Abstract
The fission yeast Dbf4 homologue Dfp1 has a well-characterized role in regulating the initiation of DNA replication. Sequence analysis of Dfp1 homologues reveals three highly conserved regions, referred to as motifs N, M, and C. To determine the roles of these conserved regions in Dfp1 function, we have generated dfp1 alleles with mutations in these regions. Mutations in motif N render cells sensitive to a broad range of DNA-damaging agents and replication inhibitors, yet these mutant proteins are efficient activators of Hsk1 kinase in vitro. In contrast, mutations in motif C confer sensitivity to the alkylating agent methyl methanesulfonate (MMS) but, surprisingly, not to UV, ionizing radiation, or hydroxyurea. Motif C mutants are poor activators of Hsk1 in vitro but can fulfill the essential function(s) of Dfp1 in vivo. Strains carrying dfp1 motif C mutants have an intact mitotic and intra-S-phase checkpoint, and epistasis analysis indicates that dfp1 motif C mutants function outside of the known MMS damage repair pathways, suggesting that the observed MMS sensitivity is due to defects in recovery from DNA damage. The motif C mutants are most sensitive to MMS during S phase and are partially suppressed by deletion of the S-phase checkpoint kinase cds1. Following treatment with MMS, dfp1 motif C mutants exhibit nuclear fragmentation, chromosome instability, precocious recombination, and persistent checkpoint activation. We propose that Dfp1 plays at least two genetically separable roles in the DNA damage response in addition to its well-characterized role in the initiation of DNA replication and that motif C plays a critical role in the response to alkylation damage, perhaps by restarting or stabilizing stalled replication forks.
DNA replication is a tightly regulated event (reviewed in references 13, 24, and 30). Eukaryotes have evolved intricate mechanisms to regulate the G1/S transition and ensure that replication occurs once and only once per cell cycle. Current models of the initiation of DNA replication depict it as an ordered process consisting of two main steps. The first step involves the sequential assembly of a multiprotein complex (the prereplicative complex [pre-RC]) at DNA replication origins. The pre-RC contains the origin recognition complex, Cdt1, Cdc18, and the hexameric complex of minichromosomal maintenance proteins (MCMs) (2, 11, 43, 58). The second step of initiation involves the activation of the pre-RC by two protein kinases, resulting in the formation of two replication forks and the transition into S phase. The first kinase, cyclin-dependent kinase, is required for the recruitment of the replication protein Cdc45 onto chromatin (64, 65) and also negatively regulates Cdc18 (19), the origin recognition complex, and MCMs (42). In addition to cyclin-dependent kinase, initiation requires the action of a member of the Cdc7 family of protein kinases (reviewed in references 24, 32, 38, and 53). In the fission yeast Schizosaccharomyces pombe, Hsk1 is the Cdc7 family kinase (37). Although the critical substrates of Cdc7 kinases at initiation remain to be identified, a large amount of evidence has implicated MCMs, and there is also a requirement for Cdc7 function in order for Cdc45 to associate with replication origins (7, 22, 31, 44, 47, 52, 60, 63).
In S. pombe, Dfp1 is a member of the conserved family of protein activators of the Cdc7 family kinases. This family was founded by Saccharomyces cerevisiae Dbf4, and homologues are present in all eukaryotes examined to date (7, 8, 20, 22, 27, 29, 33, 56). Dfp1 expression is cell cycle regulated at both the transcriptional and posttranscriptional levels (6, 56). Dfp1 protein is absent in G1 cells but is expressed starting at the G1/S transition and continuing through M phase. Expression of Dfp1 activates Hsk1, allowing it to phosphorylate its critical substrates in order to promote the initiation of DNA replication. S. cerevisiae Dbf4 is regulated in a similar manner, with cell cycle regulation of Dbf4 expression leading to the activation of Cdc7 kinase as cells enter S phase (9, 47, 63). Dbf4 localizes to replication origins in vivo, indicating that it may play a role in targeting Cdc7 to replication origins as well as activating the kinase (12).
In addition to participating in DNA replication, there is growing evidence that Hsk1/Dfp1 (and Cdc7/Dbf4) is involved in the response to DNA damage (reviewed in reference 21). Both Hsk1 and Dfp1 are hyperphosphorylated in a Cds1-dependent manner in response to the replication inhibitor hydroxyurea (HU) in vivo (6, 54, 56) and are substrates of Cds1 in vitro (reference 54 and G. W. Brown, unpublished data). Strains carrying the hsk1-1312 conditional allele are sensitive to a broad range of DNA-damaging agents and require functional DNA checkpoint pathways for viability (54). Studies with the hsk1-89 allele suggest that Hsk1 is required for the activation of the S-phase checkpoint kinase Cds1 (57). Mutations in the dfp1 N terminus display HU and methyl methanesulfonate (MMS) sensitivity (46, 56). Studies of S. cerevisiae indicate that some of these properties are evolutionarily conserved. Deletion of CDC7 results in HU sensitivity, and Rad53 (the budding yeast homologue of Cds1) is required for HU-induced phosphorylation of Dbf4 in vivo (63). Cdc7/Dbf4 activity in immunoprecipitates decreases following HU treatment (63), and there is some indication that Rad53 phosphorylation inhibits Cdc7 activity in vitro (26). Together, these results suggest that Cdc7 kinases function in some aspect of the pathways that respond to DNA damage.
Alignment of dfp1+ and other DBF4 homologues revealed a low overall level of sequence identity (25% between fission yeast and budding yeast) (56). There are, however, three small regions of high sequence identity located in the N terminus (amino acids [aa] 151 to 195), the middle (aa 276 to 323), and the C terminus (aa 494 to 535) of Dfp1, termed motifs N, M, and C (29, 36, 46). To investigate the role of these highly conserved motifs, we have generated a series of mutations in the dfp1+ N and C termini. We find that the mutations in the C terminus of Dfp1 uniquely confer sensitivity to the alkylating agent MMS. The MMS sensitivity of motif C mutants was not due to defects in mitotic or intra-S-phase checkpoints, and these mutants function outside of known MMS repair pathways, suggesting that dfp1+ contributes to the recovery from alkylation damage rather than to the repair of lesions. The MMS sensitivity of motif C mutants was characterized by chromosome instability, hyperrecombination, and abnormal persistence of activated checkpoint pathways during recovery. We propose that dfp1+ has important roles outside of the initiation of DNA replication. dfp1+ is required for stable chromosome transmission, for suppression of recombination, and for appropriate downregulation of checkpoint responses during recovery from MMS-induced DNA damage during S phase.
MATERIALS AND METHODS
Yeast strains and methods.
General fission yeast genetic and molecular biology methods were used (40). All fission yeast strains are listed in Table 1 and were maintained in Edinburgh minimal media or in yeast extract (YE) with the required supplements. Flow cytometry and microscopy were performed as described previously (7). The dfp1-D1 allele, which is an unmarked deletion of the entire dfp1+ open reading frame, was generated by selection of ura4− diploids of the strain GBY405 (which carries dfp1+/dfp1::ura4+) (6) on plates containing 1 g of 5-fluoroorotic acid/liter. Excision of the ura4+ gene that had replaced one dfp1+ open reading frame in the diploid was confirmed by PCR. Depletion of Dfp1 was performed by culture of the strain AFY4, which carries dfp1+ on a plasmid, under the control of the medium-strength nmt1 promoter in the presence of 5 μg of thiamine/ml for 4 h.
TABLE 1.
S. pombe strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| AFY4 | h+dfp1-D1 leu1-32 ura4-D18 ade6-M216 [pJKars42Xdfp1-6his3HA] | This study |
| AFY6 | h+leu1::(dfp11-459-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| AFY7 | h+leu1::(dfp11-376-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| AFY16 | h+leu1::(dfp1Δ460-494-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| AFY22 | h+leu1::(dfp11-494-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| AFY23 | h+leu1::(dfp1Δ497-505-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| AFY24 | h+leu1::(dfp1-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 [pSLF172] | This study |
| AFY25 | h+leu1::(dfp1-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 [pSLF172-hsk1] | This study |
| AFY26 | h+leu1::(dfp11-376-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 [pSLF172] | This study |
| AFY27 | h+leu1::(dfp11-376-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 [pSLF172-hsk1] | This study |
| AFY28 | h+leu1::(dfp1-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 [pIRT2U] | This study |
| AFY29 | h+leu1::(dfp1-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 [pIRT2Udfp1] | This study |
| AFY30 | h+leu1::(dfp11-376-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 [pIRT2U] | This study |
| AFY31 | h+leu1::(dfp11-376-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 [pIRT2Udfp1] | This study |
| AFY32 | h−cds1::ura4+leu1::(dfp1-6his3HA leu1+) dfp1-D1 ura4-D18 | This study |
| AFY34 | h+cds1::ura4+leu1::(dfp11-376-6his3HA leu1+) dfp1-D1 ura4-D18 | This study |
| AFY48 | h+rhp51::ura4+leu1::(dfp1-6his3HA leu1+) dfp1-D1 ura4-D18 ade6− | This study |
| AFY49 | h+rad13::ura4+leu1::(dfp1-6his3HA leu1+) dfp1-D1 ura4-D18 ade6− | This study |
| AFY54 | h+rhp51::ura4+leu1::(dfp11-376-6his3HA leu1+) dfp1-D1 ura4-D18 ade6− | This study |
| AFY55 | h+rad13::ura4+leu1::(dfp11-376-6his3HA leu1+) dfp1-D1 ura4-D18 ade6− | This study |
| AFY57 | h+/h−leu1::(dfp1-6his3HA leu1+)/leu1::(dfp1-6his3HA leu1+) dfp1-D1/dfp1-D1 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | This study |
| AFY60 | h+/h−leu1::(dfp11-459-6his3HA leu1+)/leu1::(dfp11-459-6his3HA leu1+) dfp1-D1/dfp1-D1 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | This study |
| AFY61 | h+rad2::ura4+leu1::(dfp1-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| AFY63 | h−rad2::ura4+leu1::(dfp11-376-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| AFY68 | h+/h−leu1::(dfp11-376-6his3HA leu1+)/leu1::(dfp11-376-6his3HA leu1+) dfp1-D1/dfp1-D1 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | This study |
| AFY69 | h+mag1::ura4+leu1::(dfp1-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| AFY71 | h+mag1::ura4+leu1::(dfp11-376-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| AFY72 | h+chk1::ura4+leu1::(dfp1-6his3HA leu1+) dfp1-D1 ura4-D18 | This study |
| AFY74 | h+chk1::ura4+leu1::(dfp11-376-6his3HA leu1+) dfp1-D1 ura4-D18 | This study |
| FY382 | h−rad3-h1.5 ura4-D18 | S. Forsburg |
| GBY180 | h−cds1::ura4+ura4-D18 leu1-32 | A. Carr |
| GBY190 | h+rad1::ura4+ura4-D18 leu1-32 ade6-M216 | A. Carr |
| GBY405 | h+/h−dfp1::ura4+/dfp1+leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade2-M216 | 6 |
| GBY391 | h+ura4-D18 | 972h− derivative |
| GBY562 | h+dfp1-D1 leu1-32 ura4-D18 ade6-M216 [pJKars-dfp1B-3HA-3′ dfp1] | This study |
| GBY563 | h+dfp1-D1 leu1-32 ura4-D18 ade6-M216 [pJKarsdfp1B(Δ13-98)3HA-3′dfp1] | This study |
| GBY564 | h+dfp1-D1 leu1-32 ura4-D18 ade6-M216 [pJKarsdfp1B(Δ13-193)3HA-3′dfp1] | This study |
| GBY565 | h+dfp1-D1 leu1-32 ura4-D18 ade6-M216 [pJKarsdfp1B(Δ13-240)3HA-3′dfp1] | This study |
| GBY566 | h+dfp1-D1 leu1-32 ura4-D18 ade6-M216 [pJKarsdfp1B(Δ183-191)3HA-3′dfp1] | This study |
| GBY572 | h+leu1::(dfp1-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| GBY574 | h+cds1::ura4+ura4-D18 | This study |
| GBY581 | h+cdc10-V50 leu1::(dfp1-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| GBY583 | h−cdc10-V50 leu1::(dfp11-376-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| JOY6 | h−hsk1::(hsk1-HA-TAP ura4+) leu1-32 ura4-D18 | This study |
| JOY7 | h−hsk1::(hsk1-HA-TAP ura4+) leu1::(dfp1-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| JOY8 | h+hsk1::(hsk1-HA-TAP ura4+) leu1::(dfp11-459-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| JOY9 | h+hsk1::(hsk1-HA-TAP ura4+) leu1::(dfp11-376-6his3HA leu1+) dfp1-D1 ura4-D18 ade6-M216 | This study |
| TWY2 | h+dfp1-D1 ura4-D18 ade6-M216 [pIRT2Udfp1-TK] | This study |
| YJD8 | h+dfp1-D1 ura4-D18 ade6-M216 [pIRT2Udfp1] | This study |
Cloning and construction of the N- and C-terminal dfp1 truncation mutant strains.
The plasmid pJKars-dfp1B-3HA-3′dfp1 was constructed by inserting the dfp1+ promoter followed by the dfp1+ open reading frame fused to three copies of the hemagglutinin epitope tag (3HA) and the dfp1+ 3′ untranslated region into pJKars (7). Plasmids containing mutant dfp1 alleles were generated by PCR by using pJKars-dfp1B-3HA-3′dfp1 as the parent vector and were confirmed by sequencing. The plasmids were transformed into the yeast strain TWY2, which carries dfp1-D1 complemented by pIRT2U-dfp1+-TK. The plasmid pIRT2U-dfp1+-TK carries the counterselectable thymidine kinase gene (25). Strains which had lost the pIRT2U-dfp1+-TK were selected by streaking onto plates containing 10 mg of 5′-fluoro-2′-deoxyuridine (FUdR)/liter. The absence of pIRT2U-dfp1+-TK and the presence of the dfp1 deletion were confirmed by colony PCR.
Strains carrying stably integrated dfp1 motif C mutants were constructed by inserting the dfp1 alleles, including the promoter and the 3′ untranslated region, into the integration vector pJK148 (23). The plasmid was linearized in the leu1+ gene and transformed into YJD8. The strain YJD8 carries a deletion of the entire dfp1+ open reading frame, complemented by pIRT2U-dfp1+. Stable leu1+ transformants carry the dfp1 allele integrated at the leu1 locus. Strains that had lost the complementing dfp1+ plasmid were selected by streaking onto plates containing 1 g of 5-fluoroorotic acid/liter. The absence of pIRT2U-dfp1+ and the presence of the dfp1 deletion were confirmed by colony PCR.
Protein A-tagged hsk1 strains were generated by inserting a linker encoding the immunoglobulin G (IgG)-binding domain of protein A into the hsk1 tagging vector pSLFΔarsΔ5′hsk1 (7). The protein A-tagged hsk1 gene was integrated at the hsk1 locus as described previously (7).
MMS, UV, HU, and ionizing radiation sensitivity measurements.
Cells were grown in YE to mid-log phase and split into two aliquots. One was treated with 25 mM HU, and the second was mock treated. Samples were collected at the indicated time points, diluted, plated on YE, and incubated at 30°C for 5 days. The same process was used to determine sensitivity to 0.01 and 0.03% MMS. For the UV viability assay in the N-terminal mutants, cells were serially diluted, spotted onto YE plates, exposed to UV light at the indicated doses, and incubated at 30°C for 5 days. For the UV viability assay in the C-terminal mutants, equal numbers of cells were plated on YE plates, exposed to increasing doses of UV light, and incubated at 30°C for 5 days. For the gamma irradiation viability assay, a 10-ml liquid culture of log-phase cells was exposed to gamma irradiation. At the indicated dosages, aliquots were taken, diluted, plated on YE, and incubated at 30°C for 5 days. Viability was expressed as the percentage of colonies in the treated sample relative to those in the untreated sample.
Where indicated, mid-log-phase cultures were treated with 0.1% MMS for 30 min at 30°C instead of 2, 4, or 6 h with 0.03% MMS. For these samples, an equal volume of 20% (wt/vol) sodium thiosulfate was added to neutralize the MMS. Samples were then diluted, plated on YE plates, and incubated at 30°C for 5 days.
Cell cycle synchronization.
Cells were synchronized in G1 phase by shifting cdc10-129 mutants to 36°C for 4 h. Cells were shifted back to the permissive temperature (25°C) simultaneously with the addition of MMS to 0.1%. Cultures were synchronized in early S phase by incubation in 25 mM HU for 4 h. Cells were harvested, washed with cold H2O, and inoculated back into fresh media containing 0.1% MMS. Cells were synchronized in G2 by treating a mid-log-phase culture with benomyl (20 μg/ml) for 3 h. Cells were then harvested and resuspended in fresh media containing 0.1% MMS. Following 30 min in MMS, sodium thiosulfate was added (as described above) and the dilutions were plated on YE. Flow cytometry was performed to confirm that the blocks were successful (data not shown).
Kinase assays.
Wild-type and mutant Dfp1 were expressed as calmodulin-binding protein-hexahistidine fusions in Escherichia coli. Recombinant proteins were purified to near homogeneity by using Talon-Sepharose (Clontech) and calmodulin-agarose (Stratagene). Kinase assays contained 5 nM Hsk1 and 0.5 μM GST-Cdc191-222 and were performed as described previously (7). Cds1 activity gel assays were performed essentially as described previously (57). In brief, denatured extract from 107 cells per sample was fractionated on sodium dodecyl sulfate (SDS)-10% polyacrylamide gels cast with 0.5 mg of myelin basic protein (Sigma)/ml. The gel was subjected to a denaturation-renaturation protocol (57) and incubated for 2 h in kinase buffer (25 mM Tris-HCl, 10 mM MgCl2, 1 mM dithiothreitol, 50 μM [γ-32P]ATP; 50 μCi/ml). After extensive washing, the gel was dried and exposed to a storage phosphor screen.
Immunoblots and immunoprecipitation.
Native extracts were prepared from 109 cells containing protein A-tagged Hsk1 and 3HA-tagged Dfp1. Extracts were precipitated with rabbit IgG cross-linked to CNBr-activated agarose beads (Sigma) or unconjugated agarose beads (for the mock precipitations). After extensive washing, the proteins were eluted with 1% SDS and fractionated by SDS-polyacrylamide gel electrophoresis (PAGE). Immunoblots were probed with anti-hemagglutinin (HA) antibody 16B12 (Covance).
Chromosome stability and mitotic recombination.
Stable (nonsporulating) diploids were constructed that contained mutant dfp1 and complementing ade6− alleles. Diploids were grown in YE to log phase and treated with 0.03% MMS for 2 h. Samples were taken before and after MMS treatment, diluted, plated onto YE plates containing 5 mg of phyloxin B/liter, and grown at 30°C for 3 to 5 days to identify haploid colonies. In S. pombe, chromosome loss is followed rapidly by haploidization (3), and so chromosome loss rates can be inferred from haploidization rates. Recombination between complementing ade6− alleles in the diploids was measured by determining the number of ade6− diploids by replica-plating to Edinburgh minimal media lacking adenine.
RESULTS
The essential region of Dfp1 lies downstream of aa 240.
Sequence alignment of Dbf4 homologues has identified three highly conserved regions, termed motifs N, M, and C (36). In order to determine the function of these highly conserved domains, we constructed a series of site-directed mutations in dfp1+.
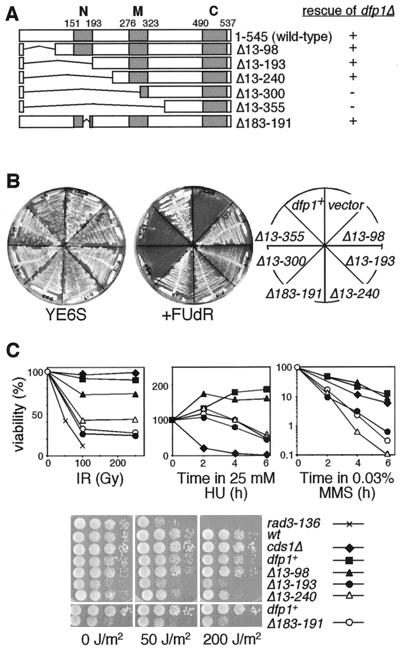
We first made a series of deletion mutations in the amino terminus of dfp1+ (Fig. 1A). Plasmids carrying mutant dfp1 alleles were introduced into a yeast strain carrying a deletion of the chromosomal dfp1+ gene rescued by dfp1+ carried on a second, counterselectable plasmid. Transformants were streaked onto media containing FUdR, which selects against cells carrying the wild-type dfp1+ plasmid (Fig. 1B). Under these conditions, cells could form colonies only if the mutant dfp1 gene could complement the dfp1 deletion. We found that mutations that deleted up to aa 240 of Dfp1, and therefore removed motif N, could fulfill the essential function of Dfp1. Further deletion, to aa 300 or 355, removed part or all of motif M and inactivated Dfp1. We confirmed that all mutants were expressed by using immunoblot analysis (data not shown). These results are in general agreement with recent data indicating that motif M but not motif N is required for the essential mitotic cell cycle function of Dfp1 (46).
FIG. 1.
The N terminus of dfp1 is dispensable for viability but is required for DNA damage resistance. (A) Schematic of the Dfp1 N-terminal mutants and summary of the dfp1Δ complementation analysis. The gray boxes indicate regions of high sequence identity with Dfp1 homologues. (B) Plasmid shuffle assay showing complementation of dfp1Δ by dfp1 N-terminal mutants. Transformants were streaked onto YE or YE plus 10 mg of FUdR/liter and grown at 30°C. Colony formation on FUdR indicates complementation. (C) dfp1 motif N mutants are sensitive to DNA-damaging agents and replication inhibitors. Wild-type (GBY562) (wt), dfp1Δ13-98 (GBY563), dfp1Δ13-193 (GBY564), dfp1Δ13-240 (GBY565), dfp1Δ183-191 (GBY566), cds1Δ (GBY180), and rad3-h1.5 (FY382) were treated with ionizing radiation (IR), HU, MMS, or UV radiation as described in Materials and Methods. Percent viability relative to that of the untreated sample is plotted.
Motif N mutants display moderate sensitivity towards a broad spectrum of DNA-damaging agents.
Previous work has indicated that mutations in motif N confer a mild sensitivity to the DNA replication inhibitor HU (56). We tested the sensitivity of the motif N mutant strains to HU, ionizing radiation, MMS, and UV radiation (Fig. 1C). Mutations that removed motif N, dfp1Δ13-193 and dfp1Δ13-240, conferred a moderate sensitivity to HU, UV, and ionizing radiation but were very MMS sensitive. These motif N mutants were less sensitive to UV and ionizing radiation than the checkpoint mutant rad3-h1.5, were less sensitive to HU than the checkpoint mutant cds1Δ, and were an order of magnitude more sensitive to MMS than cds1Δ. The dfp1Δ13-98 mutant behaved essentially like the wild type in these assays, indicating that this poorly conserved region of dfp1 is dispensable for the response to these damaging agents. We found that sensitivity to this broad spectrum of DNA-damaging agents could be recapitulated by a small 8-amino-acid deletion in motif N (dfp1Δ183-191), indicating a specific role for motif N in the response to these agents.
dfp1 mutants lacking motif C complement a deletion of dfp1+.
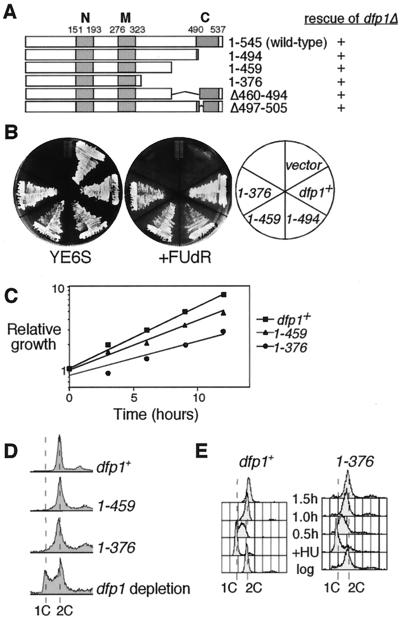
To determine whether the highly conserved C terminus was essential for viability, we constructed mutant alleles that removed regions of motif C (Fig. 2A). We again tested the ability of the mutant dfp1 alleles to rescue a dfp1+ deletion strain in a plasmid shuffle assay (Fig. 2B). As with deletion of motif N, we found that all of the truncation mutants that removed motif C could complement the dfp1 deletion. We have confirmed that the motif C mutants complement the dfp1 deletion when expressed from plasmids, either under dfp1 promoter control or under the control of a heterologous promoter, and when integrated into the chromosome (data not shown). We conclude that motif C is not required for the essential mitotic cell cycle function of dfp1. Subsequent experiments were performed with strains bearing stably integrated dfp1 alleles. Under normal growth conditions, the most extensive truncation mutants (dfp11-459 and dfp11-376) had slightly lower growth rates than those of the wild type (Fig. 2C) and had slightly lower plating efficiencies (data not shown). The flow cytometry profile of the mutants was similar to that of the wild type, although the 2C peak was somewhat broader (see below). Examination of the forward scatter flow cytometry profile of the mutants as well as microscopic examination revealed the presence of elongated cells. However, the mutants do not require the DNA damage checkpoint for viability, suggesting that most cells in the population do not accumulate large amounts of DNA damage during normal growth (data not shown). The mutants were not temperature sensitive (data not shown). It is particularly striking that approximately one-third of the Dfp1 protein is dispensable for its essential function, despite containing the most highly conserved region of the protein.
FIG. 2.
Motif C is not required for the essential function of dfp1+. (A) Schematic of the Dfp1 C-terminal mutants and summary of the dfp1Δ complementation analysis. (B) Plasmid shuffle assay showing complementation of dfp1Δ by dfp1 C-terminal mutants. Transformants were streaked onto YE or YE plus 10 mg of FUdR/liter and grown at 30°C. Colony formation on FUdR indicates complementation. (C) Relative growth of logarithmic-phase cultures of dfp1+ (GBY572), dfp11-459 (AFY6), and dfp11-376 (AFY7) cells was assessed by measuring the optical density of the cultures at the indicated time points. (D) The DNA contents of logarithmically growing cultures of dfp1+ (GBY572), dfp11-459 (AFY6), and dfp11-376 (AFY7) cells were measured by flow cytometry. The control sample (dfp1 depletion, AFY4) was obtained following the repression of dfp1 expression by growth in the presence of 5 μg of thiamine/ml for 4 h. The positions of 1C and 2C DNA contents are indicated. (E) Progression through S phase was examined by flow cytometry. dfp1+ (GBY572) and the motif C mutant dfp11-376 (AFY7) were grown to mid-logarithmic phase (log), blocked in 25 mM HU for 4 h (+HU), and then released into the cell cycle. Cultures were sampled at the indicated times following release from HU. The positions of 1C and 2C DNA contents are indicated.
Dfp1 has a well-characterized role in entry into S phase. We tested whether the dfp1 motif C mutant strains displayed defects in S-phase entry or progression. Flow cytometry profiles of the mutants were similar to that of the wild type (although the 2C peak was somewhat broader) and did not display any accumulation of G1 cells (Fig. 2D). By contrast, depletion of Dfp1 in log-phase cells resulted in an accumulation of G1 cells, indicative of a failure to enter S phase. We conclude that the motif C mutants enter S phase normally. Several lines of evidence suggest that Cdc7 kinase activity is required for appropriate S-phase progression following release from an HU block (4, 10). We blocked the motif C mutants in early S phase with HU, released them into fresh media, and monitored the transit through S phase by flow cytometry (Fig. 2E). dfp1+ cells completed DNA replication, as indicated by 2C DNA content, by 1 h following release from HU. The dfp11-376 mutant progressed through S phase with similar kinetics. These results indicate that the C terminus of Dfp1 is not essential for viability or for normal S-phase entry or S-phase progression.
Truncation mutants show an increased sensitivity to MMS.
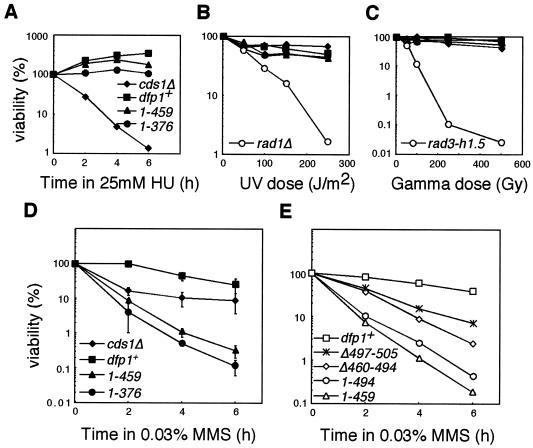
Previous studies have shown that Dfp1 may be involved in checkpoint responses (6, 46, 56). To see whether the C terminus was required for the appropriate response to DNA-damaging agents, we tested the sensitivity of the motif C mutants to HU and DNA-damaging agents. The mutants were resistant to HU, UV irradiation, and ionizing radiation, having sensitivity similar to that of the wild-type strain (Fig. 3A to C). However, they were specifically hypersensitive to the alkylating agent MMS (Fig. 3D). The most sensitive dfp1 mutant, dfp11-376, was at least 100-fold more sensitive to 0.03% MMS than was the wild-type strain. This spectrum of sensitivity is of particular interest, as most mutants that are sensitive to MMS also exhibit sensitivity to other DNA-damaging agents.
FIG. 3.
Motif C mutants are sensitive to MMS but not to HU, UV, or ionizing radiation. dfp1+ (GBY572), dfp11-459 (AFY6), dfp11-376 (AFY7), cds1Δ (GBY180), rad1Δ (GBY190), and rad3-h1.5 (FY382) were treated with HU (A), UV (B), or ionizing radiation (C) as described in Materials and Methods. Viability relative to that of the untreated culture is plotted. (D) dfp1+ (GBY572), dfp11-459 (AFY6), dfp11-376 (AFY7), and cds1Δ (GBY180) were treated with 0.03% MMS as described in Materials and Methods. Viability relative to that of the untreated culture is plotted. Points represent the means of the results of three experiments, and error bars span 1 standard deviation. (E) The region of the C terminus of dfp1+ that confers MMS resistance was mapped by treating dfp1+ (GBY572), dfp1Δ497-505 (AFY23), dfp1Δ460-494 (AFY16), dfp11-494 (AFY22), and dfp11-459 (AFY6) with MMS and plotting their viabilities relative to that of the untreated culture. A representative example of the results of two similar experiments is displayed.
To test whether the MMS sensitivity could be mapped to a smaller region of Dfp1, we generated Dfp1 mutants with smaller deletions and assayed their sensitivity to MMS (Fig. 3E). The dfp1Δ497-505 mutant, which lacks the most highly conserved amino acid segment within motif C, displayed only a mild sensitivity to MMS, indicating that these sequences do not make a major contribution to MMS resistance. Deletion of the region adjacent to motif C (dfp1Δ460-494) resulted in MMS sensitivity that was intermediate between those of dfp1Δ497-505 and dfp11-459. Removal of the carboxy terminus to aa 494 (dfp11-494) resulted in extreme MMS sensitivity. More extensive truncation of the C terminus, to aa 459 (dfp11-459) or 376 (dfp11-376), exacerbated this effect. Taken together, these results indicate that the region containing motif C (aa 495 to 545) makes a major contribution to the MMS resistance function of Dfp1. The adjacent region, aa 460 to 494, makes a lesser, yet significant, contribution, as deletion of this region results in significant MMS sensitivity. This suggests that two partially redundant regions of Dfp1 may contribute to MMS resistance or that the region extending from aa 376 to 494 is important for folding of the region containing motif C. Since dfp11-376 and dfp11-459 display similar MMS sensitivity and are more MMS sensitive than the other dfp1 motif C mutants, they were chosen for further analysis. Although these mutations delete sequences in addition to motif C, we refer to them as motif C mutants for the sake of convenience.
Motif C mutants have intact intra-S and mitotic checkpoints.
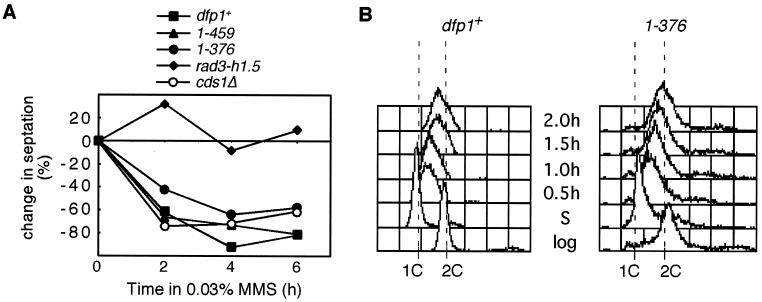
To investigate the cause of the MMS sensitivity of the motif C mutants, we tested whether the intra-S and mitotic checkpoint responses to MMS were intact in these strains. The mitotic checkpoint is activated by MMS in wild-type cells and prevents them from entering mitosis in the presence of DNA damage (reviewed in references 14, 18, 50, and 62). We monitored the mitotic progression of cultures following treatment with MMS by counting the percentage of cells with a septum (Fig. 4A). Checkpoint-defective strains, such as rad3 mutants, failed to block mitosis in the presence of MMS, showed no decrease in septation (Fig. 4A, rad3-h1.5), and displayed the classic “cut” phenotype (data not shown). The motif C mutants dfp11-459 and dfp11-376 both showed decreases in septation and displayed an elongated phenotype, like that of the wild-type strain, following MMS treatment. Recovery-defective mutants, such as cds1Δ, also showed decreases in septation (Fig. 4A) and displayed elongated cells (data not shown) in the presence of MMS. We conclude that the motif C mutants have an intact mitotic checkpoint.
FIG. 4.
dfp1 motif C mutants have intact mitotic and intra-S-phase checkpoints. (A) dfp1+ (GBY572), dfp11-459 (AFY6), dfp11-376 (AFY7), rad3-h1.5 (FY382), and cds1Δ (AFY32) were sampled at the indicated times following the addition of MMS. Cells were stained with calcofluor, and septa were visualized by fluorescence microscopy. The change in septation relative to that of the untreated culture is plotted. (B) Logarithmically growing dfp1+ (GBY572) and dfp11-376 (AFY7) cells were blocked in HU for 4 h and then released into medium containing 0.03% MMS. The samples were removed before the HU block (log), after 4 h in 25 mM HU (S), and at the indicated times after release from HU. The DNA content of the samples was analyzed by flow cytometry. The positions of 1C and 2C DNA contents are indicated.
The intra-S-phase checkpoint slows the progression of DNA replication when DNA is damaged during S phase (34, 49). In budding yeast, this slow S phase is the consequence of checkpoint-dependent repression of origin firing combined with checkpoint-independent inhibition of replication fork progression (59). We synchronized cells in early S phase by using HU (since the motif C mutant strains are not HU sensitive) and then released them into fresh media lacking HU and containing 0.03% MMS. The progression of S phase was monitored by flow cytometry. Motif C mutants released into fresh media after an HU block completed S phase within 60 min, with kinetics similar to those of the wild type (Fig. 2E). In the presence of MMS, the completion of S phase was delayed significantly (Fig. 4B) in both the wild-type and dfp11-376 strains. In both cases, cells progressed through S phase slowly, requiring over 2.5 h for the completion of S phase. We conclude that the intra-S-phase checkpoint is intact in the motif C mutant strains.
Motif C mutations are recessive and can be rescued by overexpression of hsk1+.
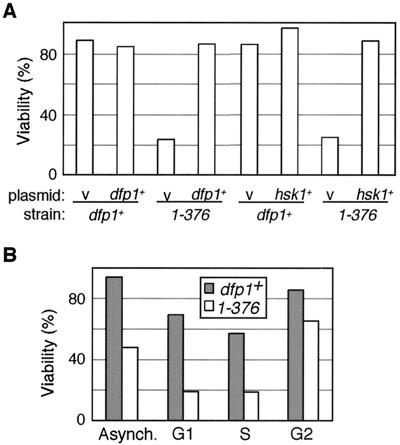
To determine whether the mutant dfp1 alleles are dominant or recessive, we expressed dfp1+ from a plasmid in the mutant strains and assayed their sensitivity to a 30-min exposure to 0.1% MMS (Fig. 5A). Under these conditions, approximately 90% of dfp1+ cells remained viable. Consistent with the results of the time course viability assays (Fig. 3), the dfp11-376 mutant was sensitive to this exposure to MMS, with only 22% of cells remaining viable. The expression of dfp1+ in the dfp11-376 mutant rescued the MMS sensitivity back to wild-type levels, indicating that the motif C mutation is recessive (Fig. 5A). We tested whether the MMS sensitivity of dfp11-376 could be suppressed by overexpressing hsk1+, which encodes the partner kinase of Dfp1 (Fig. 5A). Overexpression of hsk1+ in a dfp1+ background resulted in a slight increase in percent viability relative to that of the empty vector control. When hsk1+ was overexpressed in the mutant dfp11-376 strain, viability was restored to wild-type levels. This suggests that the role of dfp1+ in the response to MMS is mediated by the activation of Hsk1 kinase.
FIG. 5.
dfp11-376 MMS sensitivity is recessive and can be suppressed by overexpression of hsk1+. (A) Wild-type (df1+) and dfp11-376 strains carrying either the empty vector pIRT2U (v) (AFY28 and AFY30), the dfp1+ plasmid pIRT2Udfp1 (dfp1+) (AFY29 and AFY31), the empty vector pSLF172 (v) (AFY24 and AFY26), or the hsk1+ overexpression plasmid pSLF172hsk1 (hsk1+) (AFY25 and AFY27) were treated with 0.1% MMS for 30 min. Viability is plotted relative to that of the untreated culture. (B) dfp1+ and dfp11-376 cells were blocked in G1 by shifting a cdc10-129 mutant to 36°C for 4 h, in S by incubation with 25 mM HU for 4 h, and in G2 by incubation with 20 μg of benomyl/ml for 3 h. Blocked or cycling (Asynch.) cells were treated with 0.1% MMS for 30 min and plated. Viability relative to that of the untreated culture is plotted.
The dfp11-376 mutant is sensitive to DNA damage during S phase.
The level of Dfp1 protein is cell cycle regulated. Dfp1 accumulates as cells enter S phase and persists through G2 into late M phase, when levels decrease (6, 56). We asked whether the MMS sensitivity of the motif C mutants occurs during a particular phase of the cell cycle. We synchronized cells in G1, S, or G2 phase, released the cells into 0.1% MMS for 30 min, inactivated the MMS, and measured the effect on viability (Fig. 5B). Under these conditions, in an asynchronous cell culture, the dfp11-376 mutant strain showed a 50% reduction in viability relative to that of dfp1+. In G1 and S phases, dfp11-376 was twice as sensitive as it was in asynchronous culture. In G2 phase, dfp11-376 was actually less sensitive than it was in asynchronous culture. We conclude that the MMS sensitivity of dfp11-376 does not occur in G2. The greatest sensitivity to DNA damage was seen in G1 and S phases, although the sensitivity in G1 is likely the result of cells entering S phase with DNA damage that has persisted from G1. We also noted that the dfp1+ cells were most sensitive to MMS during S phase; however, the motif C mutant dfp11-376 conferred additional sensitivity to MMS during S phase compared to that of wild-type cells.
Motif C mutant Dfp1 proteins interact with Hsk1 but are poor activators of Hsk1 kinase.
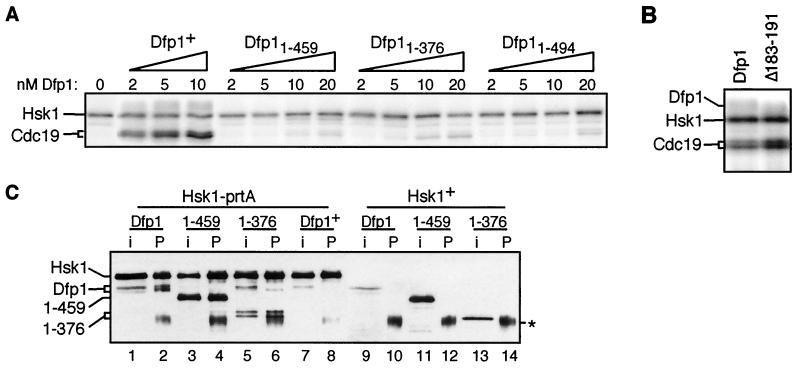
Hsk1 is capable of autophosphorylation but requires Dfp1 in order to phosphorylate exogenous substrates (6, 7, 56). We assessed the ability of increasing amounts of purified motif C mutant Dfp1 proteins to activate Hsk1 phosphorylation of the MCM protein Cdc19 in vitro. As previously reported (6, 7, 56), Hsk1 was unable to phosphorylate Cdc19 in the absence of Dfp1 (Fig. 6A). The addition of increasing amounts of Dfp1 resulted in increasing Cdc19 phosphorylation. In the presence of increasing amounts of the motif C mutants Dfp11-494, Dfp11-459, and Dfp11-376, there was little Cdc19 phosphorylation. Activation by wild-type Dfp1 was readily detectable at 2 nM, whereas mutant Dfp1 concentrations as high as 20 nM resulted in only very low levels of Cdc19 phosphorylation. It is worth noting, however, that the low levels of activity in the presence of motif C mutant Dfp1 proteins were consistently above the background levels observed in the absence of Dfp1. The defect in kinase activation in vitro is consistent with the suppression of MMS sensitivity by hsk1+ overexpression (Fig. 5A).
FIG. 6.
(A) dfp1 motif C mutants are poor Hsk1 activators in vitro. Cdc19 kinase assays were performed by incubating 5 nM Hsk1 with the indicated amounts of purified Dfp1, Dfp11-459, Dfp11-376, and Dfp11-494 protein in the presence of [γ-32P]ATP. Reaction products were fractionated by SDS-PAGE and exposed to a PhosphorImager screen. The positions of Hsk1 and Cdc19 are indicated. (B) A motif N mutant Dfp1 is an efficient activator of Hsk1 in vitro. Cdc19 kinase assays were performed with 5 nM Hsk1 and 5 nM Dfp1 or Dfp1Δ183-191. Reaction products were fractionated by SDS-PAGE and exposed to a PhosphorImager screen. (C) Dfp1 motif C mutants bind to Hsk1. Extracts were prepared from strains expressing protein A-tagged Hsk1 (lanes 1 to 8) and untagged Hsk1 (lanes 9 to 14). Extracts were incubated with IgG-agarose, and precipitates were fractionated by SDS-PAGE and transferred to nitrocellulose. Hsk1 and Dfp1 were detected with the anti-HA epitope antibody 16B12. For each strain, a sample equivalent to half of the input extract (i) was fractionated alongside the entire immunoprecipitate (P). Strains coexpressed the following proteins: lanes 1 and 2, Hsk1-prtA and Dfp1-3HA (JOY7); lanes 3 and 4, Hsk1-prtA and Dfp11-459-3HA (JOY8); lanes 5 and 6, Hsk1-prtA and Dfp11-376-3HA (JOY9); lanes 7 and 8, Hsk1-prtA and untagged Dfp1 (JOY6); lanes 9 and 10, untagged Hsk1 and Dfp1-3HA (GBY572); lanes 11 and 12, Hsk1 and Dfp11-459-3HA (AFY6); lanes 13 and 14, Hsk1 and Dfp11-376-3HA (AFY7). The asterisk indicates IgG heavy chain. Dfp11-376 appears as a doublet in some experiments (lanes 5 and 6), likely due to proteolysis in vitro.
We also assayed the ability of the motif N mutant, dfp1Δ183-191, to activate the Cdc19 kinase activity of Hsk1 (Fig. 6B). In contrast to the motif C mutants, we found that the motif N mutant is completely competent for Hsk1 activation. Therefore, motif N is not involved in kinase activation.
To determine whether the deletion of motif C of Dfp1 has an effect on its ability to bind to Hsk1, we performed coimmunoprecipitation experiments. Extracts were made from strains expressing tagged Hsk1 and either Dfp1, Dfp11-459, or Dfp11-376. The protein A-tagged Hsk1 was precipitated with IgG-agarose, and interacting Dfp1 proteins were detected by immunoblot analysis (Fig. 6C). Wild-type Dfp1 was coprecipitated essentially quantitatively with Hsk1 (Fig. 6C, lanes 1 and 2). The motif C mutants Dfp11-459 and Dfp11-376 both coprecipitated efficiently with Hsk1 (Fig. 6C, lanes 3 to 6), indicating that motif C is not required for the binding of Dfp1 to Hsk1. None of the Dfp1 proteins were precipitated from extracts of strains expressing untagged Hsk1 (Fig. 6C, lanes 9 to 14). The ability of Dfp1 proteins lacking motif C to bind Hsk1 agrees with data on both fission and budding yeast that suggest that Dfp1 has two regions that are responsible for Hsk1 binding and that are at least partially redundant (12, 46, 56). We also noted differences in the steady-state expression levels between the wild-type Dfp1 and the motif C mutant proteins. The mutant proteins accumulated to higher levels than the wild-type Dfp1 (compare Fig. 6C, lane 9 to lanes 11 and 13), even though all of the dfp1 alleles were integrated and were under the control of the dfp1+ promoter. The basis for the differences in expression levels remains to be determined. Increased expression of the mutants could result in sufficient activation of Hsk1 to fulfill the essential function of the kinase in vivo.
dfp1+ does not appear to be function in any of the major alkylation resistance pathways.
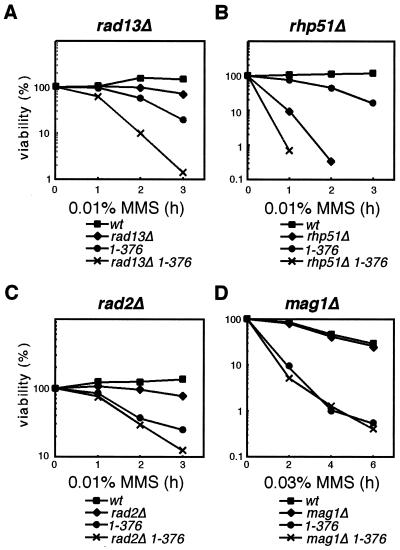
Since the motif C mutants are extremely sensitive to MMS, despite having intact intra-S and mitotic checkpoints, we tested whether dfp1 was in any of the pathways known to contribute to resistance to MMS damage in S. pombe. Recent work has identified three pathways with detectable roles in MMS repair (39). Although MMS damage is repaired by base excision repair in most eukaryotes, in fission yeast, recombination repair makes the most significant contribution to resistance to MMS damage (39). We performed epistasis analysis with mutants in nucleotide excision repair (rad13), recombination repair (rhp51), and base excision repair (mag1) pathways, as well as in the nuclease rad2 (Fig. 7). The classical interpretation of epistasis experiments in DNA repair studies is that for two genes in different pathways, the double mutant will give greater sensitivity than either mutant alone, whereas double mutants in the same pathway will be no more sensitive than the most sensitive of the single mutants.
FIG. 7.
dfp1 motif C acts outside of the known alkylation resistance pathways. Double-mutant strains carrying the motif C mutant dfp11-376 and rad13Δ (AFY49) (A), rhp51Δ (AFY54) (B), rad2Δ (AFY63) (C), or mag1Δ (AFY71) (D) were treated with 0.01 or 0.03% MMS. Colony-forming ability was analyzed at the indicated times. Viability relative to that of an untreated sample is plotted. Representative examples of the results of two similar experiments are presented. wt, wild type.
MMS viability assays were performed for the each of the single and double mutants. rad13Δ (Fig. 7A) displayed an intermediate sensitivity to MMS compared to those of dfp1+ and dfp11-376. The rad13Δ dfp11-376 double mutant was 10-fold more sensitive than the most sensitive single mutant, which suggests that rad13 and dfp1 act in distinct pathways. rhp51Δ was much more sensitive to MMS than any of the other mutants tested, in agreement with its important role in repairing MMS damage in S. pombe (39). The rhp51Δ dfp11-376 double mutant was more sensitive to MMS than rhp51Δ, by 10-fold after 1 h in MMS (Fig. 7B). This increased sensitivity of the double mutant at 1 h was observed in three separate experiments. Because of the extreme sensitivity of the double mutant to MMS, we were unable to recover any viable cells in the 2- and 3-h samples. We conclude that dfp1 functions in a separate pathway from rhp51. Similar results were obtained with rad2Δ dfp11-376 mutants (Fig. 7C), indicating that the role of dfp1 in MMS resistance is distinct from the rad2 pathway. Analysis of mag1Δ dfp11-376 is particularly interesting (Fig. 7D). mag1Δ alone did not display significant sensitivity to MMS under our experimental conditions, so it is not surprising that the mag1Δ dfp11-376 was no more sensitive than dfp11-376 alone. It is significant, however, that mag1Δ did not rescue the sensitivity of dfp11-376. This indicates that the initiation of base excision repair is not required in order for dfp1 mutants to confer MMS sensitivity. By contrast, mutations in the recombination repair pathway require mag1+ in order to confer MMS sensitivity (mag1Δ rhp51Δ double mutants are less MMS sensitive than either single mutant) (39). These results suggest that in addition to functioning outside of known MMS repair pathways, motif C of dfp1 may define an MMS response that is unrelated to the repair of the alkylated bases.
dfp1+ is in both the chk1+ and cds1+ checkpoint pathways.
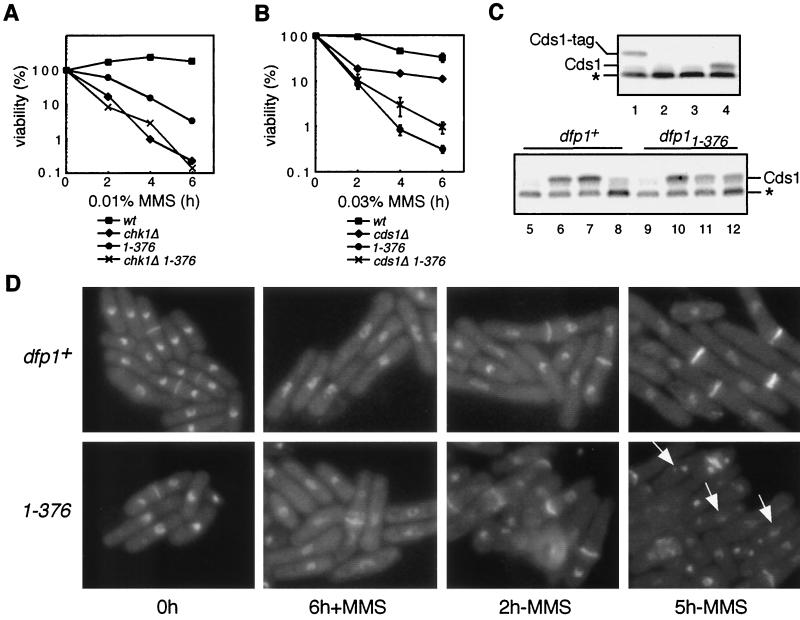
In fission yeast, the signal transduction pathway that mediates checkpoint responses bifurcates downstream of the checkpoint kinase Rad3 (45, 51). DNA-damaging agents such as ionizing radiation cause Rad3-dependent activation of the Chk1 protein kinase, whereas the DNA replication inhibitor HU causes a Rad3-dependent activation of the Cds1 protein kinase. Both Chk1 and Cds1 play roles in MMS resistance. chk1Δ and cds1Δ strains are MMS sensitive (Fig. 8A and B), Chk1 is activated by MMS (61), and Cds1 is required for the intra-S-phase checkpoint response to MMS (34). We performed epistasis analysis with dfp11-376 and chk1Δ to determine whether dfp1+ and chk1+ act in the same genetic pathway (Fig. 8A). We found that the chk1Δ and chk1Δ dfp11-376 strains had very similar MMS sensitivities, suggesting that dfp1+ and chk1+ act genetically in the same MMS resistance pathway. Furthermore, the dfp11-376 strain delays mitosis in response to MMS treatment (Fig. 4A), whereas the chk1Δ strain does not (data not shown), and the chk1Δ strain is significantly more MMS sensitive than the dfp11-376 strain (Fig. 8A). This indicates that Chk1 has roles in the MMS response pathway that Dfp1 does not (for example, induction of mitotic delay) and suggests that Chk1 is upstream of Dfp1 in the MMS response. Thus, Dfp1 may be a target of Chk1 regulation following MMS treatment.
FIG. 8.
(A) dfp11-376 is epistatic to chk1Δ. dfp1+ (GBY572) (wt), chk1Δ (AFY72), dfp11-376 (AFY7), and chk1Δ dfp11-376 (AFY74) were treated with 0.01% MMS for the indicated times. Percent viability relative to that of the untreated culture is plotted. Points represent the means of the results of three experiments. (B) cds1Δ partially suppresses the MMS sensitivity of dfp11-376. dfp1+ (GBY572) (wt), cds1Δ (AFY32), dfp11-376 (AFY7), and cds1Δ dfp11-376 (AFY34) were treated with 0.03% MMS for the indicated times. Percent viability relative to that of the untreated culture is plotted. Points represent the means of the results of two experiments, and error bars span 1 standard deviation. (C) Cds1 is not appropriately downregulated in the motif C mutant during recovery from MMS. dfp1+ and dfp11-376 cultures were treated with 0.03% MMS for 2 h. Cells were harvested and reinoculated into fresh medium lacking MMS. Cultures were sampled for extract preparation before MMS addition (lanes 5 and 9), following 2 h of MMS treatment (lanes 6 and 10), 3 h after the removal of MMS (lanes 7 and 11), and 6 h after removal of MMS (lanes 8 and 12). As controls, extracts were also prepared from an MMS-treated strain expressing tagged Cds1 (TWY3, lane 1), from an MMS-treated cds1 deletion strain (GBY180, lane 2), and from a dfp1+ strain before (lane 3) and after (lane 4) 2 h of MMS treatment. The Cds1 kinase was assayed on myelin basic protein-impregnated activity gels, as described in Materials and Methods. The positions of Cds1 and tagged Cds1 (Cds1-tag) are indicated. The asterisk indicates a phosphoprotein of unknown identity. (D) dfp11-376 displays fragmented nuclei during recovery from MMS damage. dfp1+ (GBY572) and dfp11-376 (AFY7) were treated with 0.03% MMS for 6 h. Cells were harvested and reinoculated into medium lacking MMS, and growth was continued for a further 5 h. Cultures were sampled at the indicated times, and fixed cells were stained with DAPI and calcofluor and visualized by fluorescence microscopy. Arrows indicate cells with a fragmented nucleus.
Several lines of evidence have implicated the checkpoint kinase Cds1 in the regulation of Hsk1/Dfp1. Both Hsk1 and Dfp1 are phosphorylated in vivo in a Cds1-dependent manner in response to HU (6, 54), and the temperature sensitivity of the hsk1-1312 allele is suppressed by cds1Δ, suggesting that Cds1 might negatively regulate Hsk1 (54). In addition, recent evidence indicates that hsk1+ is required for the activation of Cds1 in vivo (57). We constructed a cds1Δ dfp11-376 double mutant and compared its MMS sensitivity to those of each of the single mutants (Fig. 8B). As seen previously (Fig. 3D), dfp11-376 was considerably more MMS sensitive than cds1Δ, which suggests that Dfp1 cannot simply be a downstream effector of Cds1 in the MMS response pathway. Surprisingly, we found that deletion of cds1 partially rescued the MMS sensitivity of dfp11-376 (Fig. 8B). This result is consistent with Cds1 being a negative regulator of Dfp1, such that its deletion causes an increase in Dfp1 function and thereby a decrease in MMS sensitivity.
We next examined the activation and inactivation of Cds1 in wild-type and motif C mutant strains following treatment with MMS by using kinase activity gels (Fig. 8C). In these, the activity of Cds1 in crude extracts was assayed directly in polyacrylamide gels impregnated with the substrate myelin basic protein. With the wild-type strain, treatment with 0.03% MMS for 2 h induces activation of Cds1, as evidenced by the incorporation of 32P at the position of Cds1 (Fig. 8C, lane 4). Activated Cds1 was absent before MMS treatment (lane 3), was absent in an MMS-treated cds1Δ strain (lane 2), and was shifted to a higher apparent molecular weight in a strain carrying only a tagged cds1 gene (lane 1). In the wild-type strain, activated Cds1 persisted for 3 h following the removal of MMS (lane 7) but disappeared by 6 h (lane 8). In the motif C mutant dfp11-376 strain, Cds1 was similarly activated by MMS treatment (lanes 9 and 10). Following the removal of MMS, the activated Cds1 persisted for at least 6 h (lanes 11 and 12), albeit at slightly reduced levels relative to those of the activated sample (lane 10). Therefore, the Cds1-dependent checkpoint remained activated in the dfp11-376 mutant for a longer period of time than is evident in the wild-type strain, suggesting that the motif C mutants were defective in the inactivation of Cds1 during recovery from MMS damage. This could be the result of persistent checkpoint signals resulting from, for example, collapsed replication forks.
Nuclear fragmentation in the motif C mutant during recovery from MMS.
We examined the cellular morphology of the dfp11-376 strain following MMS treatment and during recovery from MMS-induced DNA damage (Fig. 8D) by fixing cells and incubating with DAPI (4′,6′-diamidino-2-phenylindole) to stain the nuclei and calcofluor to stain the division septa. After 6 h of MMS exposure (Fig. 8D, 6 h +MMS), elongated cells were evident in both the wild-type (dfp1+) and mutant (dfp11-376) strains, indicating that MMS damage caused a mitotic delay in both strains. Following the removal of MMS from the dfp1+ culture, dividing forms with two nuclei and a division septum were observed (Fig. 8D, 2 h −MMS and 5 h −MMS), indicating that the wild-type cells had successfully reentered the cell cycle. By contrast, following the removal of MMS from the dfp11-376 culture, cells undergoing abnormal mitosis and displaying multiple abnormal DAPI-staining bodies were observed. This morphology is reminiscent of the nuclear fragmentation phenotype observed in the recovery mutant rqh1Δ (55). Since abnormal cells appeared after the removal of MMS and at a time when wild-type cells were resuming normal cell division, this phenotype is consistent with a role for the Dfp1 C terminus in recovery from MMS damage.
Motif C is required for chromosome stability and to suppress recombination following MMS treatment.
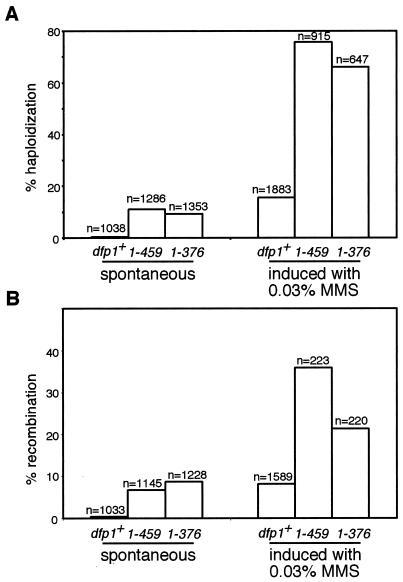
In an effort to determine the mechanism by which the dfp1 motif C mutants lose viability in MMS, we examined the spontaneous and MMS-induced rates of recombination and chromosome loss in the mutant strains (Fig. 9). In diploid S. pombe, the loss of one chromosome is followed by the rapid loss of the other two chromosomes, resulting in the formation of a haploid (3). We constructed stable homozygous diploids and assayed chromosome loss by measuring haploidization (Fig. 9A). In a log-phase population of cells, the spontaneous chromosome loss rate was significantly elevated for both motif C mutants relative to that of the wild type, with dfp11-459 showing a 23-fold increase and dfp11-376 showing a 19-fold increase. Exposure to MMS increased chromosome loss significantly in the wild-type strain (by 32-fold), indicating that alkylation destabilizes chromosomes. Following MMS treatment, the motif C mutants showed a particularly dramatic increase in chromosome loss. In fact, in this assay almost 80% of the viable mutant cells underwent chromosome loss and haploidization, indicating that motif C is important for chromosome stability following alkylation damage of DNA.
FIG. 9.
dfp1 motif C mutants have elevated chromosome loss and recombination rates. Wild-type (AFY57) (dfp1+), dfp11-459 (AFY60), and dfp11-376 (AFY68) homozygous diploids were grown from single colonies to mid-logarithmic phase, and aliquots were plated to measure spontaneous rates of chromosome loss (A) and mitotic recombination (B). A sample of the culture was treated with MMS, and aliquots were plated to measure induced rates of chromosome loss (A) and mitotic recombination (B). The number of colonies counted for each sample is indicated. Representative examples of the results of two similar experiments are shown.
The stable diploid strains also contained complementing adenine mutations that made it possible to measure mitotic recombination (Fig. 9B). The motif C mutants had significantly elevated mitotic recombination rates compared to that of the wild type. MMS treatment induced mitotic recombination rates for dfp1+ and the dfp1 mutants that were considerably higher than the spontaneous rate. Therefore, motif C of dfp1 also plays an important role in suppressing recombination following alkylation damage. A logical extension of these findings is that the tremendous loss of viability seen in dfp1 motif C mutants is due to abnormal recombination and chromosome loss during recovery from MMS-induced DNA damage.
DISCUSSION
A number of studies have focused on the essential role of dfp1+ in the initiation of DNA replication (6, 46, 56). Recent work has indicated that the C terminus of Dfp1, but not the N terminus, is required for the essential function of Dfp1 (46, 56). In contrast, we have found that the C terminus, like the N terminus, is dispensable. The discrepancy between our study and the previous work may be due to the different methods used to assess complementation by mutant dfp1 alleles. In the present study, we used a plasmid shuffle strategy in which mutant dfp1 alleles were introduced on a plasmid into a strain carrying a complete deletion of the chromosomal dfp1+ open reading frame, covered by dfp1+ on a counterselectable plasmid. Transformants carrying the mutant dfp1 genes over the dfp1 deletion were isolated after selection against the wild-type dfp1+ plasmid. We confirmed the presence of the dfp1 deletion and the absence of the wild-type dfp1+ gene by PCR. Since our finding that motif C was dispensable was surprising, we confirmed it by expressing the mutant dfp1 alleles under the control of different promoters, including a repressible one, in which case the survival of the strain was shown to require expression of the mutant dfp1 (data not shown). Finally, we integrated the mutant dfp1 genes stably into the chromosome in a dfp1 deletion strain and again confirmed that the strains were viable. We also found that the motif C mutant dfp1 alleles conferred recessive MMS sensitivity phenotypes, as they could be rescued by dfp1+, demonstrating the absence of a wild-type dfp1+ gene in our mutant strains.
Despite their dispensable nature, motif N and motif C contain the regions of highest sequence identity among Dbf4 homologues, suggesting an important conserved function for these regions. Hence, we have attempted to elucidate the roles of the nonessential motifs N and C. In characterizing the motif N and motif C mutants, we found that the motifs could be distinguished by their sensitivities to different DNA-damaging agents. Motif N mutants were moderately sensitive to a broad range of DNA-damaging agents and the DNA synthesis inhibitor HU. In contrast, motif C was dispensable for resistance to UV radiation, ionizing radiation, and HU. Motif C was required specifically for resistance to the alkylating agent MMS. Thus, Dfp1 has at least two genetically separable roles in the DNA damage response.
A postinitiation function for dfp1+.
Analysis of conditional hsk1 mutants demonstrated a role for hsk1+ that was temporally distinct from its role in promoting the initiation of DNA replication (54). We found evidence for a postinitiation function for dfp1+, and this function is critical to a cell's ability to resist alkylation damage. Mutants in motif C enter S phase normally and progress through S phase with wild-type kinetics, suggesting that the initiation activity of dfp1+ is not compromised. However, these mutants exhibit significant sensitivity to MMS. Of particular interest is the fact that cells that were synchronized with HU in early S phase, a point at which Dfp1 should have already acted at early-firing origins, exhibited the greatest sensitivity to MMS. This indicates that the role of dfp1+ in MMS resistance occurs largely in S phase, but after the bulk of initiation has occurred. While it is formally possible that a defect in Dfp1 function at initiation could cause MMS sensitivity later in S phase, the simplest interpretation of our results is that Dfp1 has a second function and that this is distinct from its function in initiation. Further evidence that dfp1+ functions in MMS resistance during S phase comes from the suppression of MMS sensitivity by the deletion of cds1. Cds1 is activated by damage specifically during S phase (34). We also found that the MMS sensitivity of the motif C mutants could be suppressed by hsk1+ overexpression, indicating that this novel function of dfp1+ involves the known activity of Dfp1 as an activator of Hsk1.
Two aspects of the novel function of the Dfp1 C terminus in alkylation resistance are of particular interest. First, this function is specific for alkylation damage yet seems unrelated to the repair of the damage. We found that the dfp1 mutants are not epistatic to mutations in the known MMS repair pathways, defined by the rhp51+, rad13+, rad2+, and mag1+ genes. While it is possible that dfp1+ functions in multiple MMS repair pathways, the simplest explanation for our results is that dfp1+ functions outside of the repair of MMS lesions. This, taken together with the evidence that the motif C mutants are particularly sensitive to MMS during S phase, suggests that the C terminus of Dfp1 may have a specific role in encounters between the replication machinery and methylated bases. It is noteworthy in this respect that MMS is known to slow replication forks in vivo (59). Secondly, there are indications that Hsk1 kinase activity is reduced in the Dfp1 motif C mutants and that this reduction affects two processes (initiation and alkylation resistance) differently. This might reflect a quantitative difference in the kinase requirements of the two processes such that initiation requires less Hsk1 kinase activity than does MMS resistance. Alternatively, this could indicate a qualitative difference between the kinase requirements of the processes. In this case, the C terminus of Dfp1 might be involved in targeting Hsk1 to effectors that are specifically involved in MMS resistance.
dfp1 mutants are defective in recovery from damage.
A number of checkpoint mutants are defective in recovery from DNA damage, including cds1Δ, rad26-T.12, rad11Δ, hus1Δ, and rqh1Δ (1, 15, 34, 41, 48, 55). The hallmark of this class of mutants is that they either are competent for activation of the mitotic checkpoint yet lose viability rapidly following exposure to DNA-damaging agents or lose viability independently of passage through an aberrant mitosis. Mutations in motif C of dfp1+ exhibit characteristics of recovery mutants. We found that damage induced a mitotic delay in the motif C mutants, as in wild-type cells, yet the motif C mutants were sensitive to very brief exposure to MMS, particularly during S phase. Models to explain this characteristic in other recovery mutants typically invoke the occurrence of some irreversible event following DNA damage from which the cells are unable to recover (1, 15, 34). In the dfp1 mutants, the best candidate for such an event is a stalled replication fork. MMS is known to cause a dramatic reduction in the rate of replication fork progression (59). In cells lacking Rad53, the budding yeast homologue of Cds1, MMS causes a high rate of irreversible replication fork collapse (59). It will be of considerable interest to determine whether dfp1 mutants suffer a further reduction in the rate of fork progression or an increase in fork collapse. Our finding that dfp1 mutants have a high rate of recombination and chromosome loss is consistent with an increase in the frequency of fork collapse, as is the nuclear fragmentation that we observed during recovery from MMS-induced DNA damage.
The role of Cds1.
Genetic suppression data from mutants in hsk1 (54) and dfp1 (this work) suggest that Cds1 is a negative regulator of the Hsk1/Dfp1 kinase. There is also an indication that Cds1 is downstream of Hsk1 and Dfp1, as the activation of Cds1 in response to HU is defective in hsk1-89 strains (57). In contrast, we find that Cds1 activation does not require motif C of Dfp1. However, the appropriate downregulation of Cds1 during recovery from MMS does require intact Dfp1. The simplest explanation for the persistent activation of Cds1 in the dfp1 mutant is that DNA damage persists in these cells. Deletion of cds1 prevents or alleviates this persistent damage by causing an increase in Hsk1/Dfp1 activity. More complex models in which Dfp1 plays a direct role in regulating Cds1 are also consistent with our results. Further experiments will be required to elucidate the relative contributions of Cds1 and Dfp1 to recovery from MMS damage in S-phase cells.
The role of Chk1.
A role for Chk1 in resisting MMS damage has not previously been described. Although Chk1 is known to be activated by MMS damage (61), Chk1 is not required for the intra-S-phase checkpoint (34) and the sensitivity of chk1Δ strains to MMS has not previously been assessed. We found that chk1Δ strains were significantly sensitive to MMS and that dfp11-376 was epistatic to chk1Δ with respect to MMS sensitivity, suggesting that Chk1 has a novel role in the DNA damage response. In addition to its well-characterized role in regulating mitosis by phosphorylation of Cdc25 (16, 17, 51), we propose that Chk1 may function in the MMS damage response by regulating Dfp1. Previous studies have shown that Chk1 activation is blocked when Cds1 is activated by HU treatment (5). We have found that MMS activates Cds1 and that MMS resistance requires Chk1 function. This suggests that the regulation of Chk1 following MMS-induced DNA damage differs from that following the inhibition of DNA replication by HU.
A model for dfp1+ function in alkylation resistance.
Our data are consistent with the following model of the role of Dfp1 in the response to MMS. Treatment of cells with MMS causes stalling of replication forks, presumably as they encounter DNA damage. In a wild-type cell, the intra-S-phase checkpoint stabilizes these stalled forks (14, 35, 59), and proteins like Rqh1 contribute to this stabilization by suppressing inappropriate recombination at the stalled forks (55). We propose that Dfp1 is important for restarting DNA synthesis following replication fork stalling. This could involve the resumption of DNA synthesis at existing forks or the firing of late origins of replication. The requirement of Cdc7/Dbf4 (the budding yeast homologues of Hsk1/Dfp1) for late origin firing is well established (4, 10), while a role for Dfp1 in restarting synthesis at a stalled fork remains speculative. It is of interest in this respect that the most likely candidates for relevant substrates of Hsk1/Dfp1 in initiation are proteins that are also believed to be present at replication forks. A great deal of circumstantial evidence suggests that MCMs, particularly Mcm2, are relevant Hsk1/Dfp1 substrates (reviewed in references 24, 32, and 53). In addition to being a component of the pre-RC, the MCM complex also appears to be at the replication fork (2, 28). Similarly, the Cdc45 protein is a putative Cdc7 family kinase target (44) and may be associated with replication forks (2). Phosphorylation of these targets by Hsk1/Dfp1 could be important in restarting stalled replication forks. In the present study, we have shown that mutations in motif C of dfp1 lead to a persistent checkpoint signal during recovery from MMS damage, aberrant recombination, and chromosome loss. All are consistent with an ineffective resumption of DNA synthesis that causes replication intermediates to persist, which results in checkpoint activation, recombination, and chromosome instability.
Alternatively, the motif C mutants could yield replication forks that are inherently unstable. Ongoing phosphorylation of Hsk1/Dfp1 targets might be important for fork processivity throughout S phase, or phosphorylation of these targets at the time of origin firing could be critical for the subsequent formation of appropriately processive replication forks. The wild-type kinetics of S-phase progression in the motif C mutants argue against such a model, however, as do the kinetics of S-phase progression in the presence of MMS, unless processivity is affected for only a small subset of forks such that the defect is not detectable by flow cytometry.
From our present study, it is evident that the role of dfp1+ in cell cycle regulation extends outside of the initiation of DNA synthesis and is more elaborate than previously expected. Two highly conserved yet dispensable regions of Dfp1 play distinct roles in the DNA damage response. The region containing motif C plays a critical role in MMS resistance during S phase, the suppression of recombination, the stabilization of chromosomes, and the inactivation of the intra-S checkpoint, allowing recovery from the effects of alkylation damage.
Acknowledgments
We thank Susan Forsburg, Greg Freyer, Joel Huberman, Janet Leatherwood, Tamar Enoch, Tony Carr, and Leona Samson for strains and plasmids and George Brush, Susan Forsburg, and Tom Kelly for critical reading of the manuscript. We also thank Christine Lugmayr and Tushara Weerasooriya for excellent technical assistance and Jianli Dai for construction of the strain YJD8.
This work was supported by Canadian Institutes of Health Research grant MOP-36360. G.W.B. is a Research Scientist of the National Cancer Institute of Canada, supported with funds from the Canadian Cancer Society.
REFERENCES
- 1.al-Khodairy, F., E. Fotou, K. S. Sheldrick, D. J. Griffiths, A. R. Lehmann, and A. M. Carr. 1994. Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol. Biol. Cell 5:147-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aparicio, O. M., D. M. Weinstein, and S. P. Bell. 1997. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell 91:59-69. [DOI] [PubMed] [Google Scholar]
- 3.Bodi, Z., A. Gysler-Junker, and J. Kohli. 1991. A quantitative assay to measure chromosome stability in Schizosaccharomyces pombe. Mol. Gen. Genet. 229:77-80. [DOI] [PubMed] [Google Scholar]
- 4.Bousset, K., and J. F. Diffley. 1998. The Cdc7 protein kinase is required for origin firing during S phase. Genes Dev. 12:480-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brondello, J. M., M. N. Boddy, B. Furnari, and P. Russell. 1999. Basis for the checkpoint signal specificity that regulates Chk1 and Cds1 protein kinases. Mol. Cell. Biol. 19:4262-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, G. W., and T. J. Kelly. 1999. Cell cycle regulation of Dfp1, an activator of the Hsk1 protein kinase. Proc. Natl. Acad. Sci. USA 96:8443-8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, G. W., and T. J. Kelly. 1998. Purification of Hsk1, a minichromosome maintenance protein kinase from fission yeast. J. Biol. Chem. 273:22083-22090. [DOI] [PubMed] [Google Scholar]
- 8.Chapman, J. W., and L. H. Johnston. 1989. The yeast gene, DBF4, essential for entry into S phase is cell cycle regulated. Exp. Cell Res. 180:419-428. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, L., T. Collyer, and C. F. Hardy. 1999. Cell cycle regulation of DNA replication initiator factor Dbf4p. Mol. Cell. Biol. 19:4270-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donaldson, A. D., W. L. Fangman, and B. J. Brewer. 1998. Cdc7 is required throughout the yeast S phase to activate replication origins. Genes Dev. 12:491-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donovan, S., J. Harwood, L. S. Drury, and J. F. Diffley. 1997. Cdc6p-dependent loading of Mcm proteins onto pre-replicative chromatin on budding yeast. Proc. Natl. Acad. Sci. USA 94:5611-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowell, S. J., P. Romanowski, and J. F. Diffley. 1994. Interaction of Dbf4, the Cdc7 protein kinase regulatory subunit, with yeast replication origins in vivo. Science 265:1243-1246. [DOI] [PubMed] [Google Scholar]
- 13.Dutta, A., and S. P. Bell. 1997. Initiation of DNA replication in eukaryotic cells. Annu. Rev. Cell Dev. Biol. 13:293-332. [DOI] [PubMed] [Google Scholar]
- 14.Elledge, S. J. 1996. Cell cycle checkpoints: preventing an identity crisis. Science 274:1664-1672. [DOI] [PubMed] [Google Scholar]
- 15.Enoch, T., A. M. Carr, and P. Nurse. 1992. Fission yeast genes involved in coupling mitosis to completion of DNA replication. Genes Dev. 6:2035-2046. [DOI] [PubMed] [Google Scholar]
- 16.Furnari, B., A. Blasina, M. N. Boddy, C. H. McGowan, and P. Russell. 1999. Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol. Biol. Cell 10:833-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furnari, B., N. Rhind, and P. Russell. 1997. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science 277:1495-1497. [DOI] [PubMed] [Google Scholar]
- 18.Hartwell, L. H., and T. A. Weinert. 1989. Checkpoints: controls that ensure the order of cell cycle events. Science 246:629-634. [DOI] [PubMed] [Google Scholar]
- 19.Jallepalli, P. V., G. W. Brown, M. Muzi-Falconi, D. Tien, and T. J. Kelly. 1997. Regulation of the replication initiator protein p65cdc18 by CDK phosphorylation. Genes Dev. 11:2767-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James, S. W., K. A. Bullock, S. E. Gygax, B. A. Kraynack, R. A. Matura, J. A. MacLeod, K. K. McNeal, K. A. Prasauckas, P. C. Scacheri, H. L. Shenefiel, H. M. Tobin, and S. D. Wade. 1999. nimO, an Aspergillus gene related to budding yeast Dbf4, is required for DNA synthesis and mitotic checkpoint control. J. Cell Sci. 112:1313-1324. [DOI] [PubMed] [Google Scholar]
- 21.Jares, P., A. Donaldson, and J. J. Blow. 2000. The Cdc7/Dbf4 protein kinase: target of the S phase checkpoint? EMBO Rep. 1:319-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang, W., D. McDonald, T. J. Hope, and T. Hunter. 1999. Mammalian Cdc7-Dbf4 protein kinase complex is essential for initiation of DNA replication. EMBO J. 18:5703-5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keeney, J. B., and J. D. Boeke. 1994. Efficient targeted integration at leu1-32 and ura4-294 in Schizosaccharomyces pombe. Genetics 136:849-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly, T. J., and G. W. Brown. 2000. Regulation of chromosome replication. Annu. Rev. Biochem. 69:829-880. [DOI] [PubMed] [Google Scholar]
- 25.Kiely, J., S. B. Haase, P. Russell, and J. Leatherwood. 2000. Functions of fission yeast orp2 in DNA replication and checkpoint control. Genetics 154:599-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kihara, M., W. Nakai, S. Asano, A. Suzuki, K. Kitada, Y. Kawasaki, L. H. Johnston, and A. Sugino. 2000. Characterization of the yeast Cdc7/Dbf4 complex purified from insect cells: its protein kinase activity is regulated by Rad53p. J. Biol. Chem. 275:35051-35062. [DOI] [PubMed] [Google Scholar]
- 27.Kumagai, H., N. Sato, M. Yamada, D. Mahony, W. Seghezzi, E. Lees, K. Arai, and H. Masai. 1999. A novel growth- and cell cycle-regulated protein, ASK, activates human Cdc7-related kinase and is essential for G1/S transition in mammalian cells. Mol. Cell. Biol. 19:5083-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labib, K., J. A. Tercero, and J. F. Diffley. 2000. Uninterrupted MCM2-7 function required for DNA replication fork progression. Science 288:1643-1647. [DOI] [PubMed] [Google Scholar]
- 29.Landis, G., and J. Tower. 1999. The Drosophila chiffon gene is required for chorion gene amplification, and is related to the yeast Dbf4 regulator of DNA replication and cell cycle. Development 126:4281-4293. [DOI] [PubMed] [Google Scholar]
- 30.Leatherwood, J. 1998. Emerging mechanisms of eukaryotic DNA replication initiation. Curr. Opin. Cell Biol. 10:742-748. [DOI] [PubMed] [Google Scholar]
- 31.Lei, M., Y. Kawasaki, M. R. Young, M. Kihara, A. Sugino, and B. K. Tye. 1997. Mcm2 is a target of regulation by Cdc7-Dbf4 during the initiation of DNA synthesis. Genes Dev. 11:3365-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei, M., and B. K. Tye. 2001. Initiating DNA synthesis: from recruiting to activating the MCM complex. J. Cell Sci. 114:1447-1454. [DOI] [PubMed] [Google Scholar]
- 33.Lepke, M., V. Putter, C. Staib, M. Kneissl, C. Berger, K. Hoehn, I. Nanda, M. Schmid, and F. Grummt. 1999. Identification, characterization and chromosomal localization of the cognate human and murine DBF4 genes. Mol. Gen. Genet. 262:220-229. [DOI] [PubMed] [Google Scholar]
- 34.Lindsay, H. D., D. J. F. Griffiths, R. Edwards, J. M. Murray, P. U. Christensen, N. Walworth, and A. M. Carr. 1998. S-phase specific activation of Cds1 kinase defines a subpathway of the checkpoint response in S. pombe. Genes Dev. 12:382-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopes, M., C. Cotta-Ramusino, A. Pellicioli, G. Liberi, P. Plevani, M. Muzi-Falconi, C. S. Newlon, and M. Foiani. 2001. The DNA replication checkpoint response stabilizes stalled replication forks. Nature 412:557-561. [DOI] [PubMed] [Google Scholar]
- 36.Masai, H., and K. Arai. 2000. Dbf4 motifs: conserved motifs in activation subunits for Cdc7 kinases essential for S-phase. Biochem. Biophys. Res. Commun. 275:228-232. [DOI] [PubMed] [Google Scholar]
- 37.Masai, H., T. Miyake, and K. Arai. 1995. hsk1+, a Schizosacchromyces pombe gene related to Saccharomyces cerevisiae CDC7, is required for chromosomal replication. EMBO J. 14:3094-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masai, H., N. Sato, T. Takeda, and K. Arai. 1999. CDC7 kinase complex as a molecular switch for DNA replication. Front. Biosci. 4:D834-D840. [DOI] [PubMed] [Google Scholar]
- 39.Memisoglu, A., and L. Samson. 2000. Contribution of base excision repair, nucleotide excision repair, and DNA recombination to alkylation resistance of the fission yeast Schizosaccharomyces pombe. J. Bacteriol. 182:2104-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795-823. [DOI] [PubMed] [Google Scholar]
- 41.Murray, J. M., H. D. Lindsay, C. A. Munday, and A. M. Carr. 1997. Role of Schizosaccharomyces pombe RecQ homolog, recombination, and checkpoint genes in UV damage tolerance. Mol. Cell. Biol. 17:6868-6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen, V. Q., C. Co, and J. J. Li. 2001. Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature 411:1068-1073. [DOI] [PubMed] [Google Scholar]
- 43.Nishitani, H., Z. Lygerou, T. Nishimoto, and P. Nurse. 2000. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature 404:625-628. [DOI] [PubMed] [Google Scholar]
- 44.Nougarede, R., F. Della Seta, P. Zarzov, and E. Schwob. 2000. Hierarchy of S-phase-promoting factors: yeast Dbf4-Cdc7 kinase requires prior S-phase cyclin-dependent kinase activation. Mol. Cell. Biol. 20:3795-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Connell, M. J., N. C. Walworth, and A. M. Carr. 2000. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 10:296-303. [DOI] [PubMed] [Google Scholar]
- 46.Ogino, K., T. Takeda, E. Matsui, H. Iiyama, C. Taniyama, K. Arai, and H. Masai. 2001. Bipartite binding of a kinase activator activates Cdc7-related kinase essential for S phase. J. Biol. Chem. 276:31376-31387. [DOI] [PubMed] [Google Scholar]
- 47.Oshiro, G., J. C. Owens, Y. Shellman, R. A. Sclafani, and J. J. Li. 1999. Cell cycle control of Cdc7p kinase activity through regulation of Dbf4p stability. Mol. Cell. Biol. 19:4888-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker, A. E., R. K. Clyne, A. M. Carr, and T. J. Kelly. 1997. The Schizosaccharomyces pombe rad11+ gene encodes the large subunit of replication protein A. Mol. Cell. Biol. 17:2381-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paulovich, A. G., and L. H. Hartwell. 1995. A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell 82:841-847. [DOI] [PubMed] [Google Scholar]
- 50.Paulovich, A. G., D. P. Toczyski, and L. H. Hartwell. 1997. When checkpoints fail. Cell 88:315-321. [DOI] [PubMed] [Google Scholar]
- 51.Rhind, N., B. Furnari, and P. Russell. 1997. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 11:504-511. [DOI] [PubMed] [Google Scholar]
- 52.Sato, N., K.-I. Arai, and H. Masai. 1997. Human and Xenopus cDNAs encoding budding yeast Cdc7-related kinases: in vitro phosphorylation of MCM subunits by a putative human homologue of Cdc7. EMBO J. 16:4340-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sclafani, R. A. 2000. Cdc7p-Dbf4p becomes famous in the cell cycle. J. Cell Sci. 113:2111-2117. [DOI] [PubMed] [Google Scholar]
- 54.Snaith, H. A., G. W. Brown, and S. L. Forsburg. 2000. Schizosaccharomyces pombe Hsk1p is a potential Cds1p target required for genome integrity. Mol. Cell. Biol. 20:7922-7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart, E., C. R. Chapman, F. Al-Khodairy, A. M. Carr, and T. Enoch. 1997. rqh1+, a fission yeast gene related to the Bloom's and Werner's syndrome genes, is required for reversible S phase arrest. EMBO J. 16:2682-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeda, T., K. Ogino, E. Matsui, M. K. Cho, H. Kumagai, T. Miyake, K. Arai, and H. Masai. 1999. A fission yeast gene, him1(+)/dfp1(+), encoding a regulatory subunit for Hsk1 kinase, plays essential roles in S-phase initiation as well as in S-phase checkpoint control and recovery from DNA damage. Mol. Cell. Biol. 19:5535-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeda, T., K. Ogino, K. Tatebayashi, H. Ikeda, K. Arai, and H. Masai. 2001. Regulation of initiation of S phase, replication checkpoint signaling, and maintenance of mitotic chromosome structures during S phase by Hsk1 kinase in the fission yeast. Mol. Biol. Cell 12:1257-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka, T., D. Knapp, and K. Nasmyth. 1997. Loading of an Mcm protein onto DNA replication origins is regulated by Cdc6p and CDKs. Cell 90:649-660. [DOI] [PubMed] [Google Scholar]
- 59.Tercero, J. A., and J. F. Diffley. 2001. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 412:553-557. [DOI] [PubMed] [Google Scholar]
- 60.Walter, J. C. 2000. Evidence for sequential action of cdc7 and cdk2 protein kinases during initiation of DNA replication in Xenopus egg extracts. J. Biol. Chem. 275:39773-39778. [DOI] [PubMed] [Google Scholar]
- 61.Walworth, N. C., and R. Bernards. 1996. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science 271:353-356. [DOI] [PubMed] [Google Scholar]
- 62.Weinert, T. 1998. DNA damage checkpoints update: getting molecular. Curr. Opin. Genet. Dev. 8:185-193. [DOI] [PubMed] [Google Scholar]
- 63.Weinreich, M., and B. Stillman. 1999. Cdc7p-Dbf4p binds to chromatin during S phase and is regulated by both the APC and the RAD53 checkpoint pathway. EMBO J. 18:5334-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zou, L., and B. Stillman. 2000. Assembly of a complex containing Cdc45p, replication protein A, and Mcm2p at replication origins controlled by S-phase cyclin-dependent kinases and Cdc7p-Dbf4p kinase. Mol. Cell. Biol. 20:3086-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zou, L., and B. Stillman. 1998. Formation of a preinitiation complex by S-phase cyclin CDK-dependent loading of Cdc45p onto chromatin. Science 280:593-596. [DOI] [PubMed] [Google Scholar]