Abstract
PTEN is mutated at high frequency in many primary human cancers and several familial cancer predisposition disorders. Activation of AKT is a common event in tumors in which the PTEN gene has been inactivated. We previously showed that deletion of the murine Pten gene in embryonic stem (ES) cells led to increased phosphatidylinositol triphosphate (PIP3) accumulation, enhanced entry into S phase, and better cell survival. Since PIP3 controls multiple signaling molecules, it was not clear to what degree the observed phenotypes were due to deregulated AKT activity. In this study, we mutated Akt-1 in Pten−/− ES cells to directly assess the role of AKT-1 in PTEN-controlled cellular processes, such as cell proliferation, cell survival, and tumorigenesis in nude mice. We showed that AKT-1 is one of the major downstream effectors of PTEN in ES cells and that activation of AKT-1 is required for both the cell survival and cell proliferation phenotypes observed in Pten−/− ES cells. Deletion of Akt-1 partially reverses the aggressive growth of Pten−/− ES cells in vivo, suggesting that AKT-1 plays an essential role in PTEN-controlled tumorigenesis.
The PTEN (phosphatase and tensin homologue deleted on chromosome 10) tumor suppressor gene is the first phosphatase identified as being frequently mutated or deleted somatically in various human cancers (22, 23, 39). One of the primary targets of PTEN is lipid phosphatidylinositol triphosphate (PIP3), a direct product of phosphatidylinositol (PI) 3-kinase (27, 40). Loss of PTEN function, either in murine embryonic stem (ES) cells or in human cancer cell lines, results in accumulation of PIP3 and activation of its downstream effectors, such as AKT/protein kinase B (15, 36) and Rac-1/Cdc42 (24). Activation of AKT stimulates cell cycle progression by down-regulation of p27, a major inhibitor for G1 cyclin-dependent kinases (36). Activated AKT/protein kinase B is also a well-characterized survival factor in vitro and prevents cells from undergoing apoptosis by inhibiting the proapoptotic factors BAD (12, 36) and caspase 9 (8) as well as the nuclear translocation of Forkhead transcription factors (20, 38).
The cellular proto-oncogene c-Akt was first identified as a homologue of the viral oncogene v-akt, which is capable of transforming mink lung cells (CCL64) in culture and causing T-cell lymphomas when injected into newborn mice (34, 35). The Akt gene encodes a serine/threonine protein kinase that is an essential downstream target of PI 3-kinase and propagates the cell survival signals of growth factors (14). It also exerts its function in glucose metabolism and other cellular processes. Amplification of the AKT gene or increase of its expression was shown previously to be associated with a number of malignant diseases, including adenocarcinomas of the breast, prostate, and intestines (9, 29, 34). Frequent chromosomal alterations in the AKT region have also been observed in patients with lymphomas and leukemia (5). In addition, activation of AKT was observed in many primary human tumor samples or cell lines carrying PTEN mutations and in cancers developed in Pten+/− mice (16, 37). Therefore, up-regulation of AKT appears to be a common event in cancers, especially in PTEN-controlled tumorigenesis.
AKT activity is largely regulated at the posttranslational level and is dependent on its pleckstrin homology (PH) domain (4). Binding of the lipid second-messenger PIP3 to the PH domain results in translocation of the AKT molecule to the vicinity of the membrane and its subsequent phosphorylation by the PIP3-dependent protein kinases, PDK1 and PDK2 (3). Phosphorylation of AKT is required for its function. Thus, AKT activity is positively regulated by PI 3-kinase, which phosphorylates PIP2 to produce PIP3 (15). On the other hand, PTEN exerts its function by dephosphorylating PIP3 at position 3, negatively regulating AKT activity (27). To date, three mammalian Akt genes have been identified, Akt-1, Akt-2, and Akt-3, or PKBα, PKBβ, and PKBγ (10). The three Akt genes share high homology in both the nucleic acid and peptide sequences. Akt-1 is ubiquitously expressed in every tissue, and Akt-2 expression is high in the skeletal muscles, the β-islet cells of the pancreas, and the brown fat, while the expression of Akt-3 is more restricted, to the heart and placenta, with low expression in a limited number of other tissues (2, 28).
To investigate the molecular basis for the tumor suppressor function of PTEN, we previously deleted the murine Pten gene in ES cells (36). We demonstrated that PTEN acts as a negative regulator for the PI 3-kinase/AKT signaling pathway, which controls and coordinates two major cellular processes, cell cycle progression and cell death. In this study, we further delineated the PTEN-regulated pathways by deleting one of the Akt genes, Akt-1, from Pten-null ES cells. Through this genetic approach, we demonstrated that AKT-1 up-regulation is essential for the tumorigenic phenotype observed for Pten-null ES cells.
MATERIALS AND METHODS
Generation of Pten−/−;Akt−/− ES cell lines.
Genomic DNA clones corresponding to the Akt-1 locus were isolated from an isogenic 129(J1) genomic library. The targeting vector contains the PGKpuropA cassette flanked by an 8.0-kb NotI-BamHI fragment (5′ arm) and a 2.1-kb XhoI fragment (3′ arm) in the backbone of pBluescript vector. Linearized targeting plasmid (50 μg) was electroporated into 2 × 107 Pten-null ES cells. After 3 days of puromycin selection (1.5 μg/ml), drug-resistant ES clones were isolated and expanded. Genomic DNAs were prepared for Southern blot analysis with an external probe. Recombinant clones were further confirmed with an internal probe and a 5′-arm probe. To obtain ES clones homozygous for the Akt-1 deletion, heterozygous ES clones were subjected to a higher-level puromycin selection (70 μg/ml) for 3 days. At the end of selection, new feeder layers were replated and the puromycin concentration was reduced to 4 μg/ml to allow surviving ES clones to recover. The optimal concentration and the time course of puromycin selection were predetermined experimentally to allow efficient killing. Homozygous deletion was confirmed by Southern blot analysis as well as Western blot analysis.
Cells and tissue culture.
ES cells were cultured on irradiated fibroblast feeder layers as described previously (36). Briefly, feeder cells were plated 1 day prior to the seeding of ES cells. ES cells were cultured in knockout Dulbecco modified Eagle medium (Gibco BRL) containing 15% fetal calf serum supplemented with nonessential amino acids, glutamic acids (100 mM), leukemia inhibitory factor (1,000 U/ml), and β-mercaptoethanol. All cell lines were cultured under this growth condition.
For biochemical analysis, ES cells were passaged twice without feeder cells at a density of 107 cells/10-cm-diameter plate. Two days later, these cultures were further passaged at a one-to-three without feeders to minimize feeder contamination and continuously cultured for 9 to 10 h, at which point cells were either treated with Colcemid (Sigma) for cell cycle studies or lysed for Western blot analysis. For insulin-like growth factor 1 (IGF-1) and serum stimulation, cells were washed and split continuously cultured in serum-free medium (in the presence of leukemia inhibitory factor) for 16 h and then stimulated with a final concentration of 1 μg of IGF-1 (a generous gift from Amgen)/ml or 15% serum for 10 or 30 min before protein analysis.
For cell cycle analysis, ES cells were preshaken, washed, and then treated with Colcemid (0.06 g/ml) for 4 h as described previously (33, 36). After mitotic shake-off, ES cells were cultured under normal growth conditions without feeders to allow reentry into the cell cycle. Cells were collected at each time point, and cell cycle profiles were studied by fluorescence-activated cell sorting (FACS) analysis. Briefly, cells were trypsinized, washed two times with cold phosphate-buffered saline (PBS), and then permeabilized overnight with 50% ethyl alcohol in PBS. ES cells were then washed with PBS, and DNA was stained with propidium iodine (40 μg/ml) in PBS containing RNase A (100 μg/ml) for 30 min at 37°C. The cells were then stored at 4°C and later analyzed by FACS.
Growth of ES cells under reduced-serum conditions.
ES cells (5 × 104 cells/well) were plated on top of feeder layers in 24-well tissue culture plates with medium containing 15% serum. One day later, cells were washed and changed to serially reduced serum conditions. Medium was changed daily, and cell numbers were counted 4 days after the initiation of serum starvation. For caspase inhibitor treatment, 4 μM caspase inhibitor cocktail (set III in dimethyl sulfoxide; Calbiochem) was added to ES cell cultures 1 day after serum starvation, and cell numbers were counted 4 days after the initiation of serum starvation. As a control, ES cells were treated with carrier dimethyl sulfoxide for the same period of time.
Growth competition assay.
Double mutant (Pten−/−;Akt−/−) ES cells (2 × 105) were cocultivated in a six-well plate with equal numbers of wild-type (WT) or Pten−/− ES cells. When they reached 80 to 90% confluency, cultures were passaged at the original density (4 × 105 cells) as passage 2. Cultures were continuously cultured for a total of six passages, and genomic DNAs were prepared from each passage. Southern analysis was then performed to determine the contributions from each cell type.
Generation of teratomas with ES cells.
Different ES clones were expanded and grafted onto immunoincompetent nude mice. Briefly, 5 × 105 ES cells were injected subcutaneously onto the backs of nude mice. Tumor formation was then observed at different time points. Tumor volume was obtained by measuring the parameter and the height of the tumors with three independent measurements. Before reaching 1.5 cm in diameter (according to National Institutes of Health guidelines), tumors were harvested and analyzed histologically.
Antibodies and Western blot analysis.
Cell lysate preparation and Western blot analysis were carried out as described previously (36). Antibody to the AKT-1 PH domain was purchased from Upstate Biotechnology. Three additional antibodies to the AKT C-terminal peptide were purchased from Upstate Biotechnology, Cell Signaling, and Santa Cruz Biotechnology. Antibodies specific for ERK, p27KIP1 (sc-528), cyclin D1 (R-124), p21CIP1/WAF1 (sc-397), cyclin A, and mouse cyclin E (sc-481) were obtained from Santa Cruz Biotechnology. Antibodies for PTEN and phosphospecific antibodies for AKT, FKHR/FKHRL1, BAD, S6 ribosomal protein, and GSK-3α/β were from Cell Signaling. Antibodies for actin and vinculin were provided by Sigma.
Statistical analysis.
Analysis of variance was applied to all data subjected to statistical analysis, and Fisher's least significant difference test was used to determine the statistical differences among the groups. The paired Student t test was used on teratoma data to determine the differences between different groups. A P value of ≤0.05 was considered statistically significant.
RESULTS
Targeted deletion of Akt-1 via homologous recombination in WT and Pten-null ES cells.
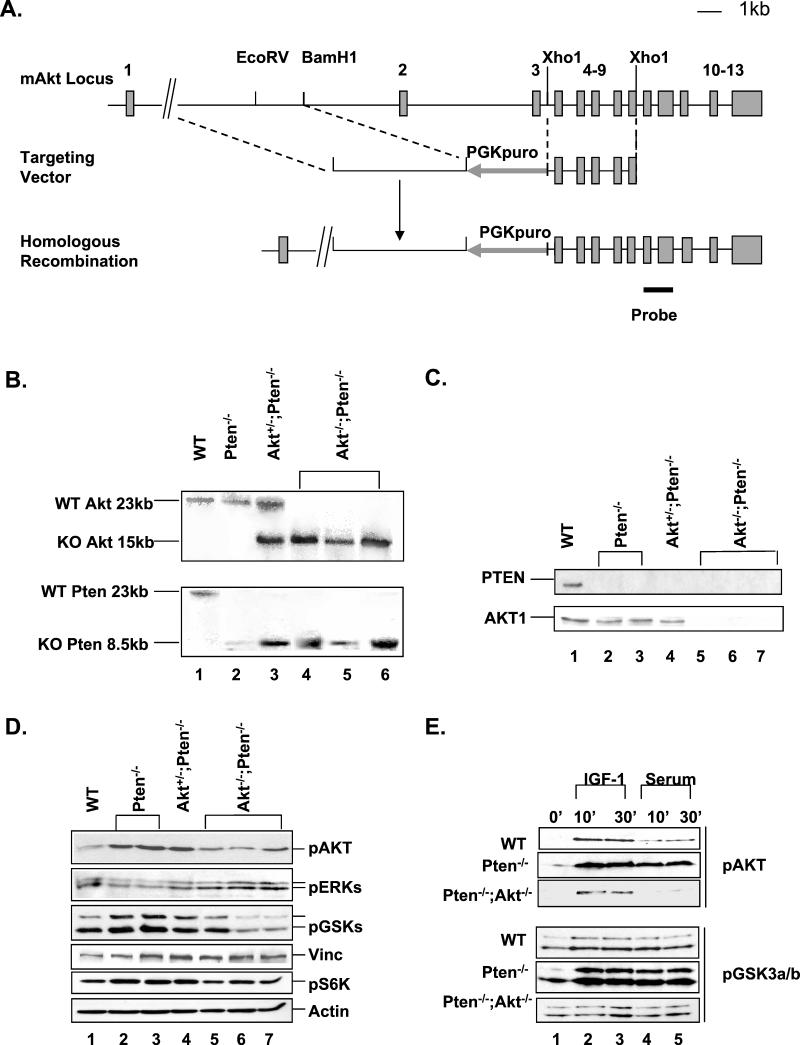
To delete the Akt-1 gene, a targeting vector was constructed to delete the entire PH domain of the AKT-1 molecule (Fig. 1A). Since AKT membrane recruitment and subsequent activation depend on its PH domain, this construct will result in inactivation of AKT-1. The knockout construct carries a puromycin-selective cassette so that Akt-1 targeting events can be selected when introduced into neomycin-resistant Pten−/− ES cells. Three out of 400 Puror colonies were identified as homologous recombinants by Southern analyses, and homozygous clones were later obtained by culturing heterozygous ES cells in a higher concentration of puromycin. Using this strategy, we obtained clones carrying the following genotypes: WT, Pten−/−, Akt+/−;Pten−/−, and Akt−/−;Pten−/−. All clones were genotyped by Southern blot analysis and confirmed by Western blot analysis using antibodies for PTEN and AKT-1 PH domain (Fig. 1B and C). Furthermore, no proteins could be detected by using three independent antibodies to the C-terminal domains of the AKT-1 molecule, suggesting that no stable truncated protein could be produced from the targeted alleles (Fig. 1C). Two independent Pten−/− clones and three independent Pten−/−;Akt−/− clones were used for further analysis.
FIG.1.
Inactivation of mouse Akt-1 gene. (A) A restriction map of the mouse Akt-1 locus is shown in the top panel with exons depicted. The middle panel shows the targeting vector with exons 2 and 3 deleted and replaced with a PGKpuro cassette. The bottom panel is the predicted recombinant harboring the deletions with the position of the 3′ external probe indicated below it. (B) Southern blot analysis of the Pten and Akt-1 locus. (C) Western analysis of PTEN and AKT-1 levels in the indicated ES cell clones. Cell lysates (20 μg) were run on a polyacrylamide gel and Western blotted with antibodies for PTEN (top) and AKT-1 (bottom). (D) Western blot analyses of phospho-AKT, ERK, GSK-3α/β, and p70S6 kinase status in indicated ES clones. Cell lysates (20 μg) were run on a polyacrylamide gel and Western blotted with antibodies for the different molecules. The same blots were reblotted with vinculin (Vinc) or actin as loading controls. (E) Serum-starved ES cells were treated with either 1 μg of IGF-1/ml or 15% fetal calf serum for 10 or 30 min. Cell lysates were analyzed for phospho-AKT (upper panels) and GSK-3 (lower panels) status. The same blots were also reblotted with actin as loading controls (data not shown). pGSK3a/b, pGSK3α/β.
As Akt-2 and Akt-3 may still compensate for the loss of Akt-1, we checked overall AKT activity with a phosphospecific antibody which can recognize all three phosphorylated forms of AKT. Our results indicated that, by deleting Akt-1 plus Pten (Fig. 1D, lanes 5 to 7), we were able to bring up-regulated AKT activity in Pten−/− cells (Fig. 1D, lanes 2 and 3) back to near-WT levels (Fig. 1D, lanes 5 to 7 versus lane 1). We also evaluated mitogen-activated protein kinase (Fig. 1D) and Jun kinase and β-catenin (data not shown) activities and were not able to find significant and consistent differences among different genotypes. On the other hand, as a direct consequence of changes in AKT activity, the phosphorylation status of GSK-3α and -3β, two of the substrates of AKT serine/threonine kinases, was affected in parallel, i.e., high in Pten−/− cells (Fig. 1D, lanes 2 and 3) but reduced in Pten−/−;Akt−/− clones (Fig. 1D, lane 1 versus lanes 5 to 7). In addition, AKT-1 deletion also appeared to diminish the enzymatic activity of p70S6 kinase as measured by the phosphorylation status of its product, S6 ribosomal protein (Fig. 1D). The changes in the phosphorylation of S6 ribosomal protein paralleled the phosphorylation status of AKT: S6 ribosomal protein was hyperphosphorylated in the Pten−/− (Fig. 1D, lanes 2 and 3) and Pten−/−;Akt+/− (lane 4) cell lines, while the phosphorylation was diminished in the Pten−/−;Akt−/− clones (Fig. 1D, lanes 5 to 7). Thus, AKT-1 seems to play an important role in GSK-3α Ser21 and GSK-3β Ser9 phosphorylation as well as in the activation of a further downstream target, p70S6 kinase in the ES cells, suggesting that AKT-1 may be essential, among the three AKT members, in controlling the PI 3-kinase/AKT axis in ES cells.
We then analyzed whether AKT and GSK-3 kinases could be further activated by growth factor stimulation in the absence of AKT-1. As shown in Fig. 1E, the effects of serum on AKT phosphorylation were significantly less than those of IGF-1 in all cell lines. AKT was hyperphosphorylated in Pten−/− cells, even under serum starvation conditions (0′), which could be further induced by IGF-1 (lanes 3 and 4) or serum stimulation (lanes 4 and 5). WT cells did respond to IGF-1 stimulation and to a lesser extent to serum treatment (Fig. 1E, top panel). Pten−/−;Akt−/− cells were almost unresponsive to serum treatment, and the remaining phosphorylation observed after IGF-1 stimulation could be due to AKT-2 and AKT-3. Similarly, IGF-1 and serum stimulation induced hyperphosphorylation of GSK-3α/β in Pten−/− cells but had less effect on WT cells and almost no effect on Pten−/−;Akt−/− cells (Fig. 1E, lower panels). These results indicated that both AKT phosphorylation and activation of the downstream target of AKT are severely impaired in Pten−/−;Akt−/− cells.
Akt-1 deletion reverses cell survival phenotype of Pten-null ES cells.
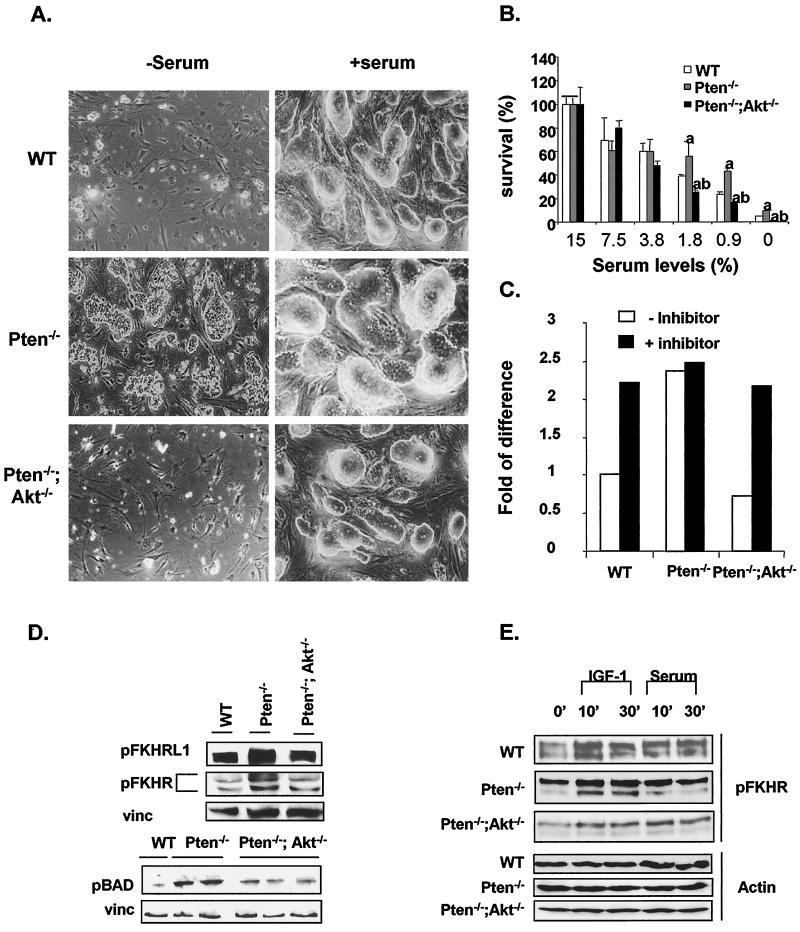
In order to assess the role of AKT-1 in PTEN/PI 3-kinase-mediated cell survival, ES clones were cultured with reduced serum concentrations. Consistent with our previous observations, Pten−/− cells demonstrated significantly enhanced cell survival compared to the WT cells under serum-starved conditions (Fig. 2A, upper and middle left panels). Deletion of Akt-1 as well as Pten completely reversed the survival phenotype seen for Pten−/− cells (Fig. 2A, lower left panel). Interestingly, Pten−/−;Akt−/− ES cells were even more sensitive to serum concentrations than were the WT cells: significantly more cell death was observed for Pten−/−;Akt−/− cultures than for WT cell cultures when serum concentrations were reduced to less than 3.8% (Fig. 2B).
FIG. 2.
Cell survival potential and apoptotic signals in ES clones carrying Pten/Akt deletions. (A) Survival of ES cells in response to serum starvation. ES cells (5 × 104) were seeded the day before serum withdrawal. Cells were cultured without serum for 4 days. Cell survival was observed under a light microscope and quantified by counting the number of surviving cells under each culture condition. Photographs are representative of three independent experiments. Left panels, cells grown under serum-free condition. Right panels, cells grown under normal growth conditions (15% serum). (B) Quantification of cell numbers at different serum concentrations. Data presented are means ± standard errors of the means of n = 3. Bars designated with the letter “a” are statistically significantly different from the WT bars at the same serum concentration (P ≤ 0.05). Bars designated with the letter “b” are statistically significantly different from the Pten−/− bars at the same serum concentration (P ≤ 0.05). (C) Quantification of cell numbers with or without caspase inhibitor cocktail. Data are presented as fold increases over cell numbers in the WT cultures in the absence of the inhibitor cocktail. (D) Western blot analyses of the phosphorylation status of FKHRL1, FKHR, and BAD under normal culture conditions. Total cell lysates (20 μg) were run on a polyacrylamide gel and Western blotted with antibodies for phosphor-FKHR/FKHRL1 and BAD. The same blots were also blotted with vinculin (vinc) as controls. (E) Serum-starved ES cells were treated with either 1 μg of IGF-1/ml or 15% fetal calf serum for 10 or 30 min. Cell lysates were then analyzed for phospho-FKHR status by Western blotting (upper panels). The same blots were also blotted with actin as controls (lower panels).
In order to determine whether the observed cell death was due to apoptosis, we treated the different ES cell lines with a cocktail of caspase inhibitors in the absence of serum. As shown in Fig. 2C, such treatment led to increased cell survival of WT and Pten−/−;Akt−/− cells to levels similar to those for Pten−/− cells.
The cell survival phenotype prompted us to study three of the downstream targets of AKT, BAD and two Forkhead transcriptional factors, FKHR and FKHRL1, which were known to regulate apoptosis through different mechanisms. FKHRL1 and FKHR phosphorylation were up-regulated in Pten−/− cells but reduced in Pten−/−;Akt−/− ES cells (Fig. 2D). Similarly, phosphorylation of BAD was returned to the WT level by deleting Akt-1 as well as Pten (Fig. 2D), suggesting that AKT-1 plays a major role in the PI 3-kinase-mediated cell survival pathway. In addition, IGF-1- and serum-induced FKHRL1 and FKHR phosphorylation was diminished in the Akt-1-deletion cell lines compared to that in WT and Pten−/− cells (Fig. 2E). Interestingly, IGF-1 and serum had similar effects on FKHR phosphorylation, which is quite different from their effects on AKT phosphorylation (Fig. 1E), suggesting that FKHR phosphorylation can be regulated by both AKT-dependent and AKT-independent pathways.
Akt-1 deletion reverses the cell growth phenotype of Pten-null ES cells.
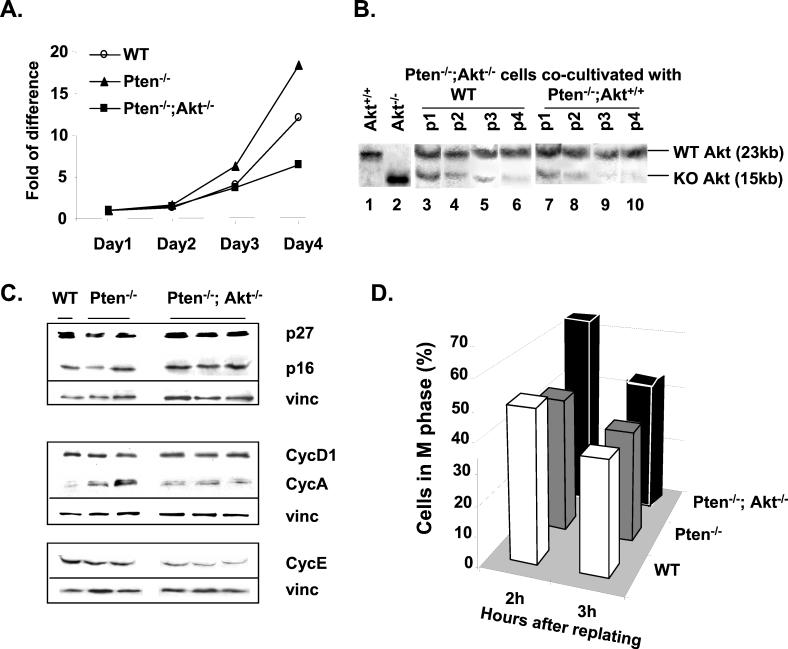
In addition to cell survival, we also noticed differences in cell growth. The growth rate of Pten−/−;Akt−/− cells appeared to be lower than that of WT and Pten−/− ES cells (Fig. 3A). In order to determine changes in the growth properties of Pten−/−;Akt−/− cells, we cocultivated Pten−/−;Akt−/− cells with equal numbers of either WT or Pten−/− ES cells. As shown in Fig. 3B, middle panel, Pten−/−;Akt−/− cells grew slower than did the WT cells, and upon passage 4, most of the culture was taken over by the WT cells (as judged by the ratio of WT band to mutant band). This is even more apparent when Pten−/−;Akt−/− cells were cocultured with Pten−/− cells (Fig. 3B, right panel). The ratio of WT band to mutant band increased significantly on passage 2, and Pten−/− cells became the dominant cell type in the cocultures between passages 2 and 3. Thus, deletion of Akt-1 not only reverses the growth advantage seen in Pten−/− cells but further decreases the cell proliferation rate to a level lower than that of the WT cells.
FIG. 3.
Growth potential, cell cycle profile, and analysis of cell cycle regulators in Pten/Akt clones. (A) Growth curve of indicated ES clones. (B) Growth competition assay. Pten−/−;Akt−/− ES cells were cocultivated with equal numbers of WT or Pten−/− cells. DNA was extracted from the cocultures after each passage and was analyzed with Southern analysis to determine the ratio of WT Akt allele (23 kb) to mutant Akt allele (15 kb). Lanes 1 and 2, WT and Pten−/−;Akt−/− cells cultured alone. Lanes 3 to 6, Pten−/−;Akt−/− ES cells cocultured with WT cells at different passages (p1 to p4). Lanes 7 to 10, Pten−/−;Akt−/− ES cells cocultured with Pten−/− cells at different passages (p1 to p4). KO, knockout. (C) Western blot analysis of cell cycle regulators. Total cell lysates (20 μg) were separated on sodium dodecyl sulfate-polyacrylamide gels and blotted with cell cycle inhibitors p27KIP1 and p16INK1 as well as cyclins A, D1, and E. Vinculin (vinc) was used on each blot as a loading control. (D) Cell cycle analysis. ES cells were synchronized at M phase with Colcemid treatment followed by replating in Colcemid-free medium to allow reentry into the cell cycle. FACS analysis was performed on each clone at different time points (2 and 3 h), and the percentages of cells remaining in M phase are shown here.
As a result of Akt-1 deletion, the levels of several cyclins and G1/S cell cycle inhibitors were altered. As reported in a previous paper (36), the level of p27 is down-regulated in Pten−/− cells but returned to near-WT level in Pten−/−;Akt−/− cells (Fig. 3C, top panel). Cyclin A levels were changed inversely (Fig. 3C, middle panel). Levels of cyclin A were elevated in Pten−/− cells and yet decreased when Akt was deleted in addition to Pten. The cyclin D1 level, on the other hand, was not affected by deleting Pten or Akt-1 (Fig. 3C, top two panels). Cyclin E levels were similar between Pten−/− and WT cells, and yet Akt-1 deletion resulted in a diminished cyclin E protein level (Fig. 3C, bottom lane). Levels of other cell cycle inhibitors were either not detectable (p15 and p21) or not changed (p16).
Akt-1 deletion delays M-phase exit in Pten−/−;Akt−/− ES cells.
Overexpression of Drosophila melanogaster PTEN (dPTEN) inhibits cell cycle progression at mitosis and promotes cell death during eye development (17). Overexpression of PTEN in mammalian cells, on the other hand, induces G1/S cell cycle arrest and cell death (21). Our previous studies also suggested that deletion of Pten in the ES cells leads to an accelerated G1/S cell cycle transition (36). To address the possible role of PTEN and PI 3-kinase/AKT in other phases of the cell cycle, we tested the rates of exit of ES cells from mitotic block. ES cells with different genotypes were treated with the M-phase blocker Colcemid, and synchronized mitotic cells were collected after mitotic shake-off (36). Cells were then cultured in Colcemid-free fresh medium, and their cell cycle status was analyzed by FACS 2 and 3 h after the release, corresponding to the beginning of S phase in ES cells (36). Approximately 45 and 38% of cells remained in M phase at 2 and 3 h, respectively, when WT ES cells were released following mitotic arrest (Fig. 3D, open bars). In contrast, Pten−/− cells showed an increased M-phase exit rate (40 and 35% at 2 and 3 h, respectively). To our surprise, the Pten−/−;Akt−/− cells remained in M phase much longer than did WT cells. Almost 70 and 50% of the Pten−/−;Akt−/− cells remained in M phase 2 and 3 h after release from mitotic block, respectively. Thus, AKT may play a critical role in cell cycle progression at M phase. The downstream targets of AKT at M phase are not yet defined. However, the recent identification of Myt1 as one of the AKT substrates suggested that AKT may be a crucial kinase involved in the phosphorylation-dephosphorylation cascades occurring though G2/M phase of the cell cycle (31).
Akt-1 deletion partially reverses the tumorigenesis phenotype of Pten-null ES cells.
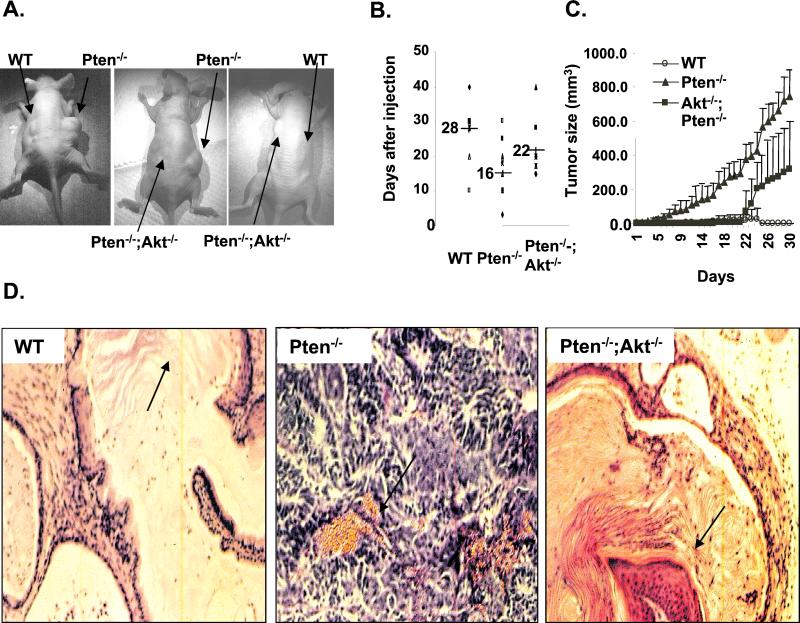
To validate the functional significance of our findings, we injected the Pten−/−;Akt−/− ES clones into nude mice to directly assess their in vivo tumorigenic potential. ES cells are known to produce benign, usually well-differentiated teratomas in this setting. However, teratomas generated from Pten−/− cells were highly proliferative, less differentiated, and well vascularized (Fig. 4D, middle panel). In contrast, deletion of Akt-1 as well as Pten significantly delayed the onset, as well as decreased the aggressive growth rate, of Pten−/− teratomas (Fig. 4B and C). The Pten−/−;Akt−/− tumors were also significantly smaller than the Pten−/− tumors (Fig. 4C). Histological analysis indicated that Pten−/−;Akt−/− tumors were more differentiated and less vascularized than Pten−/− tumors, similar to tumors generated by WT ES cells (Fig. 4D, left and right panels). Thus, deletion of Akt-1 can partially reverse the tumorigenic phenotype of Pten−/− ES cells to a level at least intermediate between those of Pten−/− and WT tumors.
FIG. 4.
Deleting Akt-1 in addition to Pten delays the onset of teratoma formation and decreases the sizes of tumors formed by Pten−/− ES cells. WT, Pten−/−;Akt+/+, and Pten−/−;Akt−/− cells (5 × 106) were injected subcutaneously onto the backs of immunoincompetent nude mice. Each mouse was injected bilaterally with cells carrying a different genotype. Teratoma formations were observed and recorded at indicated time points. (A) Examples of teratomas growing on the backs of nude mice. (B) Onset of teratomas formed from different ES clones. Each dot represents one animal; n = 9 in each group. The average time for tumor appearance is indicated as a line with the actual number beside it. (C) Progression of teratomas formed by different ES clones. The day of tumor detection is set as day 0. Data are presented as means ± standard errors of the means of n = 5. (D) Histology analysis of teratomas harvested from the nude mice. Left panel, tumors formed by injecting WT ES cells showed well-differentiated tissues with skin characteristics (arrow); middle panel, teratomas formed by Pten−/− ES cells are highly proliferative, less differentiated, and well vascularized (arrow); right panel, tumors formed by injection of Pten−/−;Akt−/− ES cells. Deletion of Akt-1 in addition to Pten partially reverses the aggressive growth of Pten−/− teratomas. The arrow indicates the more differentiated keratinized skin, similar to tumors generated by WT ES cells (left panel).
DISCUSSION
This study focuses on the PTEN/AKT pathway, which selectively targets the serine/threonine protein kinase AKT-1. Using a genetic approach, we studied the function of AKT-1 in an isogenic system. We showed that AKT-1 is the main downstream effector of PTEN and is required for both the cell survival and cell proliferation phenotype observed for the Pten-knockout ES cells.
Two interesting observations were made during our initial study of Pten−/− ES cells (36). First, the Pten−/− cells were more resistant to serum starvation-induced cell death than were the WT cells. Second, the Pten−/− ES cells had shorter doubling times and earlier S-phase entry than the WT cells, accompanied by up-regulation of AKT and down-regulation of G1 cell cycle inhibitor p27. In this study, we demonstrated that deleting Akt-1 alters both the cell survival and growth advantage of Pten−/− cells and that AKT-1 modulates the levels of proapoptotic factor BAD, transcriptional factor FHKR, and cell cycle inhibitor p27, as well as cyclins A and E.
Previous in vitro studies indicated that AKT activation might play an essential role in tumorigenesis, especially in PTEN mutation-associated tumor formation. However, most of these studies were conducted with human tumor cell lines which may carry other mutations or abnormalities (1, 6, 18, 26, 32). Using a genetic approach, we generated isogenic cell lines so that the function of AKT in PTEN-controlled signaling pathways and tumorigenesis could be directly assessed. Our data indicated that AKT plays a significant role in regulating PTEN-mediated cell growth and cell survival. First, we demonstrated that deleting Akt-1 reversed the cell survival phenotype in Pten−/− cells. Furthermore, deleting both alleles of Akt-1 appeared to have additional effects, and mutated cells were more sensitive to serum starvation-induced cell death than were the WT cells. Second, we showed that the Akt-1 knockout was able to reverse the growth advantage of Pten−/− cells. Not only did the Pten−/−; Akt−/− clones lose the ability to compete with Pten−/− cells, but they also failed to compete with the WT cells in growth competition assays, though to a lesser extent. This could be due to the presence of AKT-1 in the WT cells, which could respond to growth factor stimulation. The fact that overall AKT phosphorylation in response to IGF-1 or serum treatment is significantly diminished in the Pten−/−;Akt−/− cells, compared to that in WT cells, supported this idea. Therefore, the cell survival and cell proliferation signals are propagated more properly in the WT cells than in the Pten−/−;Akt−/− cells. It is also possible that pathways other than PI 3-kinase-dependent signals may also regulate AKT and be responsible for the additional effects seen with double-knockout cells.
Evidence from in vivo teratoma formation supported the in vitro data. ES cells can produce benign teratomas in nude mice regardless of Pten deletion. However, the onset as well as the progression of tumor formation is much earlier and more aggressive in Pten-null teratomas than in WT teratomas. By deleting Akt-1, we were able not only to reduce the size of Pten−/− teratomas but also to delay the onset of tumor formation. However, unlike the in vitro cell survival and cell growth experiments, deletion of Akt-1 as well as Pten did not completely reverse the aggressive growth phenotype of Pten−/− cells. Indeed, both the tumor size and onset of tumor development in Pten−/−;Akt−/− cells are intermediate between those of Pten−/− and WT cells, suggesting that other molecules could have taken over once the tumor development reached a certain stage. Teratomas contain many different cell types. Even though our data suggested that AKT-1 is crucial for cell survival and cell growth in ES cells, it is not surprising that the growth of the teratoma could escape the control of AKT-1, e.g., by being compensated for by AKT-2 and AKT-3 since the three AKTs were differentially regulated in different cell types (30). Together, the in vitro and in vivo characteristics of Pten−/−; Akt−/− ES clones strongly implied that AKT-1 is the main downstream effector of PTEN, although other targets may also play roles in PTEN-mediated tumorigenesis.
We demonstrated that overall phosphorylation of AKT appeared to be diminished to the WT level when Akt-1 is deleted, suggesting that possible compensation by Akt-2 and Akt-3 is minimal and is responsible only for baseline AKT phosphorylation when PTEN is intact in ES cells. This was further confirmed with treatment with IGF-1 and serum, which failed to induce an overall AKT phosphorylation in Pten−/−;Akt−/− cells similar to that in the WT or Pten−/− cells. This is not surprising, since the different AKTs are differentially activated by growth factors in a cell-type-dependent manner (30). The diminished phosphorylation of GSK-3 and S6R in the Akt-1-deletion cells further implicated AKT-1 as a general regulator for the PI 3-kinase pathway and a primary target of PTEN regulation.
Activation of AKT leads to the phosphorylation of several downstream targets. One of the unequivocal consequences of deleting Akt-1 is diminished phosphorylation of GSK-3α/β, which by itself may play a significant role in cell growth as well as other cellular processes (19). We demonstrated in this paper that deleting Akt-1 resulted in significantly diminished phosphorylation levels of GSK-3α/β in the Pten−/−;Akt−/− cells compared to those in the Pten−/− cells. Deletion of Akt-1 as well as Pten also changes the activities of several cell-death-related molecules, such as BAD and FKHR. BAD is the proapoptotic factor (12) which associates with the antiapoptosis molecule Bcl-2 to prevent Bcl-2-mediated antiapoptosis function. Phosphorylation of BAD by AKT prevents this association and shifts the apoptosis signal to antiapoptosis, thereby promoting cell survival (11). We demonstrated that BAD phosphorylation is mainly dependent on AKT-1. When Akt-1 is deleted from Pten−/− ES cells, BAD phosphorylation is brought down to the basal level. Among the other AKT targets are the Forkhead transcriptional factors, such as FKHR/FKHRL1 and AFX (7, 13, 20). Recent studies of both Drosophila and mammalian cells have firmly established Forkhead family members as substrates of AKT. FKHR/FKHRL1 phosphorylation is increased in Pten−/− ES cells, but to a much lesser extent than that of BAD. When Akt-1 is deleted, all of these downstream targets of AKT are affected in parallel, including their response to growth factor stimulation, which appears to be highly dependent on the presence of AKT-1 in the cells. Although each downstream target responded to a different extent, all of them, including the ribosomal protein kinase S6K, demonstrated reduced stimulation by IGF-1 or serum compared to that for the Pten−/− cells.
Growing evidence has drawn a connection between AKT and cell cycle progression, and yet the molecular mechanisms underlying this connection were not completely elucidated. Using mouse ES cells lacking both alleles of the Pten gene, we showed that the level of G1 cell cycle inhibitor p27 was down-regulated. These ES cells also have increased AKT activity and a selective growth advantage compared to the heterozygous and WT cells (36). In addition, deletion of another PIP3 phosphatase, Ship, was also shown previously to result in activation of AKT (25). Thus, AKT activation is a key phenomenon when PIP3 levels are elevated in both Pten- and Ship-null cells. In either event, deletions of Pten or Ship generated hyperplasia and tumor phenotype (25, 36). Recent discoveries of the AKT/Forkhead pathway also indicate that AKT may regulate cell growth by regulating the key cell cycle inhibitor p27. Therefore, we investigated the role of AKT-1 at the G1/S transition, especially its role in regulating p27 and cyclin E/Cdk2. The level of change in p27 is correlated with phosphorylation of AKT in the different ES clones. Deleting Akt-1 as well as Pten was able to reverse the diminished p27 levels in Pten−/− clones. In addition, we also observed a decrease in the level of cyclin A, which controls S-phase, G2/M, and early M-phase progressions. Furthermore, when subjected to cell cycle analysis after mitotic arrest, the Pten−/−;Akt−/− ES cells demonstrated significant delays in exiting mitosis compared to the Pten−/− or WT cells. This evidence brings up another level of regulation by which AKT may exert its function dependently or independently of PTEN and deserves future investigation. The G2/M cell cycle progression depends on the dephosphorylation and activation of cyclin B-cdc2. A number of phosphorylation-dephosphorylation events happen which lead to the final dephosphorylation of cyclin B-cdc2 and M-phase progression and eventually M-phase exit. One of the kinases in this cascade, Myt1, a Wee family member, was recently identified as a substrate for AKT (31). This study may provide mechanistic support for our observation that deleting AKT-1 results in delayed M-phase progression-exit, though the exact target responsible for this phenotype needs further investigation.
In summary, two important processes in tumorigenesis, apoptosis and cell cycle control, are regulated by AKT. Deletion of Akt-1 as well as Pten was able to at least partially reverse the Pten-deletion-associated phenotypes observed in the ES cells. We demonstrated that deleting Akt-1 had a wide spectrum of impacts on Pten-knockout cells even though the deletion was not able to completely reverse the effect of the Pten knockout, partially due to other AKTs or parallel pathways which might be active during the growth of ES cells. Importantly, AKT-1 appears to regulate not only apoptotic pathways but also a number of checkpoints during the cell cycle, including G1/S transition and S-phase progression, as well as exit from mitosis.
Acknowledgments
We thank members of our laboratories for helpful comments on the manuscript and M. Blavin for editing the manuscript.
H.W. is an Assistant Investigator of the Howard Hughes Medical Institute (HHMI). B.S. is supported by HHMI and the Department of Defense (DOD) Breast Cancer Research Program (BCRP). This work is supported, in part, by the V Foundation and by a grant from DOD, PC991538 (to H.W.).
REFERENCES
- 1.Ahmed, N. N., H. L. Grimes, A. Bellacosa, T. O. Chan, and P. N. Tsichlis. 1997. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc. Natl. Acad. Sci. USA 94:3627-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altomare, D. A., K. Guo, J. Q. Cheng, G. Sonoda, K. Walsh, and J. R. Testa. 1995. Cloning, chromosomal localization and expression analysis of the mouse Akt2 oncogene. Oncogene 11:1055-1060. [PubMed] [Google Scholar]
- 3.Anderson, K. E., J. Coadwell, L. R. Stephens, and P. T. Hawkins. 1998. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr. Biol. 8:684-691. [DOI] [PubMed] [Google Scholar]
- 4.Andielkovic, M., D. R. Alessi, R. Meier, A. Fernandez, N. J. C. Lamb, M. Frech, P. Cron, P. Cohen, J. M. Lucocq, and B. A. Hemmings. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272:31515-31524. [DOI] [PubMed] [Google Scholar]
- 5.Bertness, V. L., C. A. Felix, O. W. McBride, R. Morgan, S. D. Smith, A. A. Sandberg, and I. R. Kirsch. 1990. Characterization of the breakpoint of a t(14;14)(q11.2;q32) from the leukemic cells of a patient with T-cell acute lymphoblastic leukemia. Cancer Genet. Cytogenet. 44:47-54. [DOI] [PubMed] [Google Scholar]
- 6.Brennan, P., J. W. Babbage, B. M. T. Burgering, B. Groner, K. Relf, and D. A. Cantrell. 1997. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity 7:679-689. [DOI] [PubMed] [Google Scholar]
- 7.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 8.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318-1321. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, J. Q., A. K. Godwin, A. Bellacosa, T. Taguchi, T. F. Franke, T. C. Hamilton, P. N. Tsichlis, and J. R. Testa. 1992. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc. Natl. Acad. Sci. USA 89:9267-9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffer, P. J., J. Jin, and J. R. Woodgett. 1998. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 335:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 12.del Peso, L., M. Gonzalez-Garcia, C. Page, R. Herrera, and G. Nunez. 1997. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278:687-689. [DOI] [PubMed] [Google Scholar]
- 13.del Peso, L., V. M. Gonzalez, R. Hernandez, F. G. Barr, and G. Nunez. 1999. Regulation of the forkhead transcription factor FKHR, but not the PAX3-FKHR fusion protein, by the serine/threonine kinase Akt. Oncogene 18:7328-7333. [DOI] [PubMed] [Google Scholar]
- 14.Downward, J. 1998. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10:262-267. [DOI] [PubMed] [Google Scholar]
- 15.Franke, T. F., D. R. Kaplan, L. C. Cantley, and A. Toker. 1997. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275:665-668. [DOI] [PubMed] [Google Scholar]
- 16.Haas-Kogan, D., N. Shalev, M. Wong, G. Mills, G. Yount, and D. Stokoe. 1998. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr. Biol. 8:1195-1198. [DOI] [PubMed] [Google Scholar]
- 17.Huang, H., C. J. Potter, W. Tao, D. Li, W. Brogiolo, E. Hafen, H. Sun, and T. Xu. 1999. PTEN affects cell size, cell proliferation and apoptosis during Drosophila eye development. Development 126:5365-5372. [DOI] [PubMed] [Google Scholar]
- 18.Jung, F., J. Haendeler, C. Goebel, A. M. Zeiher, and S. Dimmeler. 2000. Growth factor-induced phosphoinositide 3-OH kinase/Akt phosphorylation in smooth muscle cells: induction of cell proliferation and inhibition of cell death. Cardiovasc. Res. 48:148-157. [DOI] [PubMed] [Google Scholar]
- 19.Kim, L., and A. R. Kimmel. 2000. GSK3, a master switch regulating cell-fate specification and tumorigenesis. Curr. Opin. Genet. Dev. 10:508-514. [DOI] [PubMed] [Google Scholar]
- 20.Kops, G. J., N. D. de Ruiter, A. M. De Vries-Smits, D. R. Powell, J. L. Bos, and B. M. Burgering. 1999. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398:630-634. [DOI] [PubMed] [Google Scholar]
- 21.Li, D. M., and H. Sun. 1997. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc. Natl. Acad. Sci. USA 95:15406-15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, J., C. Yen, D. Liaw, K. Podsypanina, S. Bose, S. I. Wang, J. Puc, C. Miliaresis, L. Rodgers, R. McCombie, S. H. Bigner, B. C. Giovanella, M. Ittmann, B. Tycko, H. Hibshoosh, M. H. Wigler, and R. Parsons. 1997. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943-1947. [DOI] [PubMed] [Google Scholar]
- 23.Liaw, D., D. J. Marsh, J. Li, P. L. Dahia, S. I. Wang, Z. Zheng, S. Bose, K. M. Call, H. C. Tsou, M. Peacocke, C. Eng, and R. Parsons. 1997. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat. Genet. 16:64-67. [DOI] [PubMed] [Google Scholar]
- 24.Liliental, J., S. Y. Moon, R. Lesche, R. Mamillapalli, D. Li, Y. Zheng, H. Sun, and H. Wu. 2000. Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr. Biol. 10:401-404. [DOI] [PubMed] [Google Scholar]
- 25.Liu, Q., T. Sasaki, I. Kozieradzki, A. Wakeham, A. Itie, D. J. Dumont, and J. M. Penninger. 1999. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev. 13:786-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muise-Helmericks, R. C., H. L. Grimes, A. Bellacosa, S. E. Malstrom, P. N. Tsichlis, and N. Rosen. 1998. Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase-dependent pathway. J. Biol. Chem. 273:29864-29870. [DOI] [PubMed] [Google Scholar]
- 27.Myers, M. P., I. Pass, I. H. Batty, J. Van der Kaay, J. P. Stolarov, B. A. Hemmings, M. H. Wigler, C. P. Downes, and N. K. Tonks. 1998. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc. Natl. Acad. Sci. USA 95:13513-13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakatani, K., H. Sakaue, D. A. Thompson, R. J. Weigel, and R. A. Roth. 1999. Identification of a human Akt3 (protein kinase B gamma) which contains the regulatory serine phosphorylation site. Biochem. Biophys. Res. Commun. 257:906-910. [DOI] [PubMed] [Google Scholar]
- 29.Nakatani, K., D. A. Thompson, A. Barthel, H. Sakaue, W. Liu, R. J. Weigel, and R. A. Roth. 1999. Up-regulation of Akt3 in estrogen receptor-deficient breast cancers and androgen-independent prostate cancer lines. J. Biol. Chem. 274:21528-21532. [DOI] [PubMed] [Google Scholar]
- 30.Okano, J., I. Gaslightwala, M. J. Birnbaum, A. K. Rustgi, and H. Nakagawa. 2000. Akt/protein kinase B isoforms are differentially regulated by epidermal growth factor stimulation. J. Biol. Chem. 275:30934-30942. [DOI] [PubMed] [Google Scholar]
- 31.Okumura, E., T. Fukuhara, H. Yoshida, S. Hanada, R. Kozutsumi, M. Mori, K. Tachibana, and T. Kishimoto. 2002. Akt inhibits Myt1 in the signalling pathway that leads to meiotic G2/M-phase transition. Nat. Cell Biol. 4:111-116. [DOI] [PubMed] [Google Scholar]
- 32.Ramaswamy, S., N. Nakamura, F. Vazques, D. B. Batt, S. Perera, T. M. Roberts, and W. R. Sellers. 1999. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 96:2110-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savatier, P., S. Huang, L. Szekely, K. G. Wiman, and J. Samarut. 1994. Contrasting patterns of retinoblastoma protein expression in mouse embryonic stem cells and embryonic fibroblasts. Oncogene 9:809-818. [PubMed] [Google Scholar]
- 34.Staal, S. 1987. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc. Natl. Acad. Sci. USA 84:5034-5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staal, S. P., and J. W. Hartley. 1988. Thymic lymphoma induction by the AKT8 murine retrovirus. J. Exp. Med. 167:1259-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun, H., R. Lesche, D. M. Li, J. Liliental, H. Zhang, J. Gao, N. Gavrilova, B. Mueller, X. Liu, and H. Wu. 1999. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc. Natl. Acad. Sci. USA 96:6199-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki, A., J. L. de la Pompa, V. Stambolic, A. J. Elia, T. Sasaki, I. del Barco Barrantes, A. Ho, A. Wakeham, A. Itie, W. Khoo, M. Fukumoto, and T. W. Mak. 1998. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr. Biol. 8:1169-1178. [DOI] [PubMed] [Google Scholar]
- 38.Tang, E. D., G. Nunez, F. G. Barr, and K. L. Guan. 1999. Negative regulation of the forkhead transcription factor FKHR by Akt. J. Biol. Chem. 274:16741-16746. [DOI] [PubMed] [Google Scholar]
- 39.Teng, D. H., R. Hu, H. Lin, T. Davis, D. Iliev, C. Frye, B. Swedlund, K. L. Hansen, V. L. Vinson, K. L. Gumpper, L. Ellis, A. El-Naggar, M. Frazier, S. Jasser, L. A. Langford, J. Lee, G. B. Mills, M. A. Pershouse, R E. Pollack, C. Tornos, P. Troncoso, W. K. Yung, G. Fujii, A. Berson, P. A. Steck, et al. 1997. MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 57:5221-5225. [PubMed] [Google Scholar]
- 40.Wu, X., K. Senechal, M. S. Neshat, Y. E. Whang, and C. L. Sawyers. 1998. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 95:15587-15591. [DOI] [PMC free article] [PubMed] [Google Scholar]