Abstract
Fusion tyrosine kinases (FTKs) such as BCR/ABL, TEL/ABL, TEL/JAK2, TEL/PDGFβR, TEL/TRKC(L), and NPM/ALK arise from reciprocal chromosomal translocations and cause acute and chronic leukemias and non-Hodgkin's lymphoma. FTK-transformed cells displayed drug resistance against the cytostatic drugs cisplatin and mitomycin C. These cells were not protected from drug-mediated DNA damage, implicating activation of the mechanisms preventing DNA damage-induced apoptosis. Various FTKs, except TEL/TRKC(L), can activate STAT5, which may be required to induce drug resistance. We show that STAT5 is essential for FTK-dependent upregulation of RAD51, which plays a central role in homology-dependent recombinational repair (HRR) of DNA double-strand breaks (DSBs). Elevated levels of Rad51 contributed to the induction of drug resistance and facilitation of the HRR in FTK-transformed cells. In addition, expression of antiapoptotic protein Bcl-xL was enhanced in cells transformed by the FTKs able to activate STAT5. Moreover, cells transformed by all examined FTKs displayed G2/M delay upon drug treatment. Individually, elevated levels of Rad51, Bcl-xL, or G2/M delay were responsible for induction of a modest drug resistance. Interestingly, combination of these three factors in nontransformed cells induced drug resistance of a magnitude similar to that observed in cells expressing FTKs activating STAT5. Thus, we postulate that RAD51-dependent facilitation of DSB repair, antiapoptotic activity of Bcl-xL, and delay in progression through the G2/M phase work in concert to induce drug resistance in FTK-positive leukemias and lymphomas.
Chromosomal translocations are responsible for the appearance of oncogenes encoding fusion tyrosine kinases (FTKs) such as BCR/ABL, TEL/ABL, TEL/JAK2, TEL/PDGFβR, TEL/TRKC(L), and NPM/ALK (6, 35). BCR/ABL is derived from relocation of the portion of the c-ABL gene from chromosome 9 to the portion of the BCR gene locus on chromosome 22 [t(9;22)] and is present in most chronic myelogenous leukemia (CML) patients and a cohort of acute lymphocytic leukemia (ALL) patients (11, 19, 64). TEL/ABL results from a t(9;12) translocation reported in ALL, acute myelogenous leukemia (AML), and atypical CML (35) and consists of the amino-terminal fragment of the TEL domain fused in-frame with exon 2 of ABL (24). TEL/JAK2 was characterized as a product of a t(9;12) translocation which includes the TEL oligomerization domain and JAK2 catalytic domain (37) and was found in ALL (37, 51). TEL/PDGFβR is associated with a t(5;12) translocation which juxtaposes the amino-terminal region of TEL with the transmembrane and tyrosine kinase domains of the platelet-derived growth factor receptor β (23). TEL/PDGFβR was found in chronic myelomonocytic leukemia (35). The consequence of t(12;15) is expression of the TEL/TRKC fusion tyrosine kinase associated with AML, infantile fibrosarcoma, and congenital mesoblastic nephroma (41). The TEL/TRKC fusion in AML [TEL/TRKC(L)] includes exons 1 to 4 of the TEL gene fused in frame to the tyrosine kinase domain of TRKC lacking a 42-bp exon near the C terminus of the TRKC moiety. NPM/ALK, formed by the t(2;5) translocation, was implicated in the pathogenesis of anaplastic large cell lymphoma (38). The NPM/ALK fusion gene encodes a 75-kDa hybrid protein that contains the amino-terminal portion of the nucleolar phosphoprotein nucleophosmin (NPM) joined to the entire cytoplasmic portion of the receptor tyrosine kinase ALK (anaplastic lymphoma kinase) (44). These FTKs (BCR/ABL-related FTKs) show structural similarities, which include an amino-terminal oligomerization domain responsible for constitutive oligomerization and activation of the associated tyrosine kinase of the carboxy-terminal fusion partner.
FTKs and other oncogenic tyrosine kinases such as v-Src and HER-2/neu activate multiple signaling pathways responsible for protection from apoptosis, induction of growth factor-independent proliferation, transformation, and resistance to therapeutic drugs and to γ-radiation (25, 41, 43, 53, 54, 61, 85). Resistance to DNA-damaging agents is a cause for failure in the therapy of human cancer, including hematological malignancies. Several mechanisms of resistance to DNA damage have been suggested (18), including overexpression of the P-glycoprotein family of membrane transporters, which decrease the intracellular accumulation of the drugs (e.g., MDR1), changes in cellular proteins involved in detoxification (e.g., glutathione S-transferase) or activation of chemotherapeutic drugs (e.g., NADP), elevation of expression of antiapoptotic proteins regulating caspase-3 (e.g., Bcl-2 and Bcl-xL), transient cell cycle arrest, and modulation of DNA repair. Existing evidence indicates that the last three mechanisms may be engaged in drug resistance induced by BCR/ABL kinase (2, 4, 17, 46, 73).
BCR/ABL enhanced the expression and phosphorylation of the RAD51 protein (73). It is notable that RAD51 has been proposed to play a central role in homology-dependent recombinational repair (HRR) of DNA double-strand breaks (DSBs) (63, 75, 76). BCR/ABL-dependent stimulation of RAD51 facilitated HRR of DSBs and induced drug resistance in leukemic cells (73). DSBs are considered the principal lethal DNA damage resulting from treatment with radiomimetic and cross-linking drugs. DSBs can be generated when replication forks encounter the drug-induced DNA lesions (34). HRR mechanisms are known to resolve such replication fork-associated DSBs (3). It is of great importance that cells recognize DSBs and act upon them rapidly and efficiently, because cell death or impaired cell function can occur if these are left unrepaired or are repaired inaccurately.
In addition to DNA repair mechanisms, cell cycle checkpoint activation processes are initiated in response to DNA damage (14). More specifically, DNA damage signals to arrest cell cycle progression, giving the cell more time to repair what might otherwise be a fatal lesion. Numerous studies have implicated a G2/M checkpoint, because its disruption sensitized tumor cells to genotoxic agents (8, 9). Delay in the G2/M transition appears to be necessary for resistance to DNA damage in BCR/ABL-positive cells (4, 46, 77).
Mechanisms preventing mitochondrion-dependent activation of caspase-3 were also implicated in drug resistance of BCR/ABL cells (2, 17). BCR/ABL can inhibit apoptosis by modulation of Bcl-2 family members (1, 59, 60), which regulate activation of caspase-3 (26). Some FTKs can enhance expression of antiapoptotic members of the family, such as Bcl-xL (1, 33) and Bcl-2 (67), and inhibit the proapoptotic function of Bad (59). These events may prevent the release of cytochrome c from the mitochondria and activation of caspase-3 (2, 17, 31).
Taken together, previous reports implicated DNA repair, activation of a cell cycle checkpoint, and dysregulation of Bcl-2 family members in resistance to DNA damage (2, 4, 17, 46, 73). In this work we demonstrate that these mechanisms can work in concert to induce drug resistance in FTK-transformed cells.
MATERIALS AND METHODS
Plasmids.
The pSRα-BCR/ABL and pSRα-NPM/ALK retroviral constructs have been described (45, 72). The MigR1-BCR/ABL retroviral construct was obtained from Warren Pear (University of Pennsylvania, Philadelphia, Pa.). The cDNAs for TEL/ABL, TEL/JAK2, and TEL/TRKC(L) were obtained from D. Gary Gilliland (Brigham and Women's Hospital, Boston, Mass.) and subcloned into the pMSCV retroviral plasmid. The pSRα-TEL/PDGFβR retroviral construct was received from Martin Carroll (University of Pennsylvania, Philadelphia, Pa.). Human RAD51 cDNA in the sense (S) and antisense (AS) orientations was cloned as RAD51-internal ribosome entry site (IRES)-green fluorescent protein (GFP) into the retroviral vector MigR1 (73). In addition, RAD51(S) and RAD51(AS) cDNAs were cloned into the pLXSP-puro and pMX-puro retroviral constructs. The pGL3-Basic vector containing the RAD51 promoter region and the pMX retroviral construct carrying the STAT5 dominant-negative mutant (STAT5-DNM, ΔSTAT5B) have been described (45, 73).
Cells.
The murine growth factor-dependent myeloid cell line 32Dcl3 and BCR/ABL-expressing clones have been described (45). The murine growth factor-dependent pro-B lymphoid cell line BaF3 and the BCR/ABL-transformed clone were obtained from Richard Van Etten (Harvard Medical School, Boston, Mass.). BaF3 cell lines transfected with NPM/ALK or with empty virus (BaF3-neo) were obtained from Steven Morris (St. Jude Children's Research Hospital, Memphis, Tenn.). BaF3 cells expressing TEL/JAK2, TEL/ABL, and TEL/TRKC(L) were obtained from D. Gary Gilliland, and BaF3 cells expressing TEL/PDGFβR were obtained from Martin Carroll. 32Dcl3 cells and BCR/ABL-positive cells expressing ectopic RAD51 were obtained after retroviral infection with the pMX-RAD51 retroviral construct and selection of the clones in puromycin. Overexpression of RAD51 protein was confirmed by Western analysis. 32Dcl3 cells overexpressing Bcl-xL were obtained from Daniel Link (Washington University School of Medicine, St. Louis, Mo.) and electroporated with the pMX-RAD51 plasmid. Clones overexpressing both Bcl-xL and RAD51 were obtained after selection in puromycin. Western analysis confirmed the elevated expression of Bcl-xL and RAD51.
Cell lines were maintained in Iscove’s Modified Dulbecco medium supplemented with 10% fetal bovine serum (FBS) and 15% WEHI-conditioned medium. Bone marrow mononuclear cells (BMCs) from C57BL/6 mice (The Jackson Laboratory, Bar Harbor, Maine) were obtained 6 days after treatment with 5-fluorouracil (67, 71). BMCs from CML blast crisis (CML-BC) patients were obtained after informed consent was given, and CD34+ cells were isolated (69). Primary hematopoietic cells were maintained in the presence of recombinant human interleukin-3 (IL-3) and stem cell factor as described (67, 69, 71). Draa-40 cells (52) were cultured in alpha minimal essential medium (α-MEM) supplemented with 10% FBS. Tk−ts13 hamster fibroblasts were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS.
Drug resistance assays.
Cisplatin (Platinol-AQ; Bristol-Myers Squibb Co., Princeton, N.J.) or mitomycin C (Sigma Chemical Co., St. Louis, Mo.) was added to cells growing in semisolid medium (103/ml) (MethoCult H4230; StemCell Technologies Inc., Vancouver, Canada) supplemented with IL-3. Colonies were scored after 7 days. Results are represented as the percentage of colony-forming cells after drug treatment in comparison to the untreated control group. Viable cells in suspension cultures were detected by trypan blue dye exclusion after 48 h of incubation with the drug. Results represent the percentage of drug-treated cells excluding trypan blue in comparison to the untreated cells.
Western analysis.
Cells were solubilized in lysis buffer (10 mM HEPES [pH 7.5], 150 mM NaCl, 1% NP-40, 10% glycerol, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride, 50 mM NaF, 1 mM Na3VO4, and 10 μg each of aprotinin and leupeptin/ml). Cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and examined by Western analysis with the following antibodies: anti-RAD51 (3C10; Upstate Biotechnology, Lake Placid, N.Y.), antiphosphotyrosine (PY20 from Oncogene Research Products, Cambridge, Mass., and 4G10 from Upstate Biotechnology, Lake Placid, N.Y.), antiactin (C11; Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), anti-Bcl-2 (N-17; Santa Cruz), and anti-Bcl-x (Transduction Laboratories, Lexington, Ky.).
Luciferase assay.
The RAD51 promoter transactivation assay was performed as described (73) with modifications. Tk−ts13 cells were transiently transfected by calcium phosphate (67, 68) with plasmids encoding FTKs or empty plasmid, STAT5-DNM or empty plasmid, as well as the RAD51 reporter plasmid containing the fused CpG-rich region of the human RAD51 promoter, the simian virus 40 (SV40) promoter, and the luciferase gene (RAD51-SV40-luc). The negative control plasmid encoded the SV40 promoter fused to the luciferase gene only (SV40-luc). A β-galactosidase plasmid was also transfected into the Tk−ts13 cells as a transfection efficiency control. A total of 20 μg of cDNA/10-cm plate was used (FTK:DNM ratio, 1:4). At 48 h after transfection, serum-free medium containing 0.1% bovine serum albumin was added to the cells, and 12 h later luciferase was quantified by the luciferase assay system (Promega, Madison, Wis.). Transfection efficiency was normalized by measuring β-galactosidase activity. Transactivation units were calculated as a ratio of the counts from RAD51-SV40-luc to the counts from SV40-luc in particular groups.
GFP-positive cells.
Cells were infected with retrovirus-based particles encoding GFP as described (45), except that ecotropic Bosc23 (obtained from Warren Pear) and amphotropic Phoenix (American Type Culture Collection [ATCC], Manassas, Va.) packaging cells were used to infect murine and human cells, respectively. Transduced cells were collected after 48 to 72 h of cocultivation with the packaging cell line. GFP-positive cells were isolated by fluorescence-activated cell sorting (Becton Dickinson).
Inhibition of BCR/ABL kinase.
ABL kinase inhibitor STI571 (16) was obtained from Novartis Pharma AG (Basel, Switzerland). Cells (106/ml) were incubated for 24 h with 1 μM STI571 in the presence of IL-3, then washed, and used for experiments.
HRR assay.
Draa-40 cells (generously provided by M. Jasin, Sloan-Kettering Cancer Center, New York, N.Y.) have integrated one or two copies of the modified gene for GFP (SceGFP) as a recombination reporter and a fragment of the GFP gene as a donor for homologous repair (52). SceGFP has an inactivating insertion containing the restriction site for the rare-cutting I-SceI endonuclease. When I-SceI is expressed in vivo, a DSB results. An HRR event with a donor GFP gene fragment restores functional GFP expression, readily detected by flow cytometry. Cells were electroporated as described (52) with 100 μg of the I-SceI plasmid, 40 μg of the plasmid carrying FTK or not (control), and 40 μg of the RAD51 antisense plasmid or empty plasmid, and 48 h later 5 × 104 cells were analyzed by flow cytometry for the expression of GFP.
Modifications of G2/M cell cycle.
G2/M delay was induced by nocodazole (46). Briefly, 15 ng of nocodazole (Sigma, St. Louis, Mo.) per ml was added to the cell culture 4 h before the drug. Twenty-four hours later, nocodazole and the drugs were removed by washing and replacing the culture medium. This protocol caused transient accumulation of cells in G2/M cell cycle detectable after 12 h and 24 h but not after 48 h of nocodazole treatment (data not shown). DNA damage-dependent G2/M delay was abolished by treatment with caffeine (84). Briefly, cisplatin or mitomycin C was added at 0 h, followed by addition of 2 mM caffeine at 12 h.
Cell cycle analysis.
Cells (106) were fixed in 70% ethanol for 15 min at 4°C, washed, and incubated in 1 ml of phosphate-buffered saline (PBS) containing 0.1% NP-40 and 1 mg of DNase-free RNase (Boehringer Mannheim Co., Indianapolis, Ind.) per ml for 10 min at room temperature. DNA was stained by propidium iodide. Cells were analyzed with a FACSCalibur (Becton Dickinson) using the CellQuest program (70).
Immunofluorescence.
RAD51 was detected by immunofluorescence as described (73). Briefly, cytospin slides were prepared from GFP+ CML-BC cells infected with the IRES-GFP or RAD51(AS)-IRES-GFP retrovirus. Cells were fixed with PBS containing 0.02% Triton X-100 and 4% formaldehyde. RAD51 was detected by mouse anti-RAD51 monoclonal antibody (Upstate Biotechnology), followed by tetramethyl rhodamine isothiocyanate (TRITC)-conjugated goat anti-mouse immunoglobulin secondary antibody (Molecular Probes Inc., Eugene, Oreg.). Negative controls were performed without primary antibodies. DNA was counterstained with the DNA fluorochrome 4′,6′-diamidino-2-phenylindole (DAPI). Cells were visualized with a Nikon Eclipse E300 fluorescence microscope equipped with a digital camera. Images were prepared with Adobe Photoshop.
RESULTS
FTK-transformed cells displayed drug-induced DNA damage but remained drug resistant.
BaF3 murine hematopoietic cells (parental), cells expressing a neomycin phosphotransferase resistance gene (BaF3-neo), and cells expressing the BCR/ABL-related FTKs were treated with different concentrations of the DNA cross-linking drug cisplatin or the radiomimetic drug mitomycin C. Cells expressing FTKs displayed resistance to these drugs in comparison to normal cells, as evaluated by the clonogenic assay (Fig. 1). FTK-transformed cells formed more colonies than parental cells after treatment with cisplatin or mitomycin C. Colonies from FTK-positive cells arose even at the drug concentrations that eliminated all colonies from parental cells. Results from the trypan blue dye exclusion test were concordant with those from the clonogenic assay. FTK-transformed cells survived the genotoxic stress better than parental cells (data not shown). TEL/TRKC(L)-positive cells displayed the lowest drug resistance among the cells transformed by various FTKs.
FIG. 1.
FTK-induced drug resistance. Parental BaF3 cells and cells expressing BCR/ABL-related FTKs were exposed to cisplatin or mitomycin C and plated in methylcellulose in the presence of IL-3. Cell viability was assessed after 7 days by the clonogenic assay. Results represent the mean ± standard deviation (SD) from three independent experiments.
To determine if FTK-induced drug resistance is attributable simply to the protection from drug-induced DNA damage, the comet assay was performed to measure the extent of DNA strand breaks (66). Similar or even higher levels of DNA damage could be detected in FTK-transformed cells in comparison to parental cells or BaF3-neo cells (data not shown). Therefore, FTKs did not protect cells from the drug-induced DNA damage.
RAD51 plays an essential role in FTK-induced drug resistance.
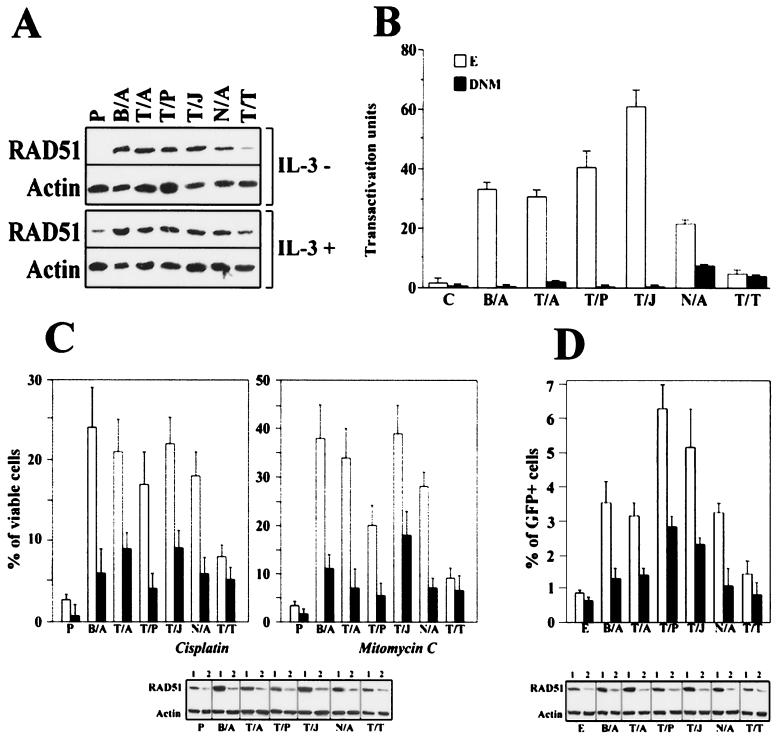
Our previous findings implicated RAD51 in resistance to radiomimetic and cross-linking drugs in BCR/ABL cells (73). Western analysis showed that cells transformed by various FTKs, except TEL/TRKC(L), expressed five to eight times more RAD51 than parental cells growing in the presence of IL-3 (Fig. 2A, panel IL-3+). High levels of RAD51 were also detected in these cells growing in the absence of IL-3 (Fig. 2A, panel IL-3−). STAT5-mediated transactivation of the RAD51 promoter and inhibition of RAD51 cleavage by caspase-3 might contribute to elevated expression of RAD51 in BCR/ABL-transformed cells (73). Interestingly, cells expressing TEL/TRKC(L), which did not activate STAT5 (41), also did not elevate RAD51 protein (Fig. 2A).
FIG. 2.
Role of RAD51 in FTK-mediated drug resistance. (A) Expression of RAD51 in FTK-transformed cells. Total cell lysates were obtained from BaF3 cells (P) and cells transformed by the indicated FTK (BCR/ABL [B/A], TEL/ABL [T/A], TEL/JAK2 [T/J], TEL/PDGFβR [T/P], NPM/ALK [N/A], and TEL/TRKC[L] [T/T]) cultured in the presence (+) or absence (−) of IL-3. RAD51 protein expression was assessed by Western analysis. Actin was detected as a loading control. Results are representative of two experiments. (B) Role of STAT5 in FTK-stimulated transactivation of the RAD51 promoter. The luciferase assay was performed in Tk−ts13 cells transiently transfected with the plasmid containing the indicated FTK or the empty plasmid (control, C) along with the plasmid carrying STAT5-DNM (DNM) or empty plasmid (E) and the plasmid encoding the luciferase reporter gene driven by the RAD51 promoter. Luciferase activity is expressed in arbitrary units. (C) Role of RAD51 in FTK-mediated inhibition of drug-induced apoptosis. BaF3 cells (P) and cells stably transfected with FTKs were infected with a retroviral construct containing RAD51(AS)-IRES-GFP (black bars) or with empty IRES-GFP virus (white bars). GFP+ cells were sorted and cultured in the presence of IL-3 with 1.5 μg of cisplatin per ml or 0.06125 μg of mitomycin C per ml for 48 h. Cell viability was assessed by the trypan blue dye exclusion test (upper panels). RAD51 expression in GFP+ cells transfected with IRES-GFP (lanes 1) or RAD51(AS)-IRES-GFP (lanes 2) was determined by Western analysis (lower panel). (D) RAD51-mediated HRR in FTK-positive cells. Draa-40 recombination-reporter cells were cotransfected with plasmids encoding FTKs or empty plasmid (E), I-SceI (to induce a DSB within one of the heterozygous GFP alleles), and either an empty plasmid (white bars) or a plasmid containing RAD51(AS) cDNA (black bars). GFP+ cells were counted after 48 h by flow cytometry. RAD51 expression in cells transfected with an empty plasmid (lanes 1) or RAD51(AS) cDNA (lanes 2) was determined by Western analysis (lower panel). Results in sections B, C, and D represent the mean ± SD from three independent experiments.
To obtain more evidence about the role of STAT5 in FTK-dependent overexpression of RAD51, transactivation of the RAD51 promoter was examined (73). Robust transactivation of the RAD51 promoter was detected in cells transfected with BCR/ABL, TEL/ABL, TEL/JAK2, TEL/PDGFβR, and NPM/ALK, but not TEL/TRKC(L) (Fig. 2B). The STAT5-DNM inhibited this effect.
Because RAD51 overexpression appeared to be directly associated with drug resistance in BCR/ABL-transformed cells (73), we sought to determine if RAD51 plays a more general role in drug resistance of the cells transformed by FTKs able to activate STAT5. Genetic studies of mammalian RAD51 are complicated by the fact that it appears to be intimately involved in cellular proliferation (75) and the mouse gene knockout model displays both an embryo- and cell-lethal phenotype (40, 78). To circumvent these problems, we developed a derivative of retroviral construct MigR1 (50) to efficiently transduce isogenic cell lines and transiently affect RAD51 expression. The MigR1 derivative encodes a variant of the murine myeloproliferative sarcoma virus long terminal repeat (LTR) promoter upstream of the RAD51 antisense [RAD51(AS)] gene, an IRES, and the GFP gene.
Parental and FTK-transformed cells were infected with the MigR1 (control) or MigR1-RAD51(AS) plasmid, respectively, and GFP+ cells were isolated by fluorescence-activated cell sorter. RAD51 protein expression in FTK (BCR/ABL, TEL/ABL, TEL/JAK2, TEL/PDGFβR, and NPM/ALK)-positive GFP+ cells transfected with RAD51 antisense cDNA was downregulated to the levels detectable in GFP+ parental cells transfected with empty plasmid (Fig. 2C, lower panel). Expression of RAD51 in GFP+ TEL/TRKC(L) cells was similar to that in parental cells. GFP+ cells were incubated with cisplatin or mitomycin C, and viable cells were detected by using the trypan blue exclusion test. The clonogenic assay could not be applied at this time because downregulation of RAD51 may have an adverse effect on hematopoietic cell proliferation after 5 to 7 days of culture (data not shown).
Both tests, the clonogenic assay and trypan blue exclusion assay, demonstrated concordance for determining drug resistance and survival following drug treatment (73). Inhibition of RAD51 expression reduced the resistance to cisplatin and mitomycin C in FTK (BCR/ABL, TEL/ABL, TEL/JAK2, TEL/PDGFβR, and NPM/ALK)-transformed cells (Fig. 2C, upper panels). Diminished levels of RAD51 did not significantly alter the sensitivity of parental cells and TEL/TRKC(L)-positive cells to these drugs. Taken together, these observations and previous findings (73) clearly implicate RAD51 in the drug resistance of FTK-positive hematopoietic malignancies overexpressing RAD51.
Drug resistance induced by the BCR/ABL→RAD51 pathway was associated with enhanced HRR of DSBs (73). Thus, we examined if other FTKs are also able to stimulate HRR. The frequency of DSB repair in cells can be quantitated by using a stable cell line which contains heteroallelic nonfunctional fragments of the GFP protein (52). In this system, recombination is induced by transfection of the I-SceI restriction enzyme, which results in a DSB within one of the GFP parental alleles. HRR of this DSB can result in the expression of a functional GFP protein, which may be identified as GFP-positive cells (52). We observed a significant (P < 0.05) increase in the percentage of GFP+ cells after cotransfection with FTKs (BCR/ABL, TEL/ABL, TEL/JAK2, TEL/PDGFβR, NPM/ALK) and I-SceI compared to empty vector and I-SceI (Fig. 2D). Moreover, cotransfection of the RAD51(AS) with the I-SceI resulted in a significant (P < 0.05) reduction of GFP repair induced by these FTKs (Fig. 2D). Interestingly, TEL/TRKC(L), which did not elevate RAD51 expression, exhibited a reduced ability to stimulate GFP repair (Fig. 2D). Taken together, these results link RAD51-dependent recombination repair with the drug resistance of FTK-transfected cells overexpressing RAD51.
Elevated level of RAD51 is not the only factor contributing to drug resistance in FTK-transformed cells.
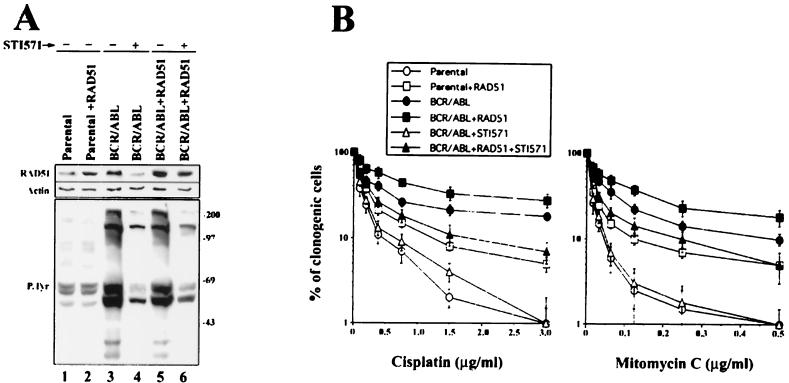
To determine if RAD51 is solely responsible for the drug resistance of FTK-transformed cells, we took advantage of the BCR/ABL-selective inhibitor STI571 (16). Inhibition of BCR/ABL kinase activity by STI571 (Fig. 3A, lower panel, compare lanes 3 and 4) was associated with abrogation of drug resistance (Fig. 3B) and downregulation of RAD51 protein (Fig. 3A, upper panel, compare lanes 3 and 4). This observation, however, did not quantitate the contribution of RAD51 to the drug resistance phenotype because other genes and mechanisms are also dysregulated after inhibition of the BCR/ABL kinase (21, 48).
FIG. 3.
Overexpression of RAD51 only partially restored therapeutic drug resistance of BCR/ABL cells treated with STI571. 32Dcl3 cells (Parental) and BCR/ABL-expressing cells were infected with a retrovirus encoding RAD51-IRES-GFP (Parental+RAD51 and BCR/ABL+RAD51 cells, respectively) or IRES-GFP (Parental and BCR/ABL cells, respectively). GFP-positive cells were isolated by fluorescence-activated cell sorting, and the ABL kinase-selective inhibitor STI571 (1 μM) was added (+) or not (−) for 24 h in the presence of IL-3. (A) Expression of RAD51 and tyrosine phosphorylation of cellular proteins were examined by immunoblotting with anti-RAD51 antibody and antiphosphotyrosine antibody, respectively. Results are representative of two independent experiments. (B) Cells were plated in methylcellulose in the presence of IL-3 with the indicated concentrations of cisplatin or mitomycin C. Colonies were counted 7 days later. Results show the percentage of clonogenic cells (mean ± SD) from three independent experiments.
To estimate the importance of RAD51 for drug resistance in BCR/ABL cells, high expression of RAD51 following STI571 treatment was rescued by an ectopic RAD51 gene driven by the LTR promoter. A mixture of clones expressing BCR/ABL and ectopic RAD51 was exposed to STI571 to inhibit the BCR/ABL kinase and its downstream effectors excluding the RAD51 protein (Fig. 3A, upper panel, compare lanes 5 and 6). STI571-treated cells expressing BCR/ABL plus ectopic RAD51 maintained their drug resistance phenotype, although not to the same extent as the untreated BCR/ABL cells (Fig. 3B). Moreover, parental 32Dcl3 cells displaying elevated levels of RAD51 (Fig. 3A, upper panel, compare lanes 1 and 2) acquired modest drug resistance, similar to that observed in STI571-treated BCR/ABL plus ectopic RAD51 cells (Fig. 3B). This suggests that another mechanism(s), in addition to that dependent on RAD51, contributes to the drug resistance in FTK-transformed cells.
Collaboration between RAD51, G2/M delay, and Bcl-xL in drug resistance.
Previous findings indicated that BCR/ABL stimulates RAD51, Bcl-xL (but not Bcl-2), and G2/M delay, which contributes to the resistance to genotoxic stress (1, 4, 73). We investigated if these processes are regulated by other FTKs. Western analysis and the DSB repair assay demonstrated that RAD51 is stimulated by the FTK→STAT5 pathway (Fig. 2A and 2B). The levels of Bcl-xL but not Bcl-2 protein were also upregulated by FTKs able to activate STAT5 (Fig. 4A). Parental BaF3 and FTK (BCR/ABL, TEL/ABL, TEL/JAK2, TEL/PDGFβR, and NPM/ALK)-positive cells growing in the presence of IL-3 accumulated in G2/M phase 12 h after drug treatment, but only the latter cells remained accumulated in G2/M after 16 h (percentages: parental, 12% ± 2%, versus BCR/ABL, 39% ± 5%; TEL/ABL, 33% ± 4%; TEL/PDGFβR, 37% ± 4%; TEL/JAK2, 44% ± 4%; and NPM/ALK, 40% ± 3%; P < 0.05) and 24 h (percentages: parental, 10% ± 3%, versus BCR/ABL, 29% ± 4%; TEL/ABL, 27% ± 4%; TEL/PDGFβR, 25% ± 5%; TEL/JAK2, 32% ± 4%; and NPM/ALK, 28% ± 3%; P < 0.05) (Fig. 4B). Similar G2/M delay was induced in BCR/ABL-transformed 32Dcl3 cells after treatment with cisplatin and mitomycin C (data not shown). TEL/TRKC(L)-transformed cells exhibited accumulation in S phase at 12 h after the treatment, but at 16 h and 24 h a significant proportion of these cells was also accumulated in G2/M (28% ± 4% and 31% ± 3%, respectively; P < 0.05 in comparison to parental cells) (Fig. 4B). At 48 h after treatment, FTK-positive cells did not display G2/M accumulation (data not shown). Thus, all FTK-positive cells examined displayed G2/M delay upon DNA damage.
FIG. 4.
Expression of Bcl-xL and induction of DNA damage-dependent G2/M delay in FTK-transformed cells. (A) Western analysis of the expression of Bcl-xL and Bcl-2 in BaF3 parental cells (Parental) and in cells transformed with the indicated FTK and cultured in the presence of IL-3. Actin was detected to show the protein load. (B) The same cells were treated with 1 μg of cisplatin per ml, and cell cycle distribution was analyzed 0, 8, 12, 16, and 24 h later by flow cytometry after staining of DNA with propidium iodide (cell cycle phases: 1, subdiploid; 2, G0/G1; 3, S; and 4, G2/M). Results represent two independent experiments.
Lack of delayed accumulation in G2/M phase was accompanied by an increased percentage of apoptotic cells containing subdiploid amounts of DNA after 16 h (percentages: parental, 28% ± 4%, versus BCR/ABL, 2% ± 1%; TEL/ABL, 0.5% ± 0.3%; TEL/PDGFβR, 1% ± 1%; TEL/JAK2, 2.5% ± 1%; NPM/ALK, 2% ± 1.5%; P < 0.05) and 24 h (percentages: parental, 56% ± 7%, versus BCR/ABL, 6% ± 3%; TEL/ABL, 7% ± 2.5%; TEL/PDGFβR, 7.5% ± 4%; TEL/JAK2, 5.5% ± 2%; NPM/ALK, 5% ± 3.5%; P < 0.05). Fewer TEL/TRKC(L)-positive cells than parental cells displayed subdiploid amounts of DNA at 16 h and 24 h after treatment (10% ± 3% and 24.5% ± 5% versus 28% ± 4% and 56% ± 7%, respectively; P < 0.05), but more than cells expressing other FTKs (BCR/ABL, TEL/ABL, TEL/JAK2, TEL/PDGFβR, and NPM/ALK).
These processes (RAD51, Bcl-xL, and G2/M delay) are regulated independently, because stimulation of a single factor did not affect the others. For example, overexpression of RAD51 did not influence Bcl-xL or G2/M, overexpression of Bcl-xL did not affect RAD51 or G2/M, and G2/M delay had no effect on RAD51 or Bcl-xL (Fig. 5A and B). Individually, elevated expression of RAD51 (Fig. 5A, lane 3) or Bcl-xL (Fig. 5A, lane 4) and G2/M delay (Fig. 5B, panel 5) caused only moderate drug resistance in parental cells (Fig. 5C).
FIG. 5.
RAD51, Bcl-xL, and G2/M delay worked in concert to induce drug resistance. (A) Expression of RAD51 and Bcl-xL (Western analysis) and (B) cell cycle (propidium iodide staining followed by flow cytometry) were examined in 32Dcl3 parental cells (parental) (lane A1, panel B1) and clones transfected with BCR/ABL (lane A2, panel B2), RAD51 (lane A3, panel B3), Bcl-xL (lane A4, panel B4), or RAD51 and Bcl-xL (lane A6, panel B6). Nocodazole was applied to induce transient G2/M arrest in parental cells (lane A5, panel B5) and in cells transfected with RAD51 (lane A7, panel B7) or RAD51 and Bcl-xL (lane A8, panel B8). (C) Cells characterized in panels A and B were plated in methylcellulose in the presence of IL-3 and cisplatin or mitomycin C. Colonies were scored after 7 days. Results represent three independent experiments (mean ± SD).
Elevated expression of RAD51 and Bcl-xL and/or G2/M delay may work in concert to protect FTK-transformed cells from drug-induced cytotoxicity. This hypothesis was tested by using three different approaches: (i) cells overexpressing RAD51 were treated with nocodazole to induce transient G2/M delay (Fig. 5B, panel 7); (ii) cells overexpressing Bcl-xL were transfected with RAD51 cDNA to enhance its expression (Fig. 5A, lane 6); and (iii) cells overexpressing RAD51 and Bcl-xL were treated with nocodazole (Fig. 5B, panel 8). Although elevated expression of Bcl-xL and RAD51 or elevated expression of RAD51 and transient G2/M delay enhanced drug resistance, its magnitude was below that observed in cells transformed with BCR/ABL (Fig. 5C). However, induction of G2/M delay (Fig. 5B, panel 8) in cells overexpressing RAD51 and Bcl-xL (Fig. 5A, lane 8) caused drug resistance similar to that observed in BCR/ABL-transformed cells (Fig. 5C). Thus, these three mechanisms can work in a synergistic or additive fashion.
Role of RAD51, Bcl-xL, and G2/M delay in drug resistance of BCR/ABL-positive primary leukemia cells.
Since the role of RAD51, Bcl-xL, and the G2/M checkpoint in FTK-dependent drug resistance has been described in cell lines, the relevance of these mechanisms in primary cells needed to be addressed. RAD51 was found to be overexpressed in CML cells (73), which exhibited drug resistance (e.g., an average of 23.5% ± 3% and 18% ± 1.5% of CD34+ bone marrow cells obtained from three CML-BC patients survived treatment with 1.5 μg of cisplatin and 0.06125 μg of mitomycin C, respectively, in comparison to 7.5% ± 2% and 5% ± 2% of bone marrow cells from three normal donors, respectively [data not shown]; P < 0.05).
Downregulation of RAD51 protein in CML cells by expression of the RAD51 antisense construct (Fig. 6A, left panel) increased their sensitivity to cisplatin and mitomycin C (Fig. 6A, right panel). Expression of BCR/ABL also induced drug resistance in primary murine bone marrow cells (e.g., 50.5% ± 5% and 32% ± 5.5% of BCR/ABL-positive cells [Fig. 6C, right panel, bars 3 and 7, respectively] versus 16.5% ± 2.5% and 2% ± 1.5% of normal cells [data not shown] were able to form colonies after treatment with 0.375 μg of cisplatin per ml or 0.1 μg of mitomycin C per ml, respectively; P < 0.05).
FIG. 6.
Overexpression of RAD51 and Bcl-xL and G2/M delay are exhibited by BCR/ABL-positive primary leukemia cells: role in drug resistance. (A) Downregulation of RAD51 increased sensitivity of CML-BC cells to cisplatin and mitomycin C. CML-BC patient cells were infected with RAD51(AS)-IRES-GFP virus (GFP+AS, •) or with IRES-GFP empty virus (GFP, ▪). Downregulation of RAD51 in GFP-positive cells was determined by immunofluorescence analysis visualizing the levels of endogenous RAD51 (left panel). Drug sensitivity was assessed by the trypan blue exclusion test after 48 h of exposure to the indicated concentrations of cisplatin or mitomycin C. (B) BCR/ABL enhances expression of Bcl-xL in primary bone marrow cells. Murine bone marrow cells were infected with BCR/ABL-IRES-GFP virus (GFP+BCR/ABL) or IRES-GFP (GFP) empty virus. Bcl-xL expression was examined by Western analysis in GFP-positive cells. (C) BCR/ABL causes G2/M delay essential for drug resistance. GFP+BCR/ABL-positive cells and GFP-positive control cells were treated with 0.375 μg of cisplatin per ml in the presence of IL-3, and cell cycle analysis was performed after 0, 12, 24, and 48 h (left panel). GFP+BCR/ABL cells cultured in the presence of IL-3 were left untreated (1) or treated with 2 mM caffeine (2), 0.375 μg of cisplatin per ml (3), 0.375 μg of cisplatin per ml plus 2 mM caffeine (4), 1.5 μg of cisplatin per ml (5), 1.5 μg of cisplatin per ml plus 2 mM caffeine (6), 0.1 μg of mitomycin C per ml (7), or 0.1 μg of mitomycin C per ml plus 2 mM caffeine (8). Twenty-four hours later, cells were plated in methylcellulose, and colonies were scored after 7 days (right panel). Results are presented as the percentage of colonies in experimental samples in comparison to the untreated control sample 1 (602 ± 37 colonies arose from 103 untreated GFP+BCR/ABL cells). ∗, P < 0.05 in comparison to the corresponding group not treated with caffeine. Cell cycle analysis of the selected samples (1, 3, and 4) was performed after 24 h of treatment to confirm that caffeine abolished accumulation of the cells in G2/M. Results represent two experiments.
Overexpression of Bcl-xL is detectable in BCR/ABL-transformed primary murine bone marrow cells (Fig. 6B), and its role in protection of BCR/ABL primary leukemia cells from apoptosis has already been reported (30). In addition, a significant difference in accumulation of BCR/ABL-positive primary murine bone marrow cells and control cells in G2/M phase in response to cisplatin treatment was detected (percentages in G2/M phase after 24 h and 48 h, 38% ± 7% and 13% ± 3% of control cells versus 61% ± 6% and 35% ± 5% of BCR/ABL-positive cells, respectively; P < 0.05) (Fig. 6C, left panel). The kinetics of G2/M accumulation of primary cells is delayed in comparison to that of cell lines (cf. Fig. 4B and 6C), probably reflecting differences in proliferation rates of BaF3 cells in comparison to primary cells.
Prolonged G2/M accumulation of BCR/ABL-positive cells in comparison to control cells observed at 48 h after the treatment was accompanied by inhibition of the former cells, exhibiting the signature of apoptosis (subdiploid amount of DNA) (4.5% ± 2.5% in comparison to 30.5% ± 5%, respectively, P < 0.05). Disruption of G2/M delay by caffeine (for example see Fig. 6C, right panel box) significantly increased drug sensitivity of BCR/ABL cells (Fig. 6C, right panel). In conclusion, all three mechanisms, overexpression of RAD51 and Bcl-xL and G2/M delay, seem to work in the primary hematopoietic cells transformed by BCR/ABL and possibly also by other FTKs, such as TEL/ABL, TEL/JAK2, TEL/PDGFβR, and NPM/ALK.
DISCUSSION
Resistance to DNA damage caused by radiation or cytostatic drugs creates a major problem for effective antitumor therapy. Usually, it does not appear to be a direct consequence of malignant transformation, but rather arises as a result of selection of the tumor cell clones which are able to develop protective mechanisms and to survive genotoxic treatment (22, 27, 28). However, malignancies induced by oncogenic tyrosine kinases such as BCR/ABL, v-SRC and HER2/neu display early drug resistance (4, 43, 53), excluding the selection process. For example, resistance to radiation and cytostatic drugs was observed in the untreated chronic phase of CML at diagnosis (4).
Here, we report that members of the family of BCR/ABL-related FTKs [BCR/ABL, TEL/ABL, TEL/JAK2, TEL/PDGFβR, NPM/ALK, and TEL/TRKC(L)] are able to induce drug resistance. FTK-transformed cells displayed similar or sometimes even more DNA strand breaks than normal cells after incubation with cisplatin or mitomycin C. These drugs do not react with DNA in a manner that would lead directly to strand breaks, and therefore the breaks must arise as indirect, secondary DNA lesions in the attempt to repair primary damage (82). When a replication fork encounters a drug-induced DNA lesion, it causes stalling of the replication process. To resolve this problem, single-strand breaks and/or DSBs occur; the latter are usually lethal if not repaired (34).
DSBs associated with replication forks are predominantly repaired by RAD51-dependent homologous recombination (3). This process is crucial for reinitiation of DNA replication on collapsed replication forks. We postulate that FTKs can stimulate HRR, resulting in drug resistance. In accordance with this hypothesis, we report that RAD51 was upregulated by various FTKs (BCR/ABL, TEL/ABL, TEL/JAK2, TEL/PDGFβR, and NPM/ALK) in a STAT5-dependent manner and played an essential role in facilitation of HRR (73; this work). This process might efficiently eliminate DSBs caused by cisplatin and mitomycin C and diminish signaling to apoptosis. In comparison, nontransformed cells express basal levels of RAD51, and thus DSBs are resolved with lower efficiency.
Interestingly, expression of RAD51 and drug resistance of TEL/TRKC(L)-transformed cells were significantly reduced in comparison to those in cells transformed by other FTKs (BCR/ABL, TEL/ABL, TEL/JAK2, TEL/PDGFβR, and NPM/ALK). Although TEL/TRKC(L) is a potent activator of the phospholipase Cγ, phosphoinositol 3-kinase, SHC, and mitogen-activated protein kinase, in striking contrast to other FTKs, it does not activate STAT5 (41). The observation implicates the FTK→STAT5→downstream effector (e.g., RAD51 and Bcl-xL) pathway in drug resistance (73, 74). This hypothesis is strengthened by the recent evidence that a STAT5 dominant-negative mutant was able to sensitize BCR/ABL-positive cells to DNA damage-induced apoptosis (29, 65).
In general, there appears to be a fundamental correlation between the expression of RAD51 and experimental resistance to therapeutic drugs (79, 81). Elevated levels of RAD51 have been linked to chlorambucil resistance in B-cell chronic lymphocytic leukemia (10) and with enhanced survival of pancreatic adenocarcinoma after treatment with the DSB-inducing therapeutic drug calicheamicin γ1 (42). Conversely, downregulation of RAD51 increased the radiosensitivity of prostate cancer cells and malignant glioma cells (13, 49).
RAD51 has also been suggested to play an essential role in the repair of chromosome breaks in normal proliferating cells (75). Therefore, a basal level of RAD51 appears to be required for the processing of spontaneous DNA lesions. Oncogenes that alter the expression, phosphorylation, nuclear localization, and/or function of RAD51 may enhance global DNA repair efficiency and lead to drug resistance. In addition to its role in drug resistance, elevated expression or phosphorylation of RAD51 may be responsible for intrachromosomal or interchromosomal deletions and chromosomal translocations, usually mediated by homologous recombination between regions of shared homology (e.g., Alu sequences) (for a review, see reference 5). This hypothesis is supported by the recent finding that large submicroscopic deletions in regions with high overall density of Alu sequences repeats have been detected in BCR/ABL-positive leukemias (36). RAD51-dependent homologous recombination can also facilitate loss of heterozygosity by gene conversion mechanisms. Moreover, in certain conditions RAD51 can permit exchange between DNA strands containing short nonhomologous sequences (7). Thus, even modest changes in the cellular levels or the activity of RAD51 may not only cause drug resistance but also promote genomic instability.
Elevated expression of RAD51 induced drug resistance, but not of the same magnitude as that observed in FTK-positive cells. This observation suggested that additional mechanisms are stimulated by FTKs which contribute to drug resistance. Previous reports implicated upregulation of Bcl-xL (1) and G2/M delay (4, 46) in BCR/ABL-dependent drug resistance. Bcl-xL can prevent apoptosis by inhibition of the release of cytochrome c from mitochondria and subsequent activation of caspase-3 (31). G2/M delay seems to provide more time to repair potentially lethal DNA lesions (8). We report here that FTKs [BCR/ABL, TEL/ABL, TEL/JAK2, TEL/PDGFβR, and NPM/ALK but not TEL/TRKC(L)] enhanced Bcl-xL expression and that all FTK-positive cells tested displayed G2/M delay upon drug treatment. Thus, at least three mechanisms can eventually contribute to drug resistance induced by FTKs: RAD51-mediated HRR, elevation of Bcl-xL expression, and G2/M delay. Each mechanism was able to induce modest drug resistance in parental cells. Interestingly, simultaneous overexpression of RAD51 and Bcl-xL and induction of G2/M delay caused the strongest drug resistance. These observations indicate that various mechanisms may work in concert to protect leukemic cells from apoptosis induced by DNA damage.
Enhanced expression of RAD51 and Bcl-xL combined with G2/M delay induced drug resistance in parental cells of the magnitude similar to that observed in cells transformed by FTKs able to activate STAT5. It is tempting to speculate that combination of these factors is sufficient for the drug resistance, especially because there is genetic evidence suggesting that cells in G2 phase repair their DSBs by using primarily the HRR mechanism (20). This hypothesis, however, should be tempered by the fact that overexpression of RAD51 and Bcl-xL proteins in parental cells (even if comparable to that observed in FTK-transformed cells) has been driven by viral promoters. Moreover, in some circumstances ectopically overexpressed Bcl-xL could partially downmodulate HRR induced by elevated levels of RAD51 (58). Also, the mechanism of G2/M delay caused by nocodazole may be at least partially different from that induced by DNA damage.
G2/M phase is generally controlled by two checkpoints, at the G2→M transition (G2 checkpoint) and at the metaphase→anaphase transition (M checkpoint) (14, 62). G2 checkpoint depends on the mechanisms regulating the Cdc2-cyclin B1 complex, such as ATM→Chk1→serine phosphorylation of Cdc25C phosphatase, driving it outside the nucleus and preventing activation of Cdc2-cyclin B1, and p53→p21 and 14-3-3σ, resulting in inactivation and sequestration of the Cdc2-cyclin B1 complex in the cytoplasm (8). The M checkpoint is controlled by the mitotic spindle assembly mechanisms (62). Genetic and biochemical evidence showed that the G2 and M checkpoints could be activated by DNA lesions to prolong G2/M phase and reduce the cytotoxicity of DNA damage (9, 32, 39, 84).
The mechanisms of G2/M delay in FTK-positive cells have not been characterized, but regulation of Cdc2-cyclin B1 could be involved (46). Although there is no direct evidence that FTKs can affect the M checkpoint, CML cells contain elevated levels of MAD2 and BUB1 (T. Skorski, unpublished observation), which inhibit the anaphase-promoting complex and cause mitotic spindle arrest (62). Thus, it seems reasonable to speculate that both the G2 and M checkpoints may contribute to G2/M delay in FTK-positive cells treated with DNA-damaging agents. Nocodazole, a mitotic spindle inhibitor (83), does not affect the Cdc2-cyclin B1 complex, but requires BUB1 and MAD2 to exert cell cycle delay (46, 80). Therefore, the G2/M delay induced in parental BaF3 cells by nocodazole probably does not exactly mimic that observed in FTK-positive cells after DNA damage, but may demonstrate some similarities. Although nocodazole arrests cells after chromosome condensation, cells can decondense their chromosomes and return to G2 phase before reentering M phase after removal of nocodazole (56). A similar decondensation of chromosomes occurs when G2/M checkpoints are triggered by DNA damage (57), perhaps allowing better access of DNA repair machinery to the damaged DNA. In agreement with these observations, we (this work) and others (32) showed that nocodazole-induced G2/M delay reduced the genotoxic effect of drugs such as ethyl methanesulfonate, cisplatin, and mitomycin C when examined after 48 h (apoptosis) and 7 days (clonogenicity). In contrast, colcemid did not provide any protective effect shortly (6 h) after DNA damage induced by irradiation (12). This discrepancy may depend on differences in the experimental protocols and/or in the DNA lesions caused by cytostatic drugs and irradiation.
Therefore, results from our experimental model may lead to overestimation of the role of RAD51, Bcl-xL, and G2/M delay in the induction of resistance of FTK-positive leukemias and lymphomas to the cross-linking and radiomimetic drugs. We cannot rule out other mechanisms, such as G1 cell cycle arrest (14), elevated expression of Bcl-2 (55), and repair of DSBs by nonhomologous end joining (47). However, we (this work) and others (4, 46) did not detect a pronounced G1 arrest in FTK-transformed cells in response to DNA damage; conversely, the role of G2/M was emphasized. Bcl-xL but not Bcl-2 was reported to have a significant inhibitory effect on the activation of caspase-3 in BCR/ABL-transformed cells treated with cytostatics (1), but we cannot exclude that other antiapoptotic pathways may also contribute to drug resistance (59). The recent finding that BCR/ABL downregulates DNA-protein kinase catalytic subunits (15) diminishes the potential role (if any) of nonhomologous end joining in the drug resistance of FTK-transformed cells. Although RAD51-mediated HRR seems to represent the major pathway of repair of lethal DNA lesions in these cells (3), mechanisms such as single-strand annealing, nucleotide excision repair, and base excision repair may be also regulated by the FTKs and contribute to DNA repair.
The collaboration between RAD51, Bcl-xL, and G2/M delay to induce drug resistance was described for murine hematopoietic cell lines. Since these three components are also functional in primary leukemia cells expressing BCR/ABL, it seems reasonable to speculate that they may collaborate to induce drug resistance in these cells and possibly in other FTK-positive hematological malignancies.
In conclusion, we hypothesize that FTKs, depending on their ability to activate STAT5, may induce common mechanisms of drug resistance, consisting of the stimulation of RAD51 (facilitated DSB repair), Bcl-xL (inhibition of caspase-3 activation), and/or prolongation of G2/M cell cycle phase (more time for DNA repair). These mechanisms can work in concert to participate in therapeutic drug resistance in leukemia/lymphoma cells and to promote genomic instability leading to a more malignant phenotype of the disease (Fig. 7).
FIG. 7.
Model of drug resistance induced by FTKs. The basal level of RAD51 in normal hematopoietic cells is regulated by the physiological signaling from the ligand (L)-receptor (R) complex. It can be further modified by DNA damage (tyrosine phosphorylation). RAD51-mediated HRR does not efficiently repair DSBs during a short G2/M phase. Remaining DSBs and other lesions trigger death pathways. This pronounced signal could not be inhibited by the basal levels of Bcl-xL resulting in the release of cytochrome c from mitochondria and activation of caspase-3. In contrast, FTK-transformed cells contain high levels of RAD51 modified by tyrosine phosphorylation. HRR is pronounced in these cells, and transient arrest in the G2/M phase provides additional time for repair. Thus, DSBs are repaired with high efficiency, and apoptotic signaling is significantly diminished. Modest apoptotic signaling in FTK-positive cells can be inhibited by elevated levels of Bcl-xL, which prevent the release of cytochrome c from mitochondria and activation of caspase-3. Because of this “antiapoptotic umbrella” provided by Bcl-xL, FTK-transformed cells can tolerate other (possibly mutagenic) DNA lesions. In addition, dysregulated HRR may result in unfaithful repair of DSBs. Taken together, FTK-positive cells can accumulate DNA lesions leading to genomic instability and malignant progression of the disease. Blue, ligand-receptor-induced events; red, FTK-induced events.
Acknowledgments
We thank D. Gary Gilliland (Harvard Medical School, Boston, Mass.) and Kristine Yoder (Kimmel Cancer Center, Philadelphia, Pa.) for critical reading of the manuscript.
This work was supported by Public Health Service grant CA89052 from the National Cancer Institute, by American Cancer Society grant RPG-98-348-01-LBC, and by Medical Center for Postgraduate Education grant 501-2-1-03-97/07 (all to T.S.). T.S. is a Scholar of the Leukemia and Lymphoma Society. A.S. is a recipient of the fellowship from the Leukemia Research Foundation and is also supported by a grant from the Elsa U. Pardee Foundation. A.B. and M.M. were recipients of fellowships from the Batory Foundation.
REFERENCES
- 1.Amarante-Mendes, G. P., A. J. McGahon, W. K. Nishioka, D. E. Afar, O. N. Witte, and D. R. Green. 1998. Bcl-2-independent Bcr-Abl-mediated resistance to apoptosis: protection is correlated with up regulation of Bcl-xL. Oncogene 16:1383-1390. [DOI] [PubMed] [Google Scholar]
- 2.Amarante-Mendes, G. P., C. Naekyung Kim, L. Liu, Y. Huang, C. L. Perkins, D. R. Green, and K. Bhalla. 1998. Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome C and activation of caspase-3. Blood 91:1700-1705. [PubMed] [Google Scholar]
- 3.Arnaudeau, C., C. Lundin, and T. Helleday. 2001. DNA double-strand breaks associated with replication forks are predominantly repaired by homologous recombination involving an exchange mechanism in mammalian cells. J. Mol. Biol. 307:1235-1245. [DOI] [PubMed] [Google Scholar]
- 4.Bedi, A., J. P. Barber, G. C. Bedi, W. S. el-Deiry, D. Sidransky, M. S. Vala, A. J. Akhtar, J. Hilton, and R. J. Jones. 1995. BCR-ABL-mediated inhibition of apoptosis with delay of G2/M transition after DNA damage: a mechanism of resistance to multiple anticancer agents. Blood 86:1148-1158. [PubMed] [Google Scholar]
- 5.Bishop, A. J., and R. H. Schiestl. 2001. Homologous recombination as a mechanism of carcinogenesis. Biochim. Biophys. Acta 1471:M109-121. [DOI] [PubMed] [Google Scholar]
- 6.Blume-Jensen, P., and T. Hunter. 2001. Oncogenic kinase signalling. Nature 411:355-365. [DOI] [PubMed] [Google Scholar]
- 7.Bucka, A., and A. Stasiak. 2001. RecA-mediated strand exchange traverses substitutional heterologies more easily than deletions or insertions. Nucleic Acids Res. 29:2464-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, T. A., H. Hermeking, C. Lengauer, K. W. Kinzler, and B. Vogelstein. 1999. 14-3-3sigma is required to prevent mitotic catastrophe after DNA damage. Nature 401:616-620. [DOI] [PubMed] [Google Scholar]
- 9.Chan, T. A., P. M. Hwang, H. Hermeking, K. W. Kinzler, and B. Vogelstein. 2000. Cooperative effects of genes controlling the G2/M checkpoint. Genes Dev. 14:1584-1588. [PMC free article] [PubMed] [Google Scholar]
- 10.Christodoulopoulos, G., A. Malapetsa, H. Schipper, E. Golub, C. Radding, and L. C. Panasci. 1999. Chlorambucil induction of HsRad51 in B-cell chronic lymphocytic leukemia. Clin. Cancer Res. 5:2178-2184. [PubMed] [Google Scholar]
- 11.Clark, S. S., J. McLaughlin, M. Timmons, A. M. Pendergast, Y. Ben-Neriah, L. W. Dow, W. Crist, G. Rovera, S. D. Smith, and O. N. Witte. 1988. Expression of a distinctive BCR-ABL oncogene in Ph1-positive acute lymphocytic leukemia (ALL). Science 239:775-777. [DOI] [PubMed] [Google Scholar]
- 12.Collins, M. K., J. Marvel, P. Malde, and A. Lopez-Rivas. 1992. Interleukin 3 protects murine bone marrow cells from apoptosis induced by DNA damaging agents. J. Exp. Med. 176:1043-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collis, S. J., A. Tighe, S. D. Scott, S. A. Roberts, J. H. Hendry, and G. P. Margison. 2001. Ribozyme minigene-mediated RAD51 down-regulation increases radiosensitivity of human prostate cancer cells. Nucleic Acids Res. 29:1534-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dasika, G. K., S. C. Lin, S. Zhao, P. Sung, A. Tomkinson, and E. Y. Lee. 1999. DNA damage-induced cell cycle checkpoints and DNA strand break repair in development and tumorigenesis. Oncogene 18:7883-7899. [DOI] [PubMed] [Google Scholar]
- 15.Deutsch, E., A. Dugray, B. AbdulKarim, E. Marangoni, L. Maggiorella, S. Vaganay, R. M'Kacher, S. D. Rasy, F. Eschwege, W. Vainchenker, A. G. Turhan, and J. Bourhis. 2001. BCR-ABL down-regulates the DNA repair protein DNA-PKcs. Blood 97:2084-2090. [DOI] [PubMed] [Google Scholar]
- 16.Druker, B. J., S. Tamura, E. Buchdunger, S. Ohno, G. M. Segal, S. Fanning, J. Zimmermann, and N. B. Lydon. 1996. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 2:561-566. [DOI] [PubMed] [Google Scholar]
- 17.Dubrez, L., B. Eymin, O. Sordet, N. Droin, A. G. Turhan, and E. Solary. 1998. BCR-ABL delays apoptosis upstream of procaspase-3 activation. Blood 91:2415-2422. [PubMed] [Google Scholar]
- 18.el-Deiry, W. S. 1997. Role of oncogenes in resistance and killing by cancer therapeutic agents. Curr. Opin. Oncol. 9:79-87. [DOI] [PubMed] [Google Scholar]
- 19.Epner, D. E., and H. P. Koeffler. 1990. Molecular genetic advances in chronic myelogenous leukemia. Ann. Intern. Med. 113:3-6. [DOI] [PubMed] [Google Scholar]
- 20.Fukushima, T., M. Takata, C. Morrison, R. Araki, A. Fujimori, M. Abe, K. Tatsumi, M. Jasin, P. K. Dhar, E. Sonoda, T. Chiba, and S. Takeda. 2001. Genetic analysis of the DNA-dependent protein kinase reveals an inhibitory role of Ku in late S-G2 phase DNA double-strand break repair. J. Biol. Chem. 276:44413-44418. [DOI] [PubMed] [Google Scholar]
- 21.Gesbert, F., W. R. Sellers, S. Signoretti, M. Loda, and J. D. Griffin. 2000. BCR/ABL regulates expression of the cyclin-dependent kinase inhibitor p27Kip1 through the phosphatidylinositol 3-Kinase/AKT pathway. J. Biol. Chem. 275:39223-39230. [DOI] [PubMed] [Google Scholar]
- 22.Goldie, J. H. 1994. Modelling the process of drug resistance. Lung Cancer 10:S91-S96. [DOI] [PubMed] [Google Scholar]
- 23.Golub, T. R., G. F. Barker, M. Lovett, and D. G. Gilliland. 1994. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell 77:307-316. [DOI] [PubMed] [Google Scholar]
- 24.Golub, T. R., A. Goga, G. F. Barker, D. E. Afar, J. McLaughlin, S. K. Bohlander, J. D. Rowley, O. N. Witte, and D. G. Gilliland. 1996. Oligomerization of the ABL tyrosine kinase by the Ets protein TEL in human leukemia. Mol. Cell. Biol. 16:4107-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenland, C., C. Touriol, G. Chevillard, S. W. Morris, R. Bai, J. Duyster, G. Delsol, and M. Allouche. 2001. Expression of the oncogenic NPM-ALK chimeric protein in human lymphoid T-cells inhibits drug-induced, but not Fas-induced apoptosis. Oncogene 20:7386-7397. [DOI] [PubMed] [Google Scholar]
- 26.Gross, A., J. M. McDonnell, and S. J. Korsmeyer. 1999. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13:1899-1911. [DOI] [PubMed] [Google Scholar]
- 27.Hampson, R. 1997. Selection for genome instability by DNA damage in human cells: unstable microsatellites and their consequences for tumourigenesis. Radiat. Oncol. Investig. 5:111-114. [DOI] [PubMed] [Google Scholar]
- 28.Harrison, D. J. 1995. Molecular mechanisms of drug resistance in tumours. J. Pathol. 175:7-12. [DOI] [PubMed] [Google Scholar]
- 29.Hoover, R. R., M. J. Gerlach, E. Y. Koh, and G. Q. Daley. 2001. Cooperative and redundant effects of STAT5 and Ras signaling in BCR/ABL transformed hematopoietic cells. Oncogene 20:5826-5835. [DOI] [PubMed] [Google Scholar]
- 30.Horita, M., E. J. Andreu, A. Benito, C. Arbona, C. Sanz, I. Benet, F. Prosper, and J. L. Fernandez-Luna. 2000. Blockade of the Bcr-Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing signal transducer and activator of transcription 5-dependent expression of Bcl-xL. J. Exp. Med. 191:977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson, B. W., E. Cepero, and L. H. Boise. 2000. Bcl-xL inhibits cytochrome c release but not mitochondrial depolarization during the activation of multiple death pathways by tumor necrosis factor-alpha. J. Biol. Chem. 275:31546-31553. [DOI] [PubMed] [Google Scholar]
- 32.Johnson, P. A., P. Clements, K. Hudson, and K. W. Caldecott. 1999. A mitotic spindle requirement for DNA damage-induced apoptosis in Chinese hamster ovary cells. Cancer Res. 59:2696-2700. [PubMed] [Google Scholar]
- 33.Karni, R., R. Jove, and A. Levitzki. 1999. Inhibition of pp60c-Src reduces Bcl-XL expression and reverses the transformed phenotype of cells overexpressing EGF and HER-2 receptors. Oncogene 18:4654-4662. [DOI] [PubMed] [Google Scholar]
- 34.Khanna, K. K., and S. P. Jackson. 2001. DNA double-strand breaks: signaling, repair and the cancer connection. Nat. Genet. 27:247-254. [DOI] [PubMed] [Google Scholar]
- 35.Kolibaba, K. S., and B. J. Druker. 1997. Protein tyrosine kinases and cancer. Biochim. Biophys. Acta 1333:F217-F248. [DOI] [PubMed] [Google Scholar]
- 36.Kolomietz, E., J. Al-Maghrabi, S. Brennan, J. Karaskova, S. Minkin, J. Lipton, and J. A. Squire. 2001. Primary chromosomal rearrangements of leukemia are frequently accompanied by extensive submicroscopic deletions and may lead to altered prognosis. Blood 97:3581-3588. [DOI] [PubMed] [Google Scholar]
- 37.Lacronique, V., A. Boureux, V. D. Valle, H. Poirel, C. T. Quang, M. Mauchauffe, C. Berthou, M. Lessard, R. Berger, J. Ghysdael, and O. A. Bernard. 1997. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 278:1309-1312. [DOI] [PubMed] [Google Scholar]
- 38.Ladanyi, M. 2000. Aberrant ALK tyrosine kinase signaling. Different cellular lineages, common oncogenic mechanisms. Am. J. Pathol. 157:341-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, X., and M. Cai. 1997. Inactivation of the cyclin-dependent kinase Cdc28 abrogates cell cycle arrest induced by DNA damage and disassembly of mitotic spindles in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:2723-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim, D.-S., and P. Hasty. 1996. A mutation in mouse rad51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol. Cell. Biol. 16:7133-7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu, Q., J. Schwaller, J. Kutok, D. Cain, J. C. Aster, I. R. Williams, and D. G. Gilliland. 2000. Signal transduction and transforming properties of the TEL-TRKC fusions associated with t(12;15) (p13;q25) in congenital fibrosarcoma and acute myelogenous leukemia. EMBO J. 19:1827-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maacke, H., K. Jost, S. Opitz, S. Miska, Y. Yuan, L. Hasselbach, J. Luttges, H. Kalthoff, and H. W. Sturzbecher. 2000. DNA repair and recombination factor rad51 is overexpressed in human pancreatic adenocarcinoma. Oncogene 19:2791-2795. [DOI] [PubMed] [Google Scholar]
- 43.Masumoto, N., S. Nakano, H. Fujishima, K. Kohno, and Y. Niho. 1999. v-src induces cisplatin resistance by increasing the repair of cisplatin-DNA interstrand cross-links in human gallbladder adenocarcinoma cells. Int. J. Cancer 80:731-737. [DOI] [PubMed] [Google Scholar]
- 44.Morris, S. W., M. N. Kirstein, M. B. Valentine, K. Dittmer, D. N. Shapiro, A. T. Look, and D. L. Saltman. 1995. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 267:316-317. [DOI] [PubMed] [Google Scholar]
- 45.Nieborowska-Skorska, M., M. A. Wasik, A. Slupianek, P. Salomoni, T. Kitamura, B. Calabretta, and T. Skorski. 1999. Signal transducer and activator of transcription (STAT)5 activation by BCR/ABL is dependent on intact Src homology (SH)3 and SH2 domains of BCR/ABL and is required for leukemogenesis. J. Exp. Med. 189:1229-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishii, K., J. H. Kabarowski, D. L. Gibbons, S. D. Griffiths, I. Titley, L. M. Wiedemann, and M. F. Greaves. 1996. ts BCR-ABL kinase activation confers increased resistance to genotoxic damage via cell cycle block. Oncogene 13:2225-2234. [PubMed] [Google Scholar]
- 47.Norbury, C. J., and I. D. Hickson. 2001. Cellular responses to DNA damage. Annu. Rev. Pharmacol. Toxicol. 41:367-401. [DOI] [PubMed] [Google Scholar]
- 48.Oetzel, C., T. Jonuleit, A. Gotz, H. van der Kuip, H. Michels, J. Duyster, M. Hallek, and W. E. Aulitzky. 2000. The tyrosine kinase inhibitor CGP 57148 (ST1 571) induces apoptosis in BCR-ABL-positive cells by down-regulating BCL-X. Clin. Cancer Res. 6:1958-1968. [PubMed] [Google Scholar]
- 49.Ohnishi, T., T. Taki, S. Hiraga, N. Arita, and T. Morita. 1998. In vitro and in vivo potentiation of radiosensitivity of malignant gliomas by antisense inhibition of the RAD51 gene. Biochem. Biophys. Res. Commun. 245:319-324. [DOI] [PubMed] [Google Scholar]
- 50.Pear, W. S., J. P. Miller, L. Xu, J. C. Pui, B. Soffer, R. C. Quackenbush, A. M. Pendergast, R. Bronson, J. C. Aster, M. L. Scott, and D. Baltimore. 1998. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood 92:3780-3792. [PubMed] [Google Scholar]
- 51.Peeters, P., S. D. Raynaud, J. Cools, I. Wlodarska, J. Grosgeorge, P. Philip, F. Monpoux, L. Van Rompaey, M. Baens, H. Van den Berghe, and P. Marynen. 1997. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood 90:2535-2540. [PubMed] [Google Scholar]
- 52.Pierce, A. J., R. D. Johnson, L. H. Thompson, and M. Jasin. 1999. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 13:2633-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pietras, R. J., B. M. Fendly, V. R. Chazin, M. D. Pegram, S. B. Howell, and D. J. Slamon. 1994. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene 9:1829-1838. [PubMed] [Google Scholar]
- 54.Raitano, A. B., Y. E. Whang, and C. L. Sawyers. 1997. Signal transduction by wild-type and leukemogenic Abl proteins. Biochim. Biophys. Acta 1333:F201-F216. [DOI] [PubMed] [Google Scholar]
- 55.Reed, J. C. 1997. Bcl-2 family proteins: regulators of apoptosis and chemoresistance in hematologic malignancies. Semin. Hematol. 34:9-19. [PubMed] [Google Scholar]
- 56.Rieder, C. L., and R. Cole. 2000. Microtubule disassembly delays the G2-M transition in vertebrates. Curr. Biol. 10:1067-1070. [DOI] [PubMed] [Google Scholar]
- 57.Rieder, C. L., and R. W. Cole. 1998. Entry into mitosis in vertebrate somatic cells is guarded by a chromosome damage checkpoint that reverses the cell cycle when triggered during early but not late prophase. J. Cell Biol. 142:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saintigny, Y., A. Dumay, S. Lambert, and B. S. Lopez. 2001. A novel role for the Bcl-2 protein family: specific suppression of the RAD51 recombination pathway. EMBO J. 20:2596-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salomoni, P., F. Condorelli, S. M. Sweeney, and B. Calabretta. 2000. Versatility of BCR/ABL-expressing leukemic cells in circumventing proapoptotic BAD effects. Blood 96:676-684. [PubMed] [Google Scholar]
- 60.Sanchez-Garcia, I., and D. Martin-Zanca. 1997. Regulation of Bcl-2 gene expression by BCR-ABL is mediated by Ras. J. Mol. Biol. 267:225-228. [DOI] [PubMed] [Google Scholar]
- 61.Sattler, M., and R. Salgia. 1997. Activation of hematopoietic growth factor signal transduction pathways by the human oncogene BCR/ABL. Cytokine Growth Factor Rev. 8:63-79. [DOI] [PubMed] [Google Scholar]
- 62.Shah, J. V., and D. W. Cleveland. 2000. Waiting for anaphase: Mad2 and the spindle assembly checkpoint. Cell 103:997-1000. [DOI] [PubMed] [Google Scholar]
- 63.Shinohara, A., and T. Ogawa. 1995. Homologous recombination and the roles of double-strand breaks. Trends Biochem. Sci. 20:387-391. [DOI] [PubMed] [Google Scholar]
- 64.Shtivelman, E., B. Lifshitz, R. P. Gale, B. A. Roe, and E. Canaani. 1986. Alternative splicing of RNAs transcribed from the human abl gene and from the bcr-abl fused gene. Cell 47:277-284. [DOI] [PubMed] [Google Scholar]
- 65.Sillaber, C., F. Gesbert, D. A. Frank, M. Sattler, and J. D. Griffin. 2000. STAT5 activation contributes to growth and viability in Bcr/Abl-transformed cells. Blood 95:2118-2125. [PubMed] [Google Scholar]
- 66.Singh, N. P., M. T. McCoy, R. R. Tice, and E. L. Schneider. 1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175:184-191. [DOI] [PubMed] [Google Scholar]
- 67.Skorski, T., A. Bellacosa, M. Nieborowska-Skorska, M. Majewski, R. Martinez, J. K. Choi, R. Trotta, P. Wlodarski, D. Perrotti, T. O. Chan, M. A. Wasik, P. N. Tsichlis, and B. Calabretta. 1997. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. EMBO J. 16:6151-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skorski, T., P. Kanakaraj, D. H. Ku, M. Nieborowska-Skorska, E. Canaani, G. Zon, B. Perussia, and B. Calabretta. 1994. Negative regulation of p120GAP GTPase promoting activity by p210bcr/abl: implication for RAS-dependent Philadelphia chromosome positive cell growth. J. Exp. Med. 179:1855-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skorski, T., P. Kanakaraj, M. Nieborowska-Skorska, M. Z. Ratajczak, S. C. Wen, G. Zon, A. M. Gewirtz, B. Perussia, and B. Calabretta. 1995. Phosphatidylinositol-3 kinase activity is regulated by BCR/ABL and is required for the growth of Philadelphia chromosome-positive cells. Blood 86:726-736. [PubMed] [Google Scholar]
- 70.Skorski, T., M. Nieborowska-Skorska, K. Campbell, R. V. Iozzo, G. Zon, Z. Darzynkiewicz, and B. Calabretta. 1995. Leukemia treatment in severe combined immunodeficiency mice by antisense oligodeoxynucleotides targeting cooperating oncogenes. J. Exp. Med. 182:1645-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skorski, T., M. Nieborowska-Skorska, P. Wlodarski, D. Perrotti, R. Martinez, M. A. Wasik, and B. Calabretta. 1996. Blastic transformation of p53-deficient bone marrow cells by p210bcr/abl tyrosine kinase. Proc. Natl. Acad. Sci. USA 93:13137-13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slupianek, A., M. Nieborowska-Skorska, G. Hoser, A. Morrione, M. Majewski, L. Xue, S. W. Morris, M. A. Wasik, and T. Skorski. 2001. Role of phosphatidylinositol 3-kinase-Akt pathway in nucleophosmin/anaplastic lymphoma kinase-mediated lymphomagenesis. Cancer Res. 61:2194-2199. [PubMed] [Google Scholar]
- 73.Slupianek, A., C. Schmutte, G. Tombline, M. Nieborowska-Skorska, G. Hoser, M. O. Nowicki, A. J. Pierce, R. Fishel, and T. Skorski. 2001. BCR/ABL regulates mammalian recA homologs, resulting in drug resistance. Mol. Cell 8:795-806. [DOI] [PubMed] [Google Scholar]
- 74.Socolovsky, M., A. E. Fallon, S. Wang, C. Brugnara, and H. F. Lodish. 1999. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/− 5b−/− mice: a direct role for Stat5 in Bcl-X(L) induction. Cell 98:181-191. [DOI] [PubMed] [Google Scholar]
- 75.Sonoda, E., M. S. Sasaki, J. M. Buerstedde, O. Bezzubova, A. Shinohara, H. Ogawa, M. Takata, Y. Yamaguchiiwai, and S. Takeda. 1998. Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J. 17:598-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sonoda, E., M. S. Sasaki, C. Morrison, Y. Yamaguchi-Iwai, M. Takata, and S. Takeda. 1999. Sister chromatid exchanges are mediated by homologous recombination in vertebrate cells. Mol. Cell. Biol. 19:5166-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stiewe, T., K. Parssanedjad, H. Esche, B. Opalka, and B. M. Putzer. 2000. E1A overcomes the apoptosis block in BCR-ABL+ leukemia cells and renders cells susceptible to induction of apoptosis by chemotherapeutic agents. Cancer Res. 60:3957-3964. [PubMed] [Google Scholar]
- 78.Tsuzuki, T., Y. Fujii, K. Sakumi, Y. Tominaga, K. Nakao, M. Sekiguchi, A. Matsushiro, Y. Yoshimura, and T. Morita. 1996. Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc. Natl. Acad. Sci. USA 93:6236-6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vispe, S., C. Cazaux, C. Lesca, and M. Defais. 1998. Overexpression of Rad51 protein stimulates homologous recombination and increases resistance of mammalian cells to ionizing radiation. Nucleic Acids Res. 26:2859-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang, Y., and D. J. Burke. 1995. Checkpoint genes required to delay cell division in response to nocodazole respond to impaired kinetochore function in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 15:6838-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xia, S. J., M. A. Shammas, and R. J. S. Reis. 1997. Elevated recombination in immortal human cells is mediated by HsRAD51 recombinase. Mol. Cell. Biol. 17:7151-7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zdraveski, Z. Z., J. A. Mello, M. G. Marinus, and J. M. Essigmann. 2000. Multiple pathways of recombination define cellular responses to cisplatin. Chem. Biol. 7:39-50. [DOI] [PubMed] [Google Scholar]
- 83.Zhai, Y., P. J. Kronebusch, P. M. Simon, and G. G. Borisy. 1996. Microtubule dynamics at the G2/M transition: abrupt breakdown of cytoplasmic microtubules at nuclear envelope breakdown and implications for spindle morphogenesis. J. Cell Biol. 135:201-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou, B. B., P. Chaturvedi, K. Spring, S. P. Scott, R. A. Johanson, R. Mishra, M. R. Mattern, J. D. Winkler, and K. K. Khanna. 2000. Caffeine abolishes the mammalian G2/M DNA damage checkpoint by inhibiting ataxia-telangiectasia-mutated kinase activity. J. Biol. Chem. 275:10342-10348. [DOI] [PubMed] [Google Scholar]
- 85.Zou, X., and K. Calame. 1999. Signaling pathways activated by oncogenic forms of Abl tyrosine kinase. J. Biol. Chem. 274:18141-18144. [DOI] [PubMed] [Google Scholar]