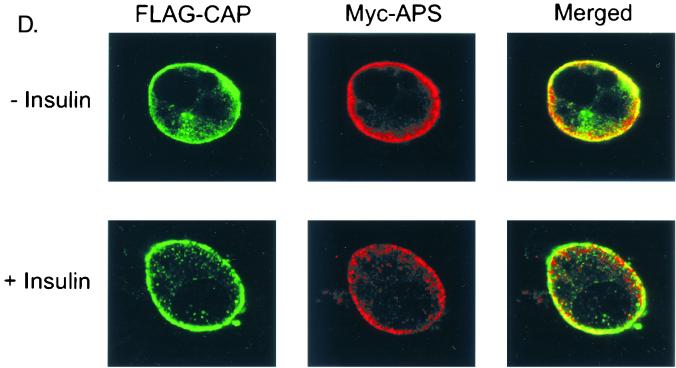
In vivo and in vitro interactions between APS and CAP. (A) COS-1 cells were cotransfected with Myc-APS and FLAG-tagged wild-type CAP (WT), CAPΔSH3, or CAPΔSoHo. Cells were lysed 20 h after transfection, and immunoprecipitation (i.p.) was performed using an anti-FLAG antibody. Immunoprecipitates were analyzed by anti-Myc, anti-Cbl, and anti-FLAG immunoblotting (upper panels). Myc-APS was detected in cell lysates by immunoblotting with an anti-Myc antibody (lower panels). (B) 3T3-L1 cells were used for the same experiment as described for panel A except that cells were treated with or without 100 nM insulin for 2 min prior to the preparation of lysates. (C) Myc-APS was overexpressed in COS-1 cells. Lysates were incubated with glutathione-Sepharose-bound GST or GST-CAPSH3 fusion proteins. Precipitates were subjected to immunoblotting with anti-Myc or anti-Cbl antibodies. The GST fusion proteins used were stained with Coomassie blue. (D) 3T3-L1 adipocytes were electroporated with Myc-tagged wild-type APS or FLAG-CAP and allowed to recover for 30 h. The cells were treated with or without 100 nM insulin for 2 min. FLAG-CAP and Myc-APS were visualized by indirect immunofluorescent staining.
To evaluate the effect of APS on the binding of Cbl to CAP, COS-1 cells were also transfected with different FLAG-CAP constructs in the absence of Myc-APS (Fig. 4A). Lysates were precipitated with anti-FLAG antibodies. Anti-Cbl immunoblotting showed that the amount of endogenous Cbl coprecipitated with wild-type CAP was significantly greater when Myc-APS was not coexpressed (Fig. 4A). Moreover, deletion of the SoHo domain of CAP greatly decreased the binding affinity of CAP for Cbl in COS-1 cells, while concomitantly enhancing the association of CAP with Myc-APS in both COS-1 cells and 3T3-L1 adipocytes (Fig. 4A and B). These results suggest that the interaction regions in CAP for Cbl and APS overlap with each other, and the SoHo domain may serve a critical role in deciding the binding specificity of CAP for APS and Cbl.
To define the interaction between CAP and APS in more detail, each of the SH3 domains of CAP was expressed as a GST fusion protein in bacteria either alone or together. Lysates prepared from COS-1 cells transfected with Myc-APS were incubated with these GST fusion proteins and GST protein alone as a control. Coomassie blue staining demonstrated that comparable amounts of GST fusion proteins were included in all assays. The bound proteins were immunoblotted with anti-Myc and anti-Cbl antibodies. As shown in Fig. 4C, a GST fusion protein containing all three SH3 domains bound specifically to both Myc-APS and endogenous Cbl. Each of three SH3 domains of CAP was able to bind Myc-APS, with the carboxyl-terminal SH3 domain (SH3C) displaying the highest affinity for binding. On the other hand, SH3C was the only SH3 domain showing detectable association with endogenous Cbl, in agreement with our previous report (33).
Previous studies have found that ectopically expressed APS is present throughout the cytoplasm in NIH 3T3 cells under basal conditions and is translocated to the plasma membrane and especially the peripheral ruffling regions upon PDGF stimulation (45). To determine the cellular compartment in which APS is localized and whether APS and CAP are colocalized in 3T3-L1 adipocytes, cells were cotransfected with Myc-APS and FLAG-CAP followed by double immunofluorescence labeling. Transfected cells were treated with or without insulin, fixed, and incubated with anti-Myc and anti-FLAG antibodies followed by labeling with complementary fluorescence-conjugated secondary antibodies. Under basal conditions, FLAG-CAP and Myc-APS were both predominantly confined to the plasma membrane, with lower levels detected in cytoplasm (Fig. 4D). Insulin treatment of cells did not change this rim-like distribution of either protein. These results suggest that APS and CAP display a similar gross cellular distribution that is independent of insulin stimulation.
Overexpression of APS in 3T3-L1 adipocytes inhibits insulin-stimulated tyrosine phosphorylation of Cbl and subsequent Cbl-Crk interaction.
Insulin stimulates the tyrosine phosphorylation of Cbl in 3T3-L1 adipocytes (36). In these cells c-Cbl has also been presumed to be a substrate of the Src family kinase Fyn as well as the insulin receptor (25). We thus evaluated the effect of overexpression of wild-type APS or the Y618F mutant of APS on insulin-stimulated tyrosine phosphorylation of Cbl in 3T3-L1 adipocytes. Both forms of Myc-APS protein were expressed at similar levels, as detected in the cell lysates by immunoblotting with anti-Myc antibodies. Expression of either form of APS had no effect on the ability of insulin to stimulate the phosphorylation of ERK that is activated as a consequence of a well-characterized kinase pathway (Fig. 5A). Immunoprecipitation of Cbl from vector-transfected cells followed by antiphosphotyrosine immunoblotting showed that insulin produced an eightfold increase in Cbl tyrosine phosphorylation. Expression of either form of APS led to a significant reduction in insulin-stimulated tyrosine phosphorylation of Cbl, with the Y618F mutant producing a stronger effect than the wild-type protein (Fig. 5A). Considering that the average efficiency of transfection in these experiments was 60%, the observed reduction in Cbl phosphorylation suggests that the expression of APS/Y618F almost completely inhibited the tyrosine phosphorylation of Cbl in response to insulin. Therefore, tyrosine phosphorylation of Cbl is facilitated by APS and catalyzed by the insulin receptor. In addition, exposure of 3T3-L1 adipocytes to the specific Src kinase inhibitor PP2 was found to have no effect on insulin-stimulated tyrosine phosphorylation of Cbl (data not shown), excluding Fyn as a Cbl kinase that works downstream of the insulin receptor.
FIG. 5.
Overexpression of APS blocks insulin-stimulated tyrosine phosphorylation of Cbl and Crk-Cbl interaction. 3T3-L1 adipocytes were transfected with vector alone, Myc-tagged wild-type APS (Myc-APS/WT), or Myc-APS/Y618F. (A) Following treatment with or without 100 nM insulin for 2 min, cells were lysed and immunoprecipitation (i.p.) was performed with an anti-Cbl antibody. Immunoprecipitates were analyzed by antiphosphotyrosine and anti-Cbl immunoblotting (upper panels). Cell lysates were immunoblotted with anti-Myc and anti-phospho-ERK antibodies (lower panels). (B) Following treatment with or without 100 nM insulin for 2 min, cells were lysed and immunoprecipitation was performed with an anti-Crk antibody. Immunoprecipitates were analyzed by anti-Cbl and anti-Crk immunoblotting (upper panels). Cell lysates were immunoblotted with anti-Myc, anti-Cbl, and anti-phospho-ERK antibodies (lower panels).
Stimulation of the tyrosine phosphorylation of Cbl by insulin generates a specific docking site for Crk (36). To determine whether APS inhibits the insulin-induced association of Crk with Cbl, we immunoprecipitated endogenous Crk from lysates of APS-transfected cells. Anti-Crk immunoblotting showed that the same amount of Crk was present in all immunoprecipitates (Fig. 5B). Expression of endogenous Cbl was not affected by the expression of either wild-type APS or the Y618F mutant of APS. While insulin robustly produced the coprecipitation of Cbl with Crk in the absence of ectopic APS expression, expression of either wild-type APS or APS/Y618F significantly inhibited insulin-stimulated association of Cbl with Crk (Fig. 5B). This result is in good agreement with the inhibitory effect of APS expression on Cbl tyrosine phosphorylation (Fig. 5A).
It is thought that in order for adapter proteins to facilitate signaling, the relative concentrations of the components must be in balance. For example, knockout of the p85 subunit of PI 3-kinase enhances the activation of Akt in liver, presumably due to the fact that endogenous p85 exists in excess over both p110 catalytic subunits and IRS proteins (39). Overexpression of Myc-APS enhanced insulin-stimulated tyrosine phosphorylation of coexpressed HA-Cbl. On the other hand, when expressed alone, Myc-APS inhibited the phosphorylation of the endogenous Cbl in response to insulin. The difference may result from the relative excess of Myc-APS over endogenous Cbl and insulin receptor. Therefore, we next examined the effects of various amounts of APS expression on insulin-stimulated Cbl phosphorylation. The amount of Myc-APS DNA (300 μg) used in the experiment described in Fig. 5 was set as the maximum. Expression of wild-type APS dose-dependently inhibited the tyrosine phosphorylation of Cbl in response to insulin. At the lowest concentration of APS (30 μg), an increase in Cbl phosphorylation was observed. In contrast, there was no enhancement of Cbl phosphorylation observed with transfection of APS/Y618F. The expression of APS/Y618F dose-dependently inhibited Cbl phosphorylation, with an efficacy more potent than that observed for the wild-type protein (Fig. 6). Thus, the APS/Y618F mutant behaves as a dominant negative mutant through interfering with the endogenous APS, whereas wild-type APS inhibits only at higher levels of expression, presumably due to the disruption of the stoichiometric relationship with its partners such as Cbl and the insulin receptor.
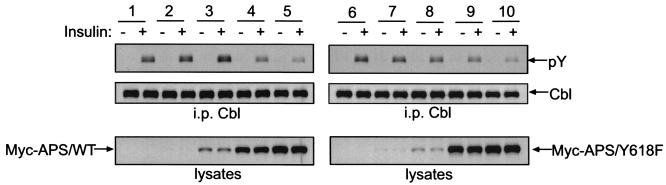
FIG. 6.
Dose-dependent effects of ectopic APS expression on insulin-stimulated Cbl phosphorylation. 3T3-L1 adipocytes were cotransfected with various amount of vector DNA and Myc-tagged wild-type APS (Myc-APS/WT) or Myc-APS/Y618F. Lanes 1 and 6, 300 μg of vector plus 0 μg of APS; lanes 2 and 7, 285 μg of vector plus 15 μg of APS; lanes 3 and 8, 270 μg of vector plus 30 μg of APS; lanes 4 and 9, 200 μg of vector plus 100 μg of APS; lanes 5 and 10, 0 μg of vector and 300 μg of APS. Following treatment with or without 100 nM insulin for 2 min, cells were lysed and immunoprecipitation (i.p.) was performed with an anti-Cbl antibody. Immunoprecipitates were analyzed by antiphosphotyrosine and anti-Cbl immunoblotting (upper panels). Cell lysates were immunoblotted with anti-Myc antibody (lower panels).
Overexpression of APS in 3T3-L1 adipocytes attenuates insulin-stimulated GLUT4 translocation.
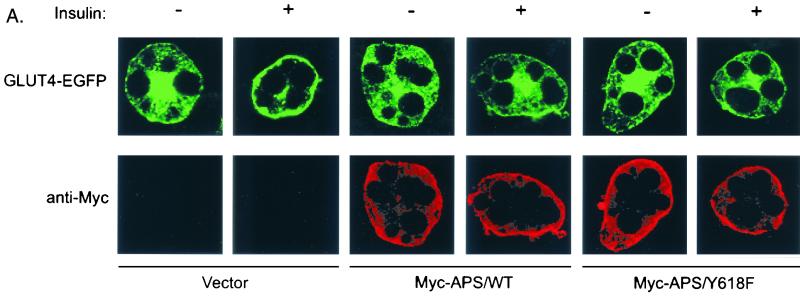
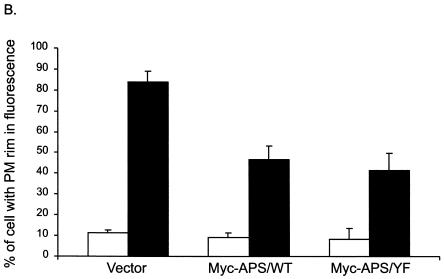
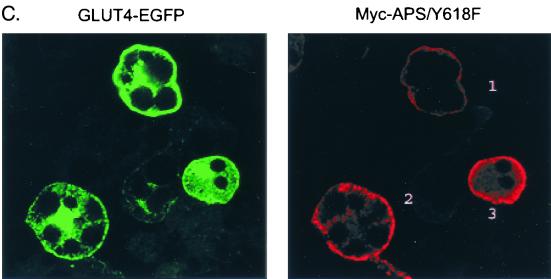
We have recently observed that the CAP/Cbl pathway is necessary for the translocation of GLUT4 to the plasma membrane in response to insulin (3, 15). To further confirm the essential role of APS in the CAP/Cbl pathway, we next examined whether expression of the APS/Y618F mutant would have a dominant negative effect on insulin-stimulated GLUT4 translocation. 3T3-L1 adipocytes were electroporated with vector alone, Myc-tagged wild-type APS, or Myc-tagged APS/Y618F together with a construct encoding an enhanced green fluorescent protein fusion of GLUT4 (GLUT4-EGFP). The amounts of Myc plasmid and GLUT4-EGFP plasmid were first used at a 3:1 ratio to ensure that cells transfected with GLUT4-EGFP would also highly express Myc-APS. The transfected cells were treated with or without insulin, and the localization of GLUT4-EGFP was examined by fluorescence microscopy. The coexpressed Myc-APS protein was visualized by indirect immunofluorescence. As expected, insulin stimulated the translocation of GLUT4-EGFP to the plasma membrane in empty vector-transfected cells. In contrast, expression of either Myc-tagged wild-type APS or Myc-tagged APS/Y618F protein resulted in marked inhibition of insulin-stimulated GLUT4-EGFP translocation (Fig. 7A). Quantitation of these data demonstrated that the number of the cells displaying rim green fluorescence in response to insulin decreased by 48 and 51%, respectively, with the coexpression of wild-type APS or APS/Y618F (Fig. 7B). To determine whether the blockade of insulin-stimulated GLUT4-EGFP translocation correlates with the level of Myc-APS, we electroporated the cells with Myc-APS/Y618F and GLUT4-EGFP at 1:1 ratio. A representative field that includes GLUT4EGFP-transfected cells expressing different amount of Myc-APS/Y618F protein is shown is Fig. 7C. In cell 1, low-level expression of the APS mutant protein did not have any visible effect on insulin-stimulated translocation of GLUT4-EGFP. However, higher expression of APS/Y618F in cells 2 and 3 almost completely inhibited the appearance of rim fluorescence in response to insulin. Thus, Myc-APS/Y618F inhibited insulin-stimulated GLUT4-EGFP translocation in an expression level-dependent pattern. Taken together, the results indicate that APS operates upstream of the CAP/Cbl pathway in the stimulation of GLUT4 translocation by insulin.
FIG. 7.
Overexpression of APS blocks insulin-stimulated GLUT4 translocation to the plasma membrane. (A) 3T3-L1 adipocytes were electroporated with 100 μg of GLUT4-EGFP plus 300 μg of vector, Myc-tagged wild-type APS (Myc-APS/WT), or Myc-APS/Y618F and allowed to recover for 30 h. The cells were treated with or without 100 nM insulin for 30 min. Cells were fixed, and fluorescence was visualized by confocal microscopy. GLUT4-EGFP was visualized by direct fluorescence, and Myc-APS was visualized by indirect immunofluorescence. These are representative images of middle sections of cells obtained from three independent experiments. (B) Numbers of GLUT4-EGFP-transfected cells displaying visually detectable plasma membrane (PM) rim fluorescence. These data were obtained by blind counting of more than 80 cells from three independent experiments. Error bars indicate standard deviations. (C) 3T3-L1 adipocytes were electroporated with 100 μg of GLUT4-EGFP plus 100 μg of Myc-APS/Y618F. After treatment with 100 nM insulin for 30 min, cells were fixed and fluorescence was visualized by confocal microscopy as described for panel A. This is a representative field of a middle section of cells obtained from three independent experiments.
DISCUSSION
Following receptor engagement, the formation of tyrosine phosphorylation-dependent multimeric complexes involving adapter and effector proteins plays an important role in signal transduction. Upon cell activation through different stimuli, the adapter protein Cbl becomes phosphorylated on tyrosine residues, producing its interaction with SH2-containing signaling molecules (2, 7, 8, 22). In differentiated adipocytes, insulin stimulates Cbl tyrosine phosphorylation and its association with the adapter protein Crk (36). Moreover, insulin induces translocation of the Cbl/CAP complex to lipid rafts, where CAP directly binds to the hydrophobic protein flotillin. The insulin-dependent localization of phospho-Cbl to subdomains of the plasma membrane results in the generation of signaling pathways involved in GLUT4 translocation (3). One major pathway involves the activation of TC10 by C3G that is complexed with Cbl/Crk (6).
In adipocytes Cbl phosphorylation seems to be catalyzed by the insulin receptor rather than by Src family tyrosine kinases such as Fyn. Previous studies have also shown that Cbl is able to associate with tyrosine-phosphorylated EGF and PDGF receptors (5, 9, 23). However, there has been no evidence that the insulin receptor binds to Cbl directly upon insulin stimulation, indicating the requirement of an adapter protein to assist in the complex formation. CAP does not appear to target Cbl directly to the insulin receptor for phosphorylation. In fact, coexpression of CAP with Cbl in 3T3-L1 cells had no effect on Cbl phosphorylation in response to insulin. Therefore, we suspected that 3T3-L1 adipocytes might express a separate adapter protein that is directly involved in the tyrosine phosphorylation of Cbl. Experiments described here revealed that tyrosine-phosphorylated APS is the adapter that couples Cbl to the insulin receptor for phosphorylation. Like CAP, APS is expressed primarily in skeletal muscle, heart, and adipose tissue and in differentiated 3T3-L1 adipocytes (28). We demonstrate that APS facilitates tyrosine phosphorylation of Cbl on tyrosines 371, 700, and 774. This phosphorylation event is required for the recruitment of Crk to the CAP/Cbl complex and for the subsequent activation of GLUT4 translocation.
APS belongs to the Lnk adapter protein family, which includes Lnk and SH2-B. Members of this protein family contain a PH domain, an SH2 domain, a C-terminal tyrosine-containing motif, and several proline-rich motifs. While Lnk functions predominantly in T-cell receptor (TCR) activation (12), APS and SH2-B are involved in signaling by various receptors for growth factors such as insulin, insulin-like growth factor 1, PDGF, and nerve growth factor (31, 32). Although both APS and SH2-B are substrates of numerous tyrosine kinases, APS is the preferential target for the insulin receptor kinase in differentiated adipocytes (16, 28). Expression of APS in CHO cells overexpressing insulin receptor was previously shown to induce a rapid ubiquitination of the insulin receptor upon ligand binding, presumably through coupling Cbl to the receptor (1). Our results demonstrate that Y371 is one of the major sites for APS-facilitated phosphorylation in response to insulin. Phosphorylation of Y371 was suggested to play an important role in the suppression of EGF receptor signaling by Cbl (18). However, preliminary data indicate that expression of APS has no effect on insulin receptor stability or MAP kinase signaling in 3T3-L1 adipocytes, suggesting that this may not be a mechanism for the down regulation of the insulin receptor in physiologically relevant insulin-responsive cells. Indeed, earlier studies indicated that the insulin receptor does not undergo degradation via the ubiquitination pathway and, furthermore, that down regulation of the insulin receptor is a slow process (12 to 24 h) compared to that of EGF and PDGF receptors (29).
The inhibition of tyrosine phosphorylation of endogenous Cbl and GLUT4 translocation through overexpression of the APS/Y618F dominant negative mutant is consistent with its role as an upstream signaling intermediate in the CAP/Cbl pathway. However, it was somewhat surprising that low- and high-level expression of the wild-type APS protein conferred opposite biological effects. It is possible that a multiprotein complex is present in 3T3-L1 adipocytes required for the coordinate regulation of Cbl phosphorylation by insulin, in which APS is responsible for the direct targeting of Cbl to the insulin receptor. The expression levels of proteins involved in such complexes are usually delicately regulated in cells. Therefore, excess expression of a single component like APS may simply interfere with the stoichiometry of the complex formation, resulting in the inhibition instead of enhancement of Cbl phosphorylation. Indeed, it was demonstrated in a recent study that APS was capable of forming multimeric structures and may exist in large protein complexes in vivo (31). Similarly, ectopic overexpression of Lnk in Jurkat T cells caused inhibition of anti-CD3-induced TCR activation of NF-AT transcription activity (19). On the other hand, earlier studies suggest a positive role of endogenous Lnk in TCR signaling, because upon TCR activation Lnk becomes tyrosine phosphorylated and signals to the PI 3-kinase, phospholipase Cγ1, and Ras pathways through its multifunctional tyrosine phosphorylation site (12). In addition, overexpression of either MP1 or JIP1, two adapter proteins that promote the activation of different MAP kinases, has been shown to have inhibitory effects on the respective kinase pathways in transfected cells (38, 44).
Our study indicates that APS and CAP are localized to the plasma membrane and specifically associate with each other independently of insulin stimulation. The physiological significance of the APS-CAP interaction remains unclear. Deletion of the SoHo domain of CAP increased binding of APS but decreased binding of Cbl. Therefore, the SoHo domain appears to play an important role in controlling the overall conformation of CAP and the binding specificity of its SH3 domains for different proteins. Since the SoHo domain interacts with the lipid raft protein flotillin, it would be interesting to determine whether and how the association with flotillin influences the interaction of CAP with APS and Cbl, respectively.
Since the C-terminal SH3 domain of CAP is used by both APS and Cbl for binding, it seems unlikely that CAP would directly bridge the formation of a ternary complex including APS and Cbl. However, a multimeric complex containing these proteins may still be present, considering the possible involvement of additional molecules. Although APS is sufficient to facilitate Cbl phosphorylation in a coexpression experiment where both APS and Cbl exist in excess amounts, CAP seems to serve an indispensable role in the control of tyrosine phosphorylation of endogenous Cbl in vivo (3, 15, 34). We have previously shown that expression of a CAP mutant deficient in flotillin/lipid raft association (ΔSoHo) inhibits insulin-stimulated tyrosine phosphorylation of Cbl and the translocation of GLUT4 in 3T3-L1 adipocytes (15). This apparent dominant negative effect may result from mistargeting of Cbl or APS and is consistent with a model in which Cbl phosphorylation by the insulin receptor is coordinated by a complex of proteins rather than directed by APS alone. In this regard, it is important to note that APS, CAP, and Cbl all are large multifunctional adapters, each of which possesses the ability to bind multiple proteins simultaneously.
REFERENCES
- 1.Ahmed, Z., B. J. Smith, and, T. S. Pillay. 2000. The APS adapter protein couples the insulin receptor to the phosphorylation of c-Cbl and facilitates ligand-stimulated ubiquitination of the insulin receptor. FEBS Lett. 475:31-34. [DOI] [PubMed] [Google Scholar]
- 2.Andoniou, C. E., C. B. Thien, and W. Y. Langdon. 1996. The two major sites of cbl tyrosine phosphorylation in abl-transformed cells select the crkL SH2 domain. Oncogene 12:1981-1989. [PubMed] [Google Scholar]
- 3.Baumann, C. A., V. Ribon, M. Kanzaki, D. C. Thurmond, S. Mora, S. Shigematsu, P. E. Bickel, J. E. Pessin, and A. R. Saltiel. 2000. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 407:202-207. [DOI] [PubMed] [Google Scholar]
- 4.Blake, T. J., M. Shapiro, H. C. I. Morse, and W. Y. Langdon. 1991. The sequences of the human and mouse c-cbl proto-oncogenes show v-cbl was generated by a large truncation encompassing a proline-rich domain and a leucine zipper-like motif. Oncogene 6:653-657. [PubMed] [Google Scholar]
- 5.Bowtell, D. D., and W. Y. Langdon. 1995. The protein product of the c-cbl oncogene rapidly complexes with the EGF receptor and is tyrosine phosphorylated following EGF stimulation. Oncogene 11:1561-1567. [PubMed] [Google Scholar]
- 6.Chiang, S.-H., C. A. Baumann, M. Kanzaki, D. C. Thurmond, C. L. Neudauer, I. G. Macara, J. E. Pessin, and A. R. Saltiel. 2001. Insulin-stimulated GLUT4 translocation requires the CAP-dependent but PI 3-kinase independent activation of the small GTP-binding protein TC10. Nature 410:944-948. [DOI] [PubMed] [Google Scholar]
- 7.Deckert, M., C. Elly, A. Altman, and Y. C. Liu. 1998. Coordinated regulation of the tyrosine phosphorylation of Cbl by Fyn and Syk tyrosine kinases. J. Biol. Chem. 273:8867-8874. [DOI] [PubMed] [Google Scholar]
- 8.Feshchenko, E. A., W. Y. Langdon, and A. Y. Tsygankov. 1998. Fyn, Yes and Syk phosphorylation sites in c-Cbl map the same tyrosine residues that become phosphorylated in activated T cells. J. Biol. Chem. 273:8323-8331. [DOI] [PubMed] [Google Scholar]
- 9.Galisteo, M. L., I. Dikic, A. G. Batzer, W. Y. Langdon, and J. Schlessinger. 1995. Tyrosine phosphorylation of the c-cbl proto-oncogene protein product and association with epidermal growth factor (EGF) receptor upon EGF stimulation. J. Biol. Chem. 270:20242-20245. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Guzman, M., E. Larsen, and K. Vuori. 2000. The proto-oncogene c-Cbl is a positive regulator of Met-induced MAP kinase activation: a role for the adaptor protein Crk. Oncogene 19:4058-4065. [DOI] [PubMed] [Google Scholar]
- 11.Grishin, A., S. Sinha, V. Roginskaya, M. J. Boyer, J. Gomez-Cambronero, S. Zuo, T. Kurosaki, G. Romero, and, S. J. Corey. 2000. Involvement of Shc and Cbl-PI3-kinase in Lyn-dependent proliferative signaling pathways for G-CSF. Oncogene 19:97-105. [DOI] [PubMed] [Google Scholar]
- 12.Huang, X., Y. Li, K. Tanaka, K. G. Moore, and J. I. Hayashi. 1995. Cloning and characterization of Lnk, a signal transduction protein that links T-cell receptor activation signal to phospholipase Cr1, Grb2, and phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA 92:11618-11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joazeiro, C. A., and A. M. Weissman. 2000. RING finger proteins: mediators of ubiquitin ligase activity. Cell 102:549-552. [DOI] [PubMed] [Google Scholar]
- 14.Joazeiro, C. A., S. S. Wing, H. Huang, J. D. Leverson, T. Hunter, and Y. C. Liu. 1999. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science 286:309-312. [DOI] [PubMed] [Google Scholar]
- 15.Kimura, A., C. A. Baumann, S. H. Chiang, and, A. R. Saltiel. 2001. The sorbin homology domain: a motif for the targeting of proteins to lipid rafts Proc. Natl. Acad. Sci. USA 98:9098-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotani, K., P. Wilden, and T. S. Pillay. 1998. SH2-Balpha is an insulin-receptor adapter protein and substrate that interacts with the activation loop of the insulin-receptor kinase. Biochem. J. 335:103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langdon, W. Y., J. W. Hartley, S. P. Klinken, S. K. Ruscetti, and H. C. Morse III. 1989. v-cbl, an oncogene from a dual-recombinant murine retrovirus that induces early B-lineage lymphomas. Proc. Natl. Acad. Sci. USA 86:1168-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levkowitz, G., H. Waterman, S. A. Ettenberg, M. Katz, A. Y. Tsygankov, I. Alroy, S. Lavi, K. Iwai, Y. Reiss, A. Ciechanover, S. Lipkowitz, and Y. Yarden. 1999. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sil-1. Mol. Cell 4:1029-1040. [DOI] [PubMed] [Google Scholar]
- 19.Li, Y., X. He, J. Schembri-King, S. Jakes, and J. Hayashi. 2000. Cloning and characterization of human Lnk, an adaptor protein with pleckstrin homology and Src homology 2 domains that can inhibit T cell activation. J. Immunol. 164:5199-5206. [DOI] [PubMed] [Google Scholar]
- 20.Liu, J., J. Wu, C. Oliver, S. Shenolikar, and D. L. Brautigan. 2000. Mutations of the serine phosphorylated in the protein phosphatase-1-binding motif in the skeletal muscle glycogen-targeting subunit. Biochem. J. 346:77-82. [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, Y. C., and A. Altman. 1998. Cbl: complex formation and functional implications. Cell. Signal. 10:377-385. [DOI] [PubMed] [Google Scholar]
- 22.Liu, Y.-C., C. Elly, W. Y. Langdon, and A. Altman. 1997. Ras-dependent, Calif.2+-stimulated activation of nuclear factor of activated T cells by a constitutively active Cbl mutant in T cells. J. Biol. Chem. 272:168-173. [DOI] [PubMed] [Google Scholar]
- 23.Lupher, M. L., Jr., C. E. Andoniou, D. Bonita, S. Miyake, and H. Band. 1998. The c-Cbl oncoprotein. Int. J. Biochem. Cell Biol. 30:439-444. [DOI] [PubMed] [Google Scholar]
- 24.Lupher, M. L., Jr., N. Rao, N. L. Lill, C. E. Andoniou, S. Miyake, E. A. Clark, B. Druker, and H. Band. 1998. Cbl-mediated negative regulation of the Syk tyrosine kinase. A critical role for Cbl phosphotyrosine-binding domain binding to Syk phosphotyrosine 323. J. Biol. Chem. 273:35273-35281. [DOI] [PubMed] [Google Scholar]
- 25.Mastick, C. C., and A. R. Saltiel. 1997. Insulin-stimulated tyrosine phosphorylation of caveolin is specific for the differentiated adipocyte phenotype in 3T3-L1 cells. J. Biol. Chem. 272:20706-20714. [DOI] [PubMed] [Google Scholar]
- 26.Meng, F., and C. A. Lowell. 1998. A beta1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO J. 17:4391-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Min, J., S. Okada, M. Kanzaki, J. S. Elmendorf, K. J. Coker, B. P. Ceresa, L. J. Syu, Y. Noda, A. R. Saltiel, and J. E. Pessin. 1999. Synip: a novel insulin-regulated syntaxin 4-binding protein mediating GLUT4 translocation in adipocytes. Mol. Cell 3:751-760. (Erratum, 4:192.) [DOI] [PubMed] [Google Scholar]
- 28.Moodie, S. A., J. Alleman-Sposeto, and T. A. Gustafson. 1999. Identification of the APS protein as a novel insulin receptor substrate. J. Biol. Chem. 274:11186-11193. [DOI] [PubMed] [Google Scholar]
- 29.Mori, S., L. Claesson-Welsh, Y. Okuyama, and Y. Saito. 1995. Ligand-induced polyubiquitination of receptor tyrosine kinases Biochem. Biophys. Res. Commun. 213:32-39. [DOI] [PubMed] [Google Scholar]
- 30.Ojaniemi, M., S. S. Martin, F. Dolfi, J. M. Olefsky, and K. Vuori. 1997. The proto-oncogene product p120cbl links c-Src and phosphatidylinositol 3′-kinase to the integrin signaling pathway. J. Biol. Chem. 272:3780-3787. [DOI] [PubMed] [Google Scholar]
- 31.Qian, X., and D. D. Ginty. 2001. SH2-B and APS are multimeric adapters that augment TrkA signaling. Mol. Cell. Biol. 21:1613-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian, X., A. Riccio, Y. Zhang, and D. D. Ginty. 1998. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron. 21:1017-1029. [DOI] [PubMed] [Google Scholar]
- 33.Ribon, V., R. Herrera, B. K. Kay, and A. R. Saltiel. 1998. A role for CAP, a novel, multifunctional Src homology 3 domain-containing protein in formation of actin stress fibers and focal adhesions. J. Biol. Chem. 273:4073-4080. [DOI] [PubMed] [Google Scholar]
- 34.Ribon, V., J. H. Johnson, H. S. Camp, and A. R. Saltiel. 1998. Thiazolidinediones and insulin resistance: peroxisome proliferator-activated receptor gamma activation stimulates expression of the CAP gene. Proc. Natl. Acad. Sci. USA 95:14751-14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribon, V., J. A. Printen, N. G. Hoffman, B. K. Kay, and A. R. Saltiel. 1998. A novel, multifunctional c-Cbl binding protein in insulin receptor signaling in 3T3-L1 adipocytes. Mol. Cell. Biol. 18:872-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribon, V., and A. R. Saltiel. 1997. Insulin stimulates tyrosine phosphorylation of the proto-oncogene product of c-Cbl in 3T3-L1 adipocytes. Biochem. J. 324:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin, C. S., E. Lai, and O. M. Rosen. 1977. Acquisition of increased hormone sensitivity during in vitro adipocyte development. J. Biol. Chem. 252:3554-3557. [PubMed] [Google Scholar]
- 38.Schaeffer, H. J., A. D. Catling, S. T. Eblen, L. S. Collier, A. Krauss, and M. J. Weber. 1998. MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science 281:1668-1671. [DOI] [PubMed] [Google Scholar]
- 39.Terauchi, Y., Y. Tsuji, S. Satoh, H. Minoura, K. Murakami, A. Okuno, K. Inukai, T. Asano, Y. Kaburagi, K. Ueki, H. Nakajima, T. Hanafusa, Y. Matsuzawa, H. Sekihara, Y. Yin, J. C. Barrett, H. Oda, T. Ishikawa, Y. Akanuma, I. Komuro, M. Suzuki, K. Yamamura, T. Kodama, H. Suzuki, and T. Kadowaki. 1999. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase. Nat. Genet. 21:230-235. [DOI] [PubMed] [Google Scholar]
- 40.Tezuka, T., H. Umemori, H. Fusaki, T. Yagi, M. Takada, T. Kurosaki, and T. Yamamoto. 1996. Physical and functional association of the cbl protooncogene product with an src-family protein tyrosine kinase, p53/56lyn, in the B cell antigen receptor-mediated signaling. J. Exp. Med. 183:675-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thien, C. F., and W. Y. Langdon. 2001. Cbl: many adaptations to regulate protein tyrosine kinases. Nat, Rev. Mol. Cell. Biol. 2:294-305. [DOI] [PubMed] [Google Scholar]
- 42.Ueno, H., K. Sasaki, H. Honda, T. Nakamoto, T. Yamagata, K. Miyagawa, K. Mitani, Y. Yazaki, and H. Hirai. 1998. c-Cbl is tyrosine-phosphorylated by interleukin-4 and enhances mitogenic and survival signals of interleukin-4 receptor by linking with the phosphatidylinositol 3′-kinase pathway. Blood 91:46-53. [PubMed] [Google Scholar]
- 43.Wang, Y., Y. G. Yeung, W. Y. Langdon, and E. R. Stanley. 1996. c-cbl is transiently tyrosine-phosphorylated, ubiquitinated, and membrane-targeted following CSF-1 stimulation of macrophages. J. Biol. Chem. 271:17-20. [DOI] [PubMed] [Google Scholar]
- 44.Whitmarsh, A. J., J. Cavanagh, C. Tournier, J. Yasuda, and R. J. Davis. 1998. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science 281:1671-1674. [DOI] [PubMed] [Google Scholar]
- 45.Yokouchi, M., T. Wakioka, H. Sakamoto, H. Ysaukawa, S. Ohtsuka, A. Sasaki, M. Ohtsubo, M. Valius, A. Inoue, S. Komiya, and A. Yoshimura. 1999. APS, an adaptor protein containing PH and SH2 domains, is associated with the PDGF receptor and c-Cbl and inhibits PDGF-induced mitogenesis. Oncogene 18:759-767. [DOI] [PubMed] [Google Scholar]