Abstract
Many members of the thyroid hormone/retinoid receptor subfamily (type II nuclear receptors) function as heterodimers with the retinoid X receptor (RXR). In heterodimers which are referred to as permissive, such as peroxisome proliferator activated receptor/RXR, both partners can bind cognate ligands and elicit ligand-dependent transactivation. In contrast, the thyroid hormone receptor (TR)/RXR heterodimer is believed to be nonpermissive, where RXR is thought to be incapable of ligand binding and is often referred to as a silent partner. In this report, we used a sensitive derepression assay system that we developed previously to reexamine the TR/RXR interrelationship. We provide functional evidence suggesting that in a TR/RXR heterodimer, the RXR component can bind its ligand in vivo. Ligand binding by RXR does not appear to directly activate the TR/RXR heterodimer; instead, it leads to a (at least transient or dynamic) dissociation of a cellular inhibitor(s)/corepressor(s) from its TR partner and thus may serve to modulate unliganded TR-mediated repression and/or liganded TR-mediated activation. Our results argue against the current silent-partner model for RXR in the TR/RXR heterodimer and reveal an unexpected aspect of cross regulation between TR and RXR.
The nuclear receptor superfamily consists of a large number of special transcription factors whose activities in many cases are regulated by their cognate ligands (34). The superfamily is generally divided into two groups. The type I group consists of classic steroid receptors that mediate the actions of steroid hormones such as glucocorticoids, mineralcorticoids, progestins, androgens, and estrogens. The type II group includes thyroid hormone receptors (TRs), retinoid receptors (retinoic acid receptors [RARs] and retinoid X receptors [RXRs]), 1,25-(OH)2 vitamin D3 receptor (VDR), and peroxisome proliferator activated receptors (PPARs) as well as many orphan receptors whose ligands (if any) remain to be defined. Type I receptors primarily act and bind to their palindromic hormone response elements as homodimers (1). In contrast, the situation is more complex for type II receptors, which can bind to DNA as monomers, homodimers, and heterodimers (12, 50). Their corresponding hormone response elements are also complex and can be organized as direct repeats, inverted repeats, and everted repeats (33).
The RXRs stand out as unique members of the type II receptor subfamily. RXRs clearly play an important role in mediating retinoid signaling, presumably through the RAR/RXR heterodimer as well as the RXR/RXR homodimer (21). The natural ligand for RXR is 9-cis-retinoic acid (9-cis-RA), a cellular metabolite derived from all-trans-RA (17, 29). In addition to its role as a classic receptor in mediating the action of its own ligand, RXR also appears to have a central role in the actions of many other type II receptors, such as TRs, RARs, VDR, and PPARs, all of which are believed to function as heterodimers with RXR (33). The primary dimerization interface lies in the ligand binding domain (LBD) of the receptor partners (43), while a region from the DNA-binding domain is also involved (33).
A ligand serves as a molecular switch that determines the functional state of the receptor. Generally, in the absence of the ligand, a receptor either is transcriptionally inactive or mediates repression (16, 35). Ligand binding induces a conformational change in the cognate receptor that results in the dissociation of corepressors (e.g., in the case of TRs and RARs) and/or the recruitment of coactivators, leading to receptor-mediated transactivation (16, 35). Interestingly, heterodimerization of a type II receptor with RXR differentially affects ligand binding of the two involved partners. While the partner of RXR in a heterodimer is usually able to bind its own ligand and elicit its ligand-dependent transactivation function, whether the RXR component can bind ligand seems to depend on the nature of its partner (1, 33). For example, in a PPAR/RXR heterodimer, both PPAR and RXR can bind their cognate ligands and activate transcription, with the binding of both ligands resulting in synergistic activation (25). Heterodimers like PPAR/RXR are thus often referred to as permissive. In contrast, the TR/RXR heterodimer is thought to be nonpermissive (1, 33), as it is activated by the TR ligand T3 but not by RXR-specific ligands (14). It is generally believed that in a nonpermissive heterodimer, RXR is incapable of ligand binding and thus is often referred to as a silent partner (1, 33). It has been suggested that the only apparent role of RXR in the TR/RXR heterodimer is to facilitate the binding of TR to a thyroid hormone response element(s) (TRE).
In this report, we used a sensitive derepression assay system that we developed previously (6) to reexamine the TR/RXR interrelationship. We have previously shown that a Gal4-TR-VP16 (GTV) chimera is transcriptionally inactive in cells due to the association of a cellular inhibitor(s)/corepressor(s) with the TR moiety, which in turn masks the VP16 transactivation function (6). Cotransfection of the TR LBD in the absence of T3 titrates out the inhibitor/corepressor and thus allows transactivation by the GTV chimera (6). Intriguingly, the GTV chimera can also be activated by the RXR LBD, but only in the presence of the RXR ligand 9-cis-RA (6).
In this study, we showed that a similar chimera, Gal4-TR(L372R)-VP16 (GTV L372R), containing a point mutation in the TR LBD (Leu 372 to Arg) that specifically abolishes heterodimerization with RXR (2), fails to be activated by the liganded RXR LBD, although it can still be activated by the apo-TR LBD. We also constructed another mutant chimera, GTV P158R, that contains a single point mutation (Pro 158 to Arg) in the TR hinge region. The GTV P158R chimera is transcriptionally inactive and is not activated by cotransfection of the TR LBD, an observation that is consistent with the suggestion that the P158R mutation destabilizes the structure of the resulting mutant TR (discussed in more detail later). Remarkably, cotransfection of the RXR LBD leads to activation of the GTV P158R chimera in the presence of 9-cis-RA. Interestingly, activation of GTV by the liganded RXR LBD does not appear to require the recruitment of functional coactivators by the RXR LBD, as expression of a dominant negative form of GRIP1 (a p160 coactivator) virtually abolishes ligand-dependent transactivation by a Gal4-RXR LBD chimera but has no effect on the activation of GTV by the liganded RXR LBD. These results are interpreted in a model where a liganded RXR LBD can heterodimerize with the TR LBD in vivo and induce a conformational change in TR that leads to a (at least transient or dynamic) dissociation of its bound inhibitor/corepressor.
This model was further tested with a native TRE-driven reporter and a VP16-TR chimera. In the absence of T3, VP16-TR alone does not activate the TRE reporter. Consistent with the model suggested from our studies with GTV chimeras, we found that cotransfection of a full-length RXR leads to activation of the TRE-bound VP16-TR in the presence of 9-cis-RA. Finally, we also showed that T3-mediated activation of a TRE-bound TR/RXR heterodimer can be enhanced by 9-cis-RA. Taken together, our results suggest that RXR in the TR/RXR heterodimer can bind its natural ligand 9-cis-RA in vivo and therefore argue against the current silent-partner model for the TR/RXR heterodimer. The observed functional modulation of the TR/RXR heterodimer by 9-cis-RA not only reveals an unexpected aspect of cross regulation between TR and RXR, but also may have implications for the evaluation of other RXR heterodimers that are currently deemed nonpermissive.
MATERIALS AND METHODS
Plasmids.
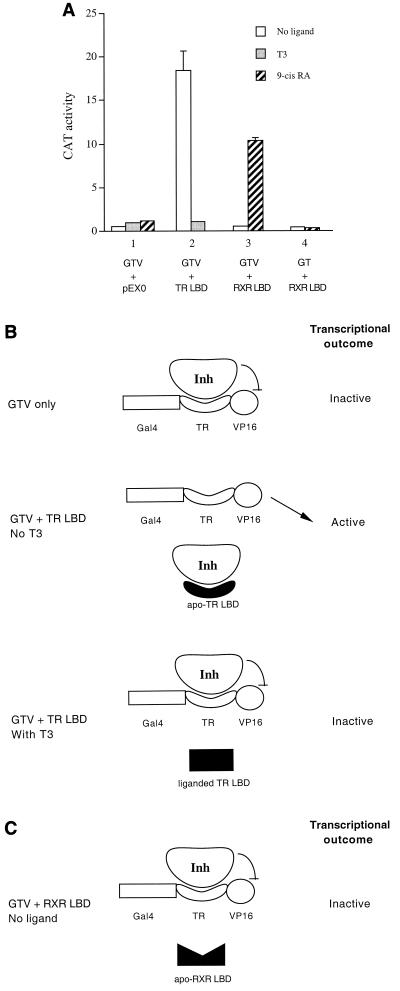
The plasmid expressing the Gal4-TR-VP16 (GTV) chimera has been described previously (6). The expressed GTV fusion protein consists of the Gal4 DNA binding domain, a major portion of the cTRα LBD comprising amino acid residues 120 to 392, and the last 68 amino acid residues of the herpes simplex virus VP16 transactivation domain. It should be noted that the TR LBD moiety in the GTV chimera lacks the last 16 amino acid residues from the wild-type LBD that contains helix 12 (the AF2 helix) (6). As a result, the TR LBD moiety (residues 120 to 392) in GTV is defective in ligand binding as well as ligand-induced dissociation of the inhibitor(s)/corepressor(s), which renders the GTV chimera transcriptionally inactive in the presence or absence of T3 (Fig. 1) (6).
FIG. 1.
(A) Activation of the Gal4-TR-VP16 (GTV) chimera by the apo-TR LBD or liganded RXR LBD. HeLa cells were transfected with 1 μg of the Gal4 reporter pMC110 and 400 ng of the GTV chimera to examine GTV-mediated transactivation. The TR LBD (2.5 μg) or RXR LBD (800 ng) or the control empty expression vector pEX0 (1.5 μg) was cotransfected as indicated. CAT activities were determined for cells without ligand (open bars) or with T3 (shaded bars) or with 9-cis-RA (hatched bars). In panel 4, Gal4-TR (GT, 400 ng) was used instead of GTV. Error bars indicate SEMs. (B) Schematic model for derepression of GTV by the apo-TR LBD. (Top) In cells transfected with GTV only, a cellular inhibitor/corepressor (Inh) associates with the TR moiety of the GTV fusion protein, which in turnmasks the transactivation function of VP16. (Middle) In cells cotransfected with the TR LBD but without ligand, the expressed apo-TR LBD competes in trans with GTV for binding to the cellular inhibitor. This results in dissociation of the inhibitor from GTV, which in turn allows VP16 to elicit its transactivation function. (Bottom) Cotransfection of the TR LBD in the presence of T3 results in the dissociation of the inhibitor from the liganded TR LBD. As a result, the inhibitor rebinds to the GTV chimera and represses VP16 activity. The TR moiety in GTV lacks helix 12 and thus is defective in ligand binding as well as ligand-induced dissociation of the inhibitor(s)/corepressor(s). Therefore, GTV alone is inactive with or without T3. (C) Schematic model for the inability of the apo-RXR LBD to activate GTV. The apo-RXR LBD has a low affinity for the inhibitor and thus cannot compete efficiently with GTV for inhibitor binding. As a result, cotransfection of the RXR LBD in the absence of ligand fails to derepress the GTV chimera.
The Gal4-TR (GT) plasmid expressing residues 120 to 392 of the TR LBD fused to the C terminus of the Gal4 DNA-binding domain was constructed by digesting Gal4-TR (residues 120 to 408) with SacI to release the region encoding residues 393 to 408 and subsequent religation of the resulting vector. To generate the mutant GTV L372R, the wild-type GTV vector was digested with EcoRV and SacI to release a region of the TR LBD encoding Leu 372. The resulting vector was then ligated to the corresponding EcoRV/SacI insert liberated from the TR L372R mutant. The GTV P158R mutant was constructed by site-directed mutagenesis with a PCR-based procedure. The oligonucleotides spanning the point mutation were oligonucleotide A (5′-CAC CGG CCC AGC CGC AGC GCA GAG GAG-3′) and oligonucleotide B (5′-CTC CTC TGC GCT GCG GCT GGG CCG GTG-3′). The changed nucleotides reflecting the P158R mutation are underlined. The primers upstream and downstream of the mutation were oligonucleotide C (5′-GAC TGG AAC AGC TAT TTC TAC-3′) and oligonucleotide D (5′-GGC AAT GGA GGC CAT GGG CGA C-3′). The first-round PCRs were carried out with the wild-type GTV plasmid as the template. Oligonucleotide pairs A and D or B and C were used in the first-round PCRs to produce fragments AD and BC, respectively. Fragments AD and BC were then gel purified. A mixture of purified fragments AD and BC was used as the template in the second round of PCR with oligonucleotides C and D as the primers. The resulting PCR product (fragment CD) was digested with XhoI and EcoRV and cloned into a GTV-derived vector digested with the same pair of enzymes. The resulting GTV P158R construct was confirmed by sequence analysis.
The Gal4-regulated reporter plasmid pMC110 was described previously (6). pEX-based plasmids expressing the cTRα LBD (residues 120 to 408) and murine RXRβ (mRXRβ) LBD (residues 185 to 438), as well as the empty control vector pEX0 have been described previously (6, 13). The plasmid expressing the Gal4-RXR LBD has been described previously (43). pcDNA3-GRIP1 NID, a plasmid expressing a dominant negative form of GRIP1, was a gift from Inez Rogatsky and Keith Yamamoto (46).
pEX-VP16-TR expresses a chimeric protein consisting of the VP16 transactivation domain inserted within the N terminus of full-length cTRα between Cys 31 and Leu 32. It was constructed as follows. First, a pEX-TR plasmid was cleaved by PflMI digestion. Primers were then designed to PCR amplify the VP16 transactivation domain in a fragment flanked by PflMI sites. The PCR product was digested with PflMI and ligated to the pEX-TR vector opened with PflMI. The resulting pEX-VP16-TR construct was confirmed by sequence analysis. The TRE-lys-CAT reporter, containing a chloramphenicol acetyltransferase (CAT) gene under the control of the ΔMTV basal promoter linked to the native TRE sequence (TGACCCCAGCTGAGGTCA) derived from the chicken lysozyme promoter (3), has been described previously (39). The TRE-DR4A-CAT reporter has been described previously (39). It contains a CAT gene under the control of the ΔMTV basal promoter linked to a synthetic direct repeat 4 (DR4) TRE sequence (AGGACANNNNAGGACA, where N is any nucleotide). Plasmids pCMV-hRXRα and pCMV-hRXRβ were obtained from Ron Evans. The empty expression vector (referred to as pCMX) that contains no insert was used as a control.
Cell culture and transfections.
HeLa cells were cultured as previously described (6). Plasmids used for transfection studies were described in the above section. Cells were transfected by either calcium phosphate coprecipitation or electroporation as previously described (6, 30). A typical calcium phosphate-mediated transfection included 1 μg of pMC110 reporter and 400 ng of GTV or GT plasmid. For the mutant GTV L372R, more plasmid DNA was required in order to detect an effect because of the intrinsic instability of the mutant chimeric protein. Therefore, up to 5 μg of GTV L372R was used in transfections. When appropriate, the plasmid expressing the TR LBD (2.5 to 5 μg) or the RXR LBD (800 ng to 4 μg) or the empty expression vector control (at comparable molar amounts) was cotransfected to examine the effect on the activities of GTV chimeras. The dominant negative effect of GRIP1 NID was examined in a transfection experiment with 400 ng of Gal4-RXR, 1.2 μg of pMC110, and 2.5 μg of pcDNA3-GRIP1 NID or 2 μg of pcDNA3 control. To examine the potential effect of GRIP1 NID on the liganded RXR LBD-mediated activation of GTV, HeLa cells were transfected with 400 ng of GTV, 1.2 μg of pMC110, 1.5 μg of RXR LBD, and 2.5 μg of pcDNA3-GRIP1 NID or 2 μg of pcDNA3 control. A typical electroporation-mediated transfection included 2.5 μg of pMC110 and 1 μg of GTV or GTV P158R. When appropriate, the plasmid expressing the TR LBD or the RXR LBD or the control plasmid pEX0 was cotransfected (at about 3 μg for each plasmid). The transfection study of the TRE-lys-CAT reporter was carried out by calcium phosphate coprecipitation, typically with 300 ng of VP16-TR, 1 μg of TRE-lys-CAT, and, when appropriate, 1 to 3 μg of pCMV-hRXRβ or the control vector pCMX. The study with the TRE-DR4A-CAT reporter was also carried out by calcium phosphate coprecipitation, with 1 μg of TRE-DR4A-CAT, 100 ng of pEX-cTRα, and 100 ng of pCMV-hRXRα. After transfection, cells were incubated at 37°C without or with ligand (1 μM T3, 9-cis-RA, or LGD1069) for about 45 h before being harvested. CAT activities were then determined as previously described (30), with a typical CAT reaction mixture containing 50 μg of cell lysates incubated at 37°C for about 14 h. Standard errors of the mean (SEMs) were calculated from two repeated experiments with duplicates.
RESULTS
Activation of GTV by liganded RXR LBD.
We have previously developed a sensitive repression/derepression assay system for TR with a Gal4-TR-VP16 (GTV) chimera (6). With such a system, we provided the first functional evidence for the association of a cellular inhibitor(s)/corepressor(s) with the TR LBD in the absence of ligand (6). The basic scheme of the system is shown in Fig. 1. As we reported previously (6), the GTV chimera failed to activate the Gal4 reporter when transfected into HeLa cells (Fig. 1A). This is due to the association of a cellular inhibitor(s)/corepressor(s) with the TR LBD moiety of the GTV chimera, which in turn represses the activation function of VP16 (Fig. 1B for the model). Consistent with this interpretation, cotransfection of the TR LBD led to activation of the Gal4 reporter (Fig. 1A), as the expressed TR LBD binds to the putative inhibitor and sequesters it (at least dynamically) from GTV, resulting in derepression of the GTV chimera (Fig. 1B).
Interestingly, derepression of GTV by the TR LBD was abolished in the presence of T3 (Fig. 1A), as ligand binding induces a conformational change in the TR LBD that dissociates the inhibitor, which now rebinds to GTV (Fig. 1B). It should be noted that the TR moiety in the GTV chimera harbors a deletion that removes residues comprising helix 12 (see Materials and Methods for details). Helix 12 is an integral part of the LBD that is engaged in ligand binding (5, 44, 49, 51) and also plays an important role in ligand-induced dissociation of corepressors (6, 8) and/or recruitment of coactivators (11, 40, 49). Consequently, the GTV chimera is defective in ligand binding and remains transcriptionally inactive even in the presence of T3 (Fig. 1A). Although the identity of the putative inhibitor was not known in our previous report (6), subsequent identification of nuclear receptor corepressors SMRT and NcoR by other groups (7, 19) suggests that the putative inhibitor is SMRT or NcoR or a related molecule.
In contrast to the derepression of GTV by the TR LBD in the absence of T3 (apo-TR LBD), cotransfection of the RXR LBD did not activate the GTV chimera in the absence of ligand (Fig. 1A). This is likely because the apo-RXR LBD binds the inhibitor/corepressor poorly and thus is not able to efficiently dissociate it from the TR moiety of the GTV chimera (Fig. 1C). The notion that the apo-RXR LBD does not associate tightly with the inhibitor/corepressor is supported by the findings that a Gal4-RXR LBD fusion mediates little repression (43, 54) and that a Gal4-RXR (LBD)-VP16 chimera is constitutively active (43).
Somewhat surprisingly, cotransfection of the RXR LBD in the presence of the RXR ligand 9-cis-RA resulted in significant activation of the Gal4 reporter by GTV (Fig. 1A). Two possible explanations for this finding are that activation of the Gal4 reporter is mediated by the activation function of the RXR LBD tethered to the TR moiety of the GTV chimera or that the activation is due to derepression of GTV by the liganded RXR LBD. We consider the first possibility very unlikely, as previous studies have suggested that a TR/RXR heterodimer cannot be activated by the RXR ligand (14). Nevertheless, we tested this possibility by transfecting HeLa cells with the Gal4 reporter, together with vectors expressing Gal4-TR (a fusion protein similar to GTV but without VP16) and the RXR LBD. As shown in Fig. 1A, we found no activation of the Gal4 reporter without or with 9-cis-RA. This result confirms the notion that the RXR component in the context of a TR/RXR heterodimer cannot be directly activated by its ligand. Taken together, our results suggest that activation of the Gal4 reporter by GTV in the presence of the liganded RXR LBD results from derepression of the GTV chimera.
GTV L372R is not activated by RXR LBD.
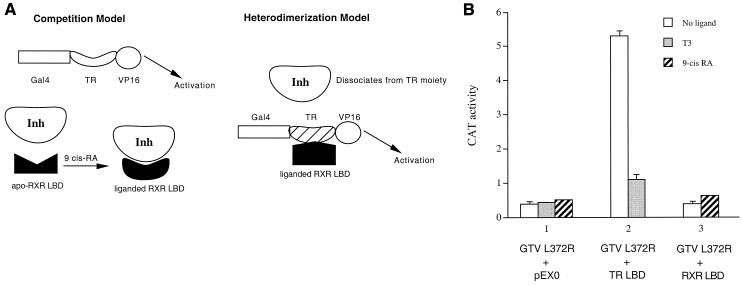
To explore the mechanism by which the liganded RXR LBD derepresses the GTV chimera, we considered two major possibilities (Fig. 2A). Since the apo-TR LBD derepresses GTV by competing for the binding of the cellular inhibitor/corepressor (Fig. 1B), one possibility (the competition model) is that a similar competition mechanism is also employed by the liganded RXR LBD (Fig. 2A, left panel). In this model, ligand binding would presumably increase the association between the RXR LBD and the inhibitor/corepressor, as the apo-RXR LBD is incapable of derepressing GTV (Fig. 1C). We believe that this model is very unlikely as ligand binding generally promotes LBD-corepressor dissociation instead of association (16). In addition, the Gal4-RXR LBD fusion elicits potent transactivation in the presence of 9-cis-RA (43), arguing strongly against the idea of an association between the liganded RXR LBD and an inhibitor/corepressor.
FIG. 2.
(A) Two alternative models for derepression of GTV by the liganded RXR LBD. Left, the competition model. Although the apo-RXR LBD does not associate with the inhibitor efficiently and thus cannot activate GTV, this model proposes that a conformational change in the RXR LBD upon ligand binding increases its affinity for the inhibitor, resulting in the derepression of GTV. Right, the heterodimerization model. This model proposes that the liganded RXR LBD heterodimerizes with the TR moiety of GTV or, equivalently, the RXR component of the GTV/RXR heterodimer binds to its ligand 9-cis-RA. In doing so, it induces a conformational change in TR that decreases its affinity for the inhibitor, resulting in the derepression of GTV. (B) Activation of the mutant GTV L372R by the apo-TR LBD but not by the liganded RXR LBD. HeLa cells were transfected with 1 μg of the Gal4 reporter pMC110 and 5 μg of the mutant GTV L372R chimera. The TR LBD (5 μg) or RXR LBD (4 μg) or the control empty expression vector pEX0 (4 μg) was cotransfected as indicated. CAT activities were determined for cells without ligand (open bars) or with T3 (shaded bars) or with 9-cis-RA (hatched bars). Error bars indicate SEMs.
An alternative possibility (the heterodimerization model) is that the liganded RXR LBD heterodimerizes with the TR moiety of the GTV chimera, which leads to a (at least transient or dynamic) dissociation of the inhibitor/corepressor from TR and thus allows VP16 to elicit its transactivation function (Fig. 2A, right panel). To distinguish between these two possibilities, we constructed a mutant chimera, GTV L372R, which is identical to the wild-type GTV except for a single amino acid change (Leu 372 to Arg) in the TR moiety of the chimera. The L372R mutation in the TR LBD has been shown previously to abolish heterodimerization of TR with RXR (2). We found that GTV L372R did not activate the Gal4 reporter in transfected HeLa cells (Fig. 2B). Cotransfection of the TR LBD led to activation (Fig. 2B), presumably through the same inhibitor competition mechanism proposed for the activation of wild-type GTV (Fig. 1A and Fig. 1B).
As expected, activation of GTV L372R by the TR LBD was abolished in the presence of T3 (Fig. 2B). We noted that the absolute CAT activity for the GTV L372R experiment was lower than that of wild-type GTV. This is likely due to the intrinsic instability of the mutant chimera in cells. Consistent with this explanation, Western blotting of lysates from cells transfected with wild-type and mutant GTVs revealed a much lower level of the expressed mutant chimera (data not shown).
To test the two possible models outlined in Fig. 2A, the RXR LBD was cotransfected with GTV L372R to examine derepression. While the competition model predicts that the liganded RXR LBD should activate GTV L372R similarly to the apo-TR LBD, the heterodimerization model predicts that GTV L372R should not be activated by the RXR LBD because its mutant TR moiety is defective in heterodimerization with RXR. As shown in Fig. 2B, the RXR LBD failed to activate GTV L372R without or with 9-cis-RA.
GTV P158R is activated by liganded RXR LBD but not apo-TR LBD.
During the course of studying repression mediated by the TR LBD, we also constructed another mutant chimera, GTV P158R. This mutant is identical to the wild-type GTV except for a single amino acid change (Pro 158 to Arg) in the hinge region of the TR moiety. The Pro-to-Arg mutation at this position was originally shown to abolish the repression function of the mutant TR (10). This result, together with the finding that this and other mutations in the hinge region of TR diminish corepressor association, had led to the suggestion that the hinge region might be the target for corepressor binding (7, 19). However, in crystal structures of TR and RAR, the hinge region (helix 1) is folded tightly into the LBD, with residues initially thought to be involved in contact with corepressors inaccessible to the surface (44, 51). Indeed, more recent studies have suggested that the receptor surface for corepressor binding substantially overlaps that for coactivators (20, 38, 41). Taken together, these studies indicate that the hinge region (helix 1) is not directly involved in corepressor binding per se; instead, the effect of mutations in this region (such as P158R) appears to be an indirect consequence reflecting the destabilization (or disruption) of the overall structure of the LBD by these mutations (42).
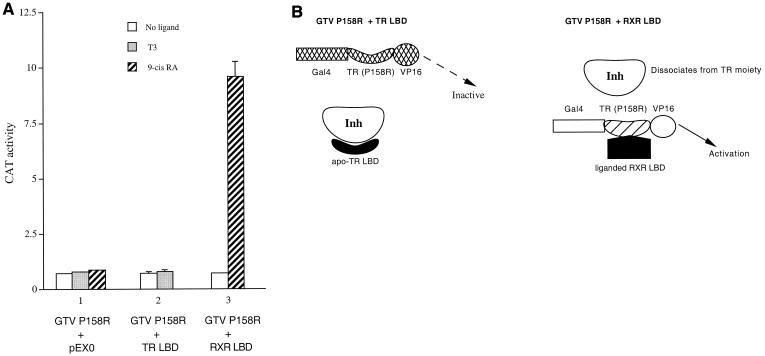
Since the equivalent Pro-to-Arg mutation (P160R in human TRα1 [hTRα1] and P214R in hTRβ1) was originally suggested to abolish corepressor binding (7, 10), we constructed the GTV P158R chimera to test whether it would be constitutively active. To our surprise, we found that the GTV P158R mutant chimera did not activate the Gal4 reporter in transfected HeLa cells (Fig. 3A). This result is explained, however, in light of a recent finding suggesting that this mutation destabilizes the overall structure of the mutant LBD (42). Thus, although the mutant TR LBD moiety in GTV P158R does not associate with the corepressor, the overall structure or conformation of the mutant GTV chimera is altered or compromised by the mutation so that it fails to activate the Gal4 reporter regardless of the state of the cellular inhibitor (Fig. 3B, left panel).
FIG. 3.
(A) Activation of the mutant GTV P158R by the liganded RXR LBD but not by the apo-TR LBD. HeLa cells were transfected with 2.5 μg of the Gal4 reporter pMC110 and 1 μg of the mutant GTV P158R chimera. The TR LBD (3 μg) or RXR LBD (3 μg) or the control empty expression vector pEX0 (3 μg) was cotransfected as indicated. CAT activities were determined for cells without ligand (open bars) or with T3 (shaded bars) or with 9-cis-RA (hatched bars). Error bars indicate SEMs. (B) Schematic model to account for the results in A. Left, the hinge region mutation P158R leads to a structural destabilization or conformational disruption of the mutant chimera, which renders it transcriptionally inactive regardless of the state of the cellular inhibitor. Thus, although the apo-TR LBD can efficiently bind the inhibitor, the GTV P158R remains inactive. Right, in cells cotransfected with the RXR LBD and treated with 9-cis-RA, heterodimerization between the liganded RXR LBD and the TR moiety of the GTV P158R chimera leads to structural stabilization of the mutant chimera and dissociation of the inhibitor from the TR moiety. As a result, GTV P158R is now transcriptionally active. More is discussed in the text.
In support of this interpretation, cotransfection of the TR LBD failed to activate GTV P158R (Fig. 3A). Since the GTV P158R chimera cannot be activated via the inhibitor competition mechanism (Fig. 3B, left panel), this provides an opportunity to further test the two alternative models depicted in Fig. 2A. Thus, if the liganded RXR LBD activates GTV via an inhibitor competition mechanism similar to that of the apo-TR LBD, it should not activate GTV P158R. Remarkably, we found that cotransfection of the RXR LBD significantly activated GTV P158R in the presence of 9-cis-RA (Fig. 3A). This finding indicates that the liganded RXR LBD and apo-TR LBD employ distinct mechanisms to activate the GTV chimera. Collectively, the results in Fig. 3A argue against the competition model and in turn provide further support for the heterodimerization model (Fig. 2A and Fig. 3B)
Coactivator recruitment by liganded RXR LBD is not required for activation of the GTV chimera.
During the revision of our manuscript, Germain et al. reported that RXR is also not a silent partner in the RAR/RXR heterodimer, as RXR can bind its ligand and recruit coactivators in a heterodimer with apo-RAR (15). However, in most cells, corepressor binding to RAR prevents liganded RXR from eliciting transactivation as a liganded RXR/apo-RAR heterodimer (15). Interestingly, while both our results and the study by Germain et al. support the notion that RXR is not a silent partner, ligand binding of RXR in the two different heterodimers (TR/RXR and RAR/RXR) appears to differentially influence the dissociation (or lack of dissociation) of corepressors.
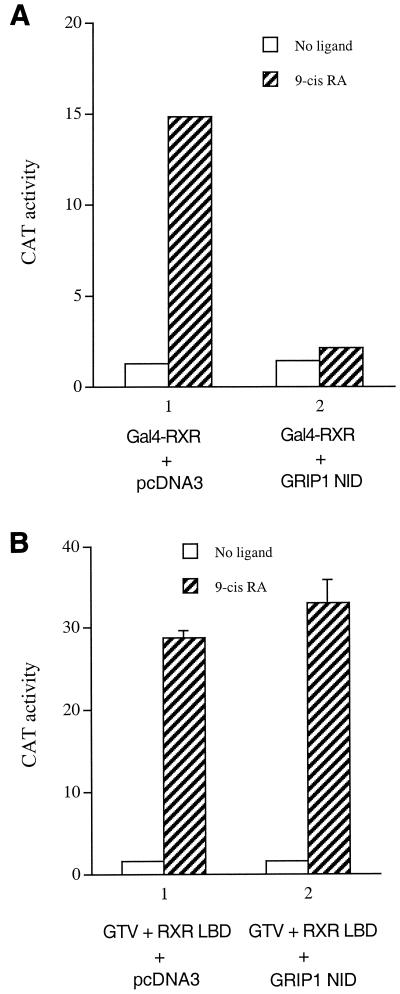
Although our results suggest that activation of GTV by the liganded RXR LBD is due to a derepression mechanism (e.g., see Fig. 2A, right panel), the study by Germain et al. raises an alternative possibility that the liganded RXR LBD in heterodimerization with GTV may recruit AF-2-interacting coactivators (such as members of the p160 family), which, in conjunction with the coactivator(s) recruited by the VP16 moiety, dominate over the function of corepressors associated with the TR LBD moiety, resulting in activation of GTV. To test this possibility, we examined whether coexpression of a dominant negative form of the p160 coactivators can block liganded RXR-mediated activation of GTV. We found that the nuclear receptor-interacting domain (NID) of GRIP1 functions as a potent dominant negative inhibitor of Gal4-RXR-mediated transactivation (Fig. 4A), presumably due to the displacement of endogenous coactivator(s) from the liganded RXR LBD by the overexpressed GRIP1 NID. Interestingly, the same GRIP1 NID was found to have no effect on liganded RXR-mediated activation of GTV (Fig. 4B). Taken together, these results suggest that a potential recruitment of functional endogenous coactivators by the liganded RXR LBD is not required for its activation of GTV per se.
FIG. 4.
Coactivator recruitment by the liganded RXR LBD is not required for its activation of GTV. (A) Expression of the nuclear receptor-interacting domain (NID) of GRIP1 blocks ligand-mediated transactivation by Gal4-RXR. HeLa cells were transfected with 1.2 μg of the Gal4 reporter pMC110 and a plasmid expressing the Gal4-RXR LBD (400 ng) to examine ligand-dependent transactivation of the RXR LBD. The plasmid pcDNA3-GRIP1 NID (2.5 μg), which expresses the nuclear receptor-interacting domain of GRIP1, or control plasmid pcDNA3 (2 μg) was cotransfected as indicated. CAT activities were determined for cells in the absence (open bars) or presence (hatched bars) of 9-cis-RA. (B) GRIP1 NID has no effect on the liganded RXR LBD-mediated activation of GTV. HeLa cells were transfected with pMC110 (1 μg), GTV (400 ng), and the RXR LBD (1.5 μg) in the absence (open bars) or presence (hatched bars) of 9-cis-RA to examine activation of GTV by the liganded RXR LBD. pcDNA3-GRIP1 NID (2.5 μg) or the control pcDNA3 (2 μg) was cotransfected as indicated. Error bars indicate SEMs.
Derepression of TR by liganded RXR on a native hormone response element.
As discussed earlier, the results of our studies with the wild-type and mutant GTV chimeras are best interpreted by a model in which a liganded RXR LBD can heterodimerize with the TR LBD in vivo and induce a conformational change in TR that leads to a (at least transient or dynamic) dissociation of its bound inhibitor (Fig. 2A, right panel). To further test whether heterodimerization between TR and liganded RXR can occur in the context of full-length receptors bound to a TRE and, should it occur, whether this too leads to the derepression of TR, a transfection study was carried out in HeLa cells with a VP16-TR chimera and the TRE-lys-CAT reporter. The VP16-TR chimera consists of the VP16 activation domain inserted within the N terminus of full-length TRα. The TRE-lys-CAT reporter contains the CAT gene under the control of the basal ΔMTV promoter linked to the native TRE sequence derived from the chicken lysozyme promoter, an everted repeat 6 (ER6) element (39).
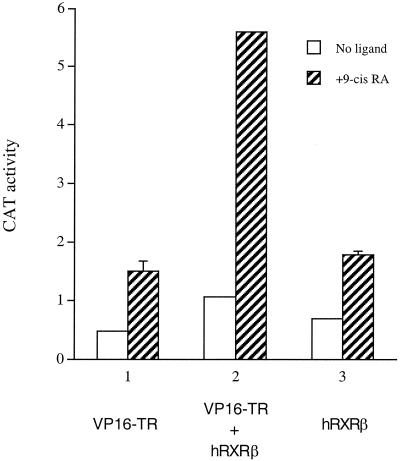
As shown in Fig. 5, VP16-TR alone did not significantly activate the TRE-lys-CAT reporter without or with 9-cis-RA. This finding is not unexpected because TR is known to bind inhibitors (or corepressors) in the absence of T3, which would then mask or repress the activity of VP16. In the presence of T3, we observed potent activation of the reporter by VP16-TR, demonstrating that the chimeric protein is capable of binding to the TRE-lys and mediating transactivation (data not shown). We then tested whether VP16-TR can be activated by RXR in the presence or absence of 9-cis-RA. As shown in Fig. 5, cotransfection of RXRβ with VP16-TR had little effect in the absence of 9-cis-RA. However, treatment with 9-cis-RA significantly activated the CAT reporter (Fig. 5). This result is consistent with the model that the liganded RXR heterodimerizes with the VP16-TR chimera and induces a (transient or dynamic) dissociation of the inhibitor from TR that allows the VP16 moiety to elicit its transactivation function.
FIG. 5.
Activation of a native TRE-bound VP16-TR chimera by RXR in the presence of 9-cis-RA. HeLa cells were transfected with the TRE-lys-CAT reporter (1 μg), together with the indicated plasmids: VP16-TR (300 ng) only, or VP16-TR (300 ng) plus hRXRβ (3 μg), or hRXRβ (3 μg) only. CAT activities were determined for cells without ligand (open bars) or with 9-cis-RA (hatched bars). Error bars indicate SEMs.
As a control, cotransfection of an empty expression vector (pCMX) with VP16-TR and TRE-lys-CAT had little effect on reporter activity (data not shown). The reporter activity observed in the presence of VP16-TR and liganded RXR was not due to cross-activation of the TRE-lys-CAT by RXRβ per se, as the control transfection of RXRβ alone with the TRE-lys-CAT reporter did not lead to significant activation with or without 9-cis-RA (Fig. 5).
RXR ligand enhances T3-mediated activation of a TRE-bound TR/RXR heterodimer.
Transcriptional regulation by nuclear receptors is determined by a dynamic network that integrates the input from multiple players, including the receptor and its ligand, the DNA-binding sites, adjacent transcription factors, coactivators/corepressors, and cellular signaling pathways (16). In this regard, the relative abundance or availability of coactivators versus corepressors may influence receptor-mediated activation by shifting the dynamic equilibrium between active (liganded and/or coactivator bound) and inactive or repressive (unliganded and/or corepressor bound) receptors. Indeed, we have previously shown that cotransfection of a truncated TR LBD (residues 120 to 392), which can still bind the inhibitor/corepressor even in the presence of ligand, markedly enhances T3-mediated activation of a Gal4-TR chimera (6). This result is consistent with the notion that even in the presence of its ligand T3, TR-mediated activation is still subject to dynamic negative modulation by the cellular inhibitor/corepressor, while the cotransfected TR LBD (residues 120 to 392) binds to and sequesters the inhibitor/corepressor, leading to superactivation of liganded TR. In further support of this dynamic view of the regulation of nuclear receptor activity by coactivators and corepressors, we and others also found that cotransfection of C-SMRT, a dominant negative form of SMRT that contains its receptor-interacting domain but lacks the transrepression domain, can further enhance liganded receptor-mediated transactivation (32; unpublished data).
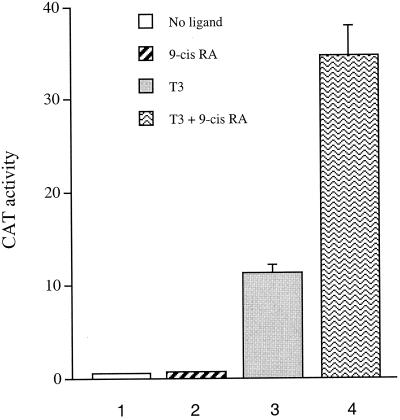
Our studies with the GTV and VP16-TR chimeras suggest that the RXR ligand 9-cis-RA can modulate the function of unliganded TR (in a TR/RXR heterodimer) by promoting the dissociation of its bound inhibitor/corepressor. In light of the dynamic view of modulation of nuclear receptor activities by coactivators and corepressors, we examined whether simultaneous treatment with T3 and 9-cis-RA would result in more activity from a TR/RXR heterodimer than treatment with T3 alone, as ligand binding of RXR may further facilitate the dynamic dissociation of a corepressor(s) from TR, which would result in superactivation of liganded TR. To this end, we transfected HeLa cells with 100 ng each of the TR and RXR expression vectors together with the TRE-DR4A-CAT reporter. As expected, the TR/RXR heterodimer did not activate the reporter in the absence of any ligand (Fig. 6). Consistent with the notion that the ligand of RXR cannot directly activate the TR/RXR heterodimer, treatment with 9-cis-RA alone also resulted in no activation of the reporter (Fig. 6).
FIG. 6.
9-cis-RA enhances T3-mediated transactivation from a TRE-bound TR/RXR heterodimer. HeLa cells were transfected with 1 μg of TRE-DR4A-CAT, and 100 ng each of the TR and RXR expression plasmids. CAT activities were determined for cells without ligand, with 9-cis-RA only, with T3 only, and with T3 and 9-cis-RA. Error bars indicate SEMs.
Interestingly, while T3 treatment led to the expected activation of the CAT reporter, incubation with T3 and 9-cis-RA simultaneously resulted in further enhancement of the reporter activity, suggesting that the TR/RXR heterodimer can be superactivated by the binding of ligands of both partners (Fig. 6). Our result in Fig. 6 differs from those in the study by Forman et al., which showed that incubation with both ligands did not lead to higher activity by the TR/RXR heterodimer than incubation with T3 alone (14). The discrepancy might have resulted from the different cellular context, different basal promoter context, or the different RXR ligands employed in the transfection studies. Nevertheless, our result supports the superactivation of the TR/RXR heterodimer by T3 and 9-cis-RA, at least under the experimental conditions described.
Activation of GTV by the RXR LBD in the presence of LGD1069, a synthetic RXR-specific ligand.
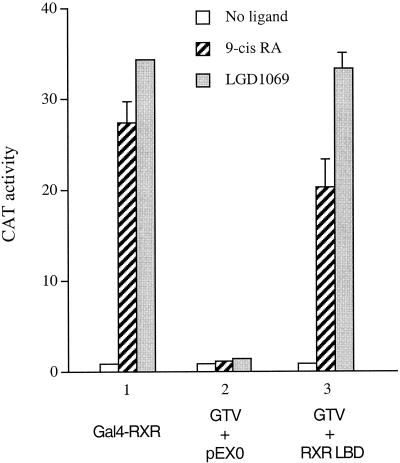
Our study suggests that the RXR component of the TR/RXR heterodimer can bind its natural ligand 9-cis-RA in cells and modulate the function of TR. To explore whether the TR/RXR heterodimer can also be modulated by synthetic RXR-specific ligands (also termed rexinoids), we examined the effect of LGD1069 on the activation of GTV by the RXR LBD. As shown in Fig. 7, both LGD1069 and 9-cis-RA efficiently activated the Gal4-RXR chimera, as they both function as RXR agonists. As expected, GTV was not activated by the RXR LBD in the absence of ligand, but incubation with 9-cis-RA resulted in significant activation (Fig. 7). A similar activation of GTV by the RXR LBD was also observed in the presence of LGD1069 (Fig. 7), suggesting that, like 9-cis-RA, this rexinoid can also mediate a derepression effect (via RXR) on unliganded TR in cells.
FIG. 7.
Activation of GTV by the RXR LBD in the presence of LGD1069. HeLa cells were transfected with 1.5 μg of pMC110 together with the indicated plasmids: Gal4-RXR LBD (500 ng), GTV (500 ng), pEX0 (1.2 μg), or RXR LBD (1.5 μg). CAT activities were determined for cells without ligand, with 9-cis-RA, or with LGD1069. Error bars indicate SEMs.
DISCUSSION
RXRs are unique members of the type II nuclear receptor subfamily. First, RXR can function as a classic receptor for the natural ligand 9-cis-RA (33, 34). In this role, RXR and RAR constitute the molecular targets that mediate retinoid signaling (21-23). In addition to its role as a classic receptor in mediating the action of its own ligand, RXR also plays an important role in the biology of many other type II receptors, such as TRs, RARs, VDR, PPARs, and a number of orphan receptors, all of which are believed to function primarily as heterodimers with RXR (4, 33). Why these type II receptors have adopted a mechanism that employs RXR as the common partner is unclear, but it may reflect an ancient requirement for heterodimerization during the evolution of nuclear receptors (52). An interesting finding is that a type II receptor often binds to its cognate DNA element(s) more efficiently as a heterodimer with RXR than as a homodimer (33). Thus, in such cases, heterodimerization with RXR appears to facilitate the DNA binding of a type II receptor.
Aside from facilitating DNA binding, heterodimerization between a type II receptor and RXR provides an opportunity for the heterodimer as an entity to respond to two different hormonal signals. Indeed, a typical example of this is the PPAR/RXR heterodimer, in which both partners can bind their cognate ligands and subsequently activate the transcription of target genes (25). The simultaneous binding of both PPAR and RXR ligands leads to synergistic activation. This property of the PPAR/RXR heterodimer that allows ligand binding to both partners is often referred to as permissive (1, 4). In contrast, the TR/RXR heterodimer has been considered to be nonpermissive (1, 4, 14), whereby only TR can bind its ligand T3, while the other partner, RXR, is generally believed to be incapable of ligand binding. It has been suggested that in a TR/RXR heterodimer, the only role of RXR is to facilitate the binding of TR to the TRE, and thus RXR is also referred to as a silent partner (1, 33). Experimental evidence supporting this silent-partner model comes primarily from a study suggesting that T3 but not RXR ligand activates the TR/RXR heterodimer and that ligand binding by RXR is decreased upon heterodimerization with TR (14).
In this study, we reexamined the functional role of RXR in the TR/RXR heterodimer by using a novel and sensitive derepression assay. This assay takes advantage of the fact that when the VP16 activation domain is fused to TR either as a direct VP16-TR fusion (Fig. 5) or as a Gal4-TR-VP16 (GTV) fusion (Fig. 1), its transactivation function is masked by the TR moiety through an inhibitor (or corepressor) that associates with the TR LBD (6). Therefore, dissociation of the inhibitor from the TR LBD (derepression) would allow VP16 to elicit its transactivation function, which can be readily detected with appropriate reporters (e.g., see Fig. 1). A somewhat unexpected finding that the liganded RXR LBD activates GTV (Fig. 1) prompted us to use this assay system to further examine the TR/RXR interrelationship.
Consistent with the notion that an RXR ligand cannot directly activate a TR/RXR heterodimer, cotransfection of Gal4-TR and the RXR LBD does not lead to activation in the presence of 9-cis-RA (Fig. 1). Therefore, activation of GTV by the liganded RXR LBD is likely due to a derepression mechanism that dissociates the inhibitor from the TR moiety of GTV. A plausible model for derepression of GTV by the liganded RXR LBD is that the liganded RXR LBD binds to the TR moiety of GTV and induces a conformational change in TR that results in a (transient or dynamic) dissociation of the inhibitor (Fig. 2A, right panel). In support of this model, the GTV L372R chimera that contains a mutant TR moiety defective in heterodimerization with RXR is not activated by the liganded RXR LBD, although it can still be activated by the apo-TR LBD (Fig. 2B).
Another interesting finding is that the GTV P158R chimera is not activated by the apo-TR LBD but is nevertheless activated by the liganded RXR LBD (Fig. 3A). As discussed in earlier sections, the inability of GTV P158R to be activated by the apo-TR LBD is likely because this specific mutation in the hinge region of TR leads to a structural alteration or conformational disruption of the mutant chimera, rendering it transcriptionally inactive regardless of the state of the cellular inhibitor (Fig. 3B). This explanation is consistent with a recent study suggesting that the Pro-to-Arg mutation at this position as well as other mutations in the hinge region tend to structurally destabilize the mutant TR LBD (42). Given this, it is remarkable that the liganded RXR LBD can efficiently activate the GTV P158R chimera. Thus, as illustrated in Fig. 3B, heterodimerization of this mutant GTV with the liganded RXR LBD appears to structurally or conformationally stabilize the mutant GTV chimera (at least to some extent) and, as in the case of wild-type GTV, lead to a dissociation of the inhibitor from the TR moiety.
Interestingly, a study by Zhang et al. showed that the Gal4 fusion with a TR hinge mutant fails to repress (54), a result also consistent with the notion that the hinge region mutation leads to a structural alteration in the LBD (42). However, cotransfection of Gal4-RXR restores the repression function of the mutant TR, although Gal4-RXR alone does not lead to repression (54). This finding was interpreted in a model in which efficient corepressor binding engages surfaces contributed by both TR and RXR (54). An alternative explanation would be that dimerization between the Gal4-TR (hinge mutant) and Gal4-RXR via the Gal4 moiety brings the TR and RXR in sufficient proximity so that subsequent heterodimerization between the RXR and the mutant TR restores (at least partially) the correct structure or conformation of the mutant TR LBD, which now can bind corepressors. Therefore, it is possible that the structural stabilization of the TR hinge mutant by heterodimerization with RXR may occur in the absence of 9-cis-RA. Nevertheless, such a stabilization effect by apo-RXR would result in the mutant TR's regaining its ability to bind inhibitors/corepressors (54) and thus would not result in activation of GTV P158R (Fig. 3A).
During the revision of the manuscript, Germain et al. showed that RXR can bind its ligand and thus is not a silent partner in the RAR/RXR heterodimer (15), adding to the accumulating evidence against a silent-partner role for RXR in the RAR/RXR heterodimer (9, 24, 28, 36, 47). Interestingly, the study by Germain et al. suggested that although liganded RXR in the heterodimer with apo-RAR can recruit coactivators, it is prohibited from doing so in the usual cellular context, as corepressors do not dissociate efficiently and thus compete with coactivators for binding (15). This study raises an alternative possibility that could account for the activation of GTV by the liganded RXR LBD observed in our experiment. In this alternative model, the liganded RXR LBD in heterodimerization with GTV may recruit AF-2-interacting coactivators (such as members of the p160 family), which, in conjunction with coactivator(s) recruited by the VP16 moiety, could dominate over the function of corepressors associated with the TR LBD moiety, resulting in activation of GTV. However, the NID of GRIP1, a potent dominant negative inhibitor that efficiently blocks ligand-dependent transactivation of Gal4-RXR (Fig. 4A), has no effect on liganded RXR-mediated activation of GTV (Fig. 4B), suggesting that the recruitment of functional endogenous coactivators by the liganded RXR LBD is not required for its activation of GTV.
Our study with wild-type and mutant GTV chimeras suggests that the liganded RXR LBD can heterodimerize with TR in cells. Although the liganded RXR in such a TR/RXR heterodimer does not directly mediate transactivation (Fig. 1A), the heterodimerization bears a functional consequence in that it induces a (transient or dynamic) dissociation of the inhibitor from TR and thus results in derepression (Fig. 2A, right panel). A further study with VP16-TR and a native TRE-controlled reporter suggests that derepression of TR by heterodimerization with liganded RXR can also occur when they are bound to TRE (Fig. 5). Finally, in a reporter assay for T3-mediated activation by a TRE-bound TR/RXR heterodimer, we provide evidence suggesting that the activity of the TR/RXR heterodimer can be superactivated by the simultaneous treatment of ligands of both partners (Fig. 6).
Collectively, our results argue against the current silent-partner model for RXR in the TR/RXR heterodimer. In contrast, we suggest that the RXR in a TR/RXR heterodimer can bind its natural ligand 9-cis-RA in cells. The binding of 9-cis-RA does not lead to direct activation from the RXR component (Fig. 1A and 6) but acts to modulate the repression function of unliganded TR (Fig. 1A and 5) and/or the activation function of liganded TR (Fig. 6). This is a potentially novel and somewhat unexpected aspect of TR-RXR cross talk, which may in turn play a role in fine-tuning the transcriptional output from a TRE-controlled target gene, contingent on the relative abundance of the two receptors and the availability of their cognate ligands, as well as the promoter and cellular context that may determine the dynamic effects of coactivators and corepressors (16, 18, 26, 31, 53).
Our suggestion that RXR in a TR/RXR heterodimer can bind its natural ligand 9-cis-RA in cells is not necessarily contradictory to the in vitro study by Forman et al. (14). In their study, Forman et al. showed that ligand binding by RXR is decreased upon heterodimerization with TR (14). However, it should be noted that significant ligand binding was still detected for RXR even in the presence of TR (14). The in vitro binding assay by Forman et al. used the synthetic RXR ligand LG69 (14), while the natural ligand 9-cis-RA was used in most of our functional studies. Nevertheless, it is interesting that the synthetic RXR-specific ligand LGD1069 (same as LG69) can also mediate activation of GTV by the RXR LBD (Fig. 7). Thus, a consensus explanation would be that although heterodimerization between TR and RXR reduces the binding of RXR to LG69 (14), a significant level of binding still occurs, resulting in a derepression effect on TR similar to that manifested by 9-cis-RA-bound RXR.
How liganded RXR induces a dissociation of the inhibitor/corepressor from its partner TR is unclear, but it presumably involves an induced conformational change in TR. Such allosteric control of partner activity by RXR and RXR ligand is reminiscent of the phantom ligand effect reported by Schulman et al. (48). In their study, Schulman et al. showed that a synthetic ligand, LG100754, binds to RXR in the RAR/RXR heterodimer but nevertheless manifests its effect through the other partner (in this case, RAR) by inducing a conformational change in RAR that results in both the dissociation of corepressors and association of coactivators (48). Indeed, synthetic ligands for RXRs (rexinoids) have been shown to exhibit diverse properties in their modulations of a variety of type II receptor/RXR heterodimers (9, 28, 37, 45) and thus are of important potential in drug development.
In this regard, it is interesting that a number of orphan receptors, such as farnesoid X receptor (FXR) and liver X receptor (LXR) can form permissive heterodimers with RXR that allow ligand binding and transactivation by RXR (4). Therefore, signaling pathways mediated by these permissive orphan receptors can potentially be subjected to intervention by rexinoids (45). Our study suggests that the property of TR/RXR, a heterodimer previously regarded as nonpermissive, can be modulated by a natural RXR ligand (9-cis-RA). Interestingly, the RAR/RXR heterodimer was also initially thought to be nonpermissive (27, 33). However, more recent studies suggest that it is at least semipermissive, whereby RXR can engage in ligand binding when its partner RAR is ligand occupied and/or when a suitable synthetic RXR ligand is presented (9, 24, 28, 36, 47). These results, together with our findings, indicate the importance of reevaluating other nonpermissive heterodimers to explore the possibility of modulating their activities by RXR ligands, especially by yet to be identified synthetic rexinoids.
Acknowledgments
We thank Richard Heyman for providing retinoids, Ken Takeshita for sharing LGD1069, Ron Evans for the pCMV-RXR plasmids, Inez Rogatsky and Keith Yamamoto for the dominant negative form of GRIP1, Fred Stanley for advice on graphic preparations, Tats Yamada for experimental assistance, and James Yopp for critical reading of the manuscript.
This work was supported by NIH grant DK16636 (to H.H.S.) and NRSA postdoctoral fellowship award DK09581 (to D.L.).
REFERENCES
- 1.Aranda, A., and A. Pascual. 2001. Nuclear hormone receptors and gene expression. Physiol. Rev. 81:1269-1304. [DOI] [PubMed] [Google Scholar]
- 2.Au-Fliegner, M., E. Helmer, J. Casanova, B. M. Raaka, and H. H. Samuels. 1993. The conserved ninth C-terminal heptad in thyroid hormone and retinoic acid receptors mediates diverse responses by affecting heterodimer but not homodimer formation. Mol. Cell. Biol. 13:5725-5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baniahmad, A., C. Steiner, A. C. Kohne, and R. Renkawitz. 1990. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell 61:505-514. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg, B., and R. M. Evans. 1998. Orphan nuclear receptors-new ligands and new possibilities. Genes Dev. 12:3149-3155. [DOI] [PubMed] [Google Scholar]
- 5.Brzozowski, A. M., A. C. Pike, Z. Dauter, R. E. Hubbard, T. Bonn, O. Engstrom, L. Ohman, G. L. Greene, J. A. Gustafsson, and M. Carlquist. 1997. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753-758. [DOI] [PubMed] [Google Scholar]
- 6.Casanova, J., E. Helmer, S. Selmi-Ruby, J.-S. Qi, M. Au-Fliegner, V. Desai-Yajnik, N. Koudinova, F. Yarm, B. M. Raaka, and H. H. Samuels. 1994. Functional evidence for ligand-dependent dissociation of thyroid hormone and retinoic acid receptors from an inhibitory cellular factor. Mol. Cell. Biol. 14:5756-5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, J. D., and R. M. Evans. 1995. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:454-457. [DOI] [PubMed] [Google Scholar]
- 8.Chen, J. D., K. Umesono, and R. M. Evans. 1996. SMRT isoforms mediate repression and anti-repression of nuclear receptor heterodimers. Proc. Natl. Acad. Sci. USA 93:7567-7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, J. Y., J. Clifford, C. Zusi, J. Starrett, D. Tortolani, J. Ostrowski, P. R. Reczek, P. Chambon, and H. Gronemeyer. 1996. Two distinct actions of retinoid-receptor ligands. Nature 382:819-822. [DOI] [PubMed] [Google Scholar]
- 10.Damm, K., and R. M. Evans. 1993. Identification of a domain required for oncogenic activity and transcriptional suppression by v-erbA and thyroid-hormone receptor alpha. Proc. Natl. Acad. Sci. USA 90:10668-10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darimont, B. D., R. L. Wagner, J. W. Apriletti, M. R. Stallcup, P. J. Kushner, D. Baxter, R. J. Fletterick, and K. R. Yamamoto. 1998. Structure and specificity of nuclear receptor-coactivator interactions. Genes Dev. 12:3343-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forman, B. M., J. Casanova, B. M. Raaka, J. Ghysdael, and H. H. Samuels. 1992. Half-site spacing and orientation determines whether thyroid hormone and retinoic acid receptors and related factors bind to DNA response elements as monomers, homodimers, or heterodimers. Mol. Endocrinol. 6:429-442. [DOI] [PubMed] [Google Scholar]
- 13.Forman, B. M., and H. H. Samuels. 1991. pEXPRESS: a family of expression vectors containing a single transcription unit active in prokaryotes, eukaryotes and in vitro. Gene 105:9-15. [DOI] [PubMed] [Google Scholar]
- 14.Forman, B. M., K. Umesono, J. Chen, and R. M. Evans. 1995. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell 81:541-550. [DOI] [PubMed] [Google Scholar]
- 15.Germain, P., J. Iyer, C. Zechel, and H. Gronemeyer. 2002. Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature 415:187-192. [DOI] [PubMed] [Google Scholar]
- 16.Glass, C. K., and M. G. Rosenfeld. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14:121-141. [PubMed] [Google Scholar]
- 17.Heyman, R. A., D. J. Mangelsdorf, J. A. Dyck, R. B. Stein, R. M. Evans, and C. Thaller. 1992. 9-cis Retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 68:397-406. [DOI] [PubMed] [Google Scholar]
- 18.Hong, S. H., and M. L. Privalsky. 2000. The SMRT corepressor is regulated by a MEK-1 kinase pathway: inhibition of corepressor function is associated with SMRT phosphorylation and nuclear export. Mol. Cell. Biol. 20:6612-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horlein, A. J., A. M. Naar, T. Heinzel, J. Torchia, B. Gloss, R. Kurokawa, A. Ryan, Y. Kamil, M. Soderstrom, C. K. Glass, and M. G. Rosenfeld. 1995. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377:397-404. [DOI] [PubMed] [Google Scholar]
- 20.Hu, X., and M. A. Lazar. 1999. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature 402:93-96. [DOI] [PubMed] [Google Scholar]
- 21.Kaster, P., M. Mark, and P. Chambon. 1995. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell 83:859-869. [DOI] [PubMed] [Google Scholar]
- 22.Kastner, P., M. Mark, N. Ghyselinck, W. Krezel, V. Dupe, J. M. Grondona, and P. Chambon. 1997. Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development 124:313-326. [DOI] [PubMed] [Google Scholar]
- 23.Kastner, P., N. Messaddeq, M. Mark, O. Wendling, J. M. Grondona, S. Ward, N. Ghyselinck, and P. Chambon. 1997. Vitamin A deficiency and mutations of RXRα, RXRβ and RARα lead to early differentiation of embryonic ventricular cardiomyocytes. Development 124:4749-4758. [DOI] [PubMed] [Google Scholar]
- 24.Kersten, S., M. I. Dawson, B. A. Lewis, and N. Noy. 1996. Individual subunits of heterodimers comprised of retinoic acid and retinoid X receptors interact with their ligands independently. Biochemistry 35:3816-3824. [DOI] [PubMed] [Google Scholar]
- 25.Kliewer, S. A., K. Umesono, D. J. Noonan, R. A. Heyman, and R. M. Evans. 1992. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature 358:771-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knutti, D., D. Kressler, and A. Kralli. 2001. Regulation of the transcriptional coactivator PGC-1 via MAPK-sensitive interaction with a repressor. Proc. Natl. Acad. Sci. USA 98:9713-9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurokawa, R., J. DiRenzo, M. Boehm, J. Sugarman, B. Gloss, M. G. Rosenfeld, R. A. Heyman, and C. K. Glass. 1994. Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature 371:528-531. [DOI] [PubMed] [Google Scholar]
- 28.Lala, D. S., R. Mukherjee, I. G. Schulman, S. S. Koch, L. J. Dardashti, A. M. Nadzan, G. E. Croston, R. M. Evans, and R. A. Heyman. 1996. Activation of specific RXR heterodimers by an antagonist of RXR homodimers. Nature 383:450-453. [DOI] [PubMed] [Google Scholar]
- 29.Levin, A. A., L. J. Sturzenbecker, S. Kazmer, T. Bosakowski, C. Huselton, G. Allenby, J. Speck, C. Kratzeisen, M. Rosenberger, A. Lovey, and J. F. Grippo. 1992. 9-cis Retinoic acid stereoisomer binds and activates the nuclear RXRα. Nature 355:359-361. [DOI] [PubMed] [Google Scholar]
- 30.Li, D., V. Desai-Yajnik, E. Lo, M. Schapira, R. Abagyan, and H. H. Samuels. 1999. NRIF3 is a novel coactivator mediating functional specificity of nuclear hormone receptors. Mol. Cell. Biol. 19:7191-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, D., F. Wang, and H. H. Samuels. 2001. Domain structure of the NRIF3 family of coregulators suggests potential dual roles in transcriptional regulation. Mol. Cell. Biol. 21:8371-8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, H., C. Leo, D. J. Schroen, and J. D. Chen. 1997. Characterization of receptor interaction and transcriptional repression by the corepressor SMRT. Mol. Endocrinol. 11:2025-2037. [DOI] [PubMed] [Google Scholar]
- 33.Mangelsdorf, D. J., and R. M. Evans. 1995. The RXR heterodimers and orphan receptors. Cell 83:841-850. [DOI] [PubMed] [Google Scholar]
- 34.Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schutz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, and R. M. Evans. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenna, N. J., R. B. Lanz, and B. W. O'Malley. 1999. Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 20:321-344. [DOI] [PubMed] [Google Scholar]
- 36.Minucci, S., M. Leid, R. Toyama, J. P. Saint-Jeannet, V. J. Peterson, V. Horn, J. E. Ishmael, N. Bhattacharyya, A. Dey, I. B. Dawid, and K. Ozato. 1997. Retinoid X receptor (RXR) within the RXR-retinoic acid receptor heterodimer binds its ligand and enhances retinoid-dependent gene expression. Mol. Cell. Biol. 17:644-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee, R., P. J. Davies, D. L. Crombie, E. D. Bischoff, R. M. Cesario, L. Jow, L. G. Hamann, M. F. Boehm, C. E. Mondon, A. M. Nadzan, J. R. Paterniti, Jr., and R. A. Heyman. 1997. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature 386:407-410. [DOI] [PubMed] [Google Scholar]
- 38.Nagy, L., H. Y. Kao, J. D. Love, C. Li, E. Banayo, J. T. Gooch, V. Krishna, K. Chatterjee, R. M. Evans, and J. W. Schwabe. 1999. Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev. 13:3209-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson, C. C., S. C. Hendy, J. S. Faris, and P. J. Romaniuk. 1994. The effects of P-box substitutions in thyroid hormone receptor on DNA binding specificity. Mol. Endocrinol. 8:829-840. [DOI] [PubMed] [Google Scholar]
- 40.Nolte, R. T., G. B. Wisely, S. Westin, J. E. Cobb, M. H. Lambert, R. Kurokawa, M. G. Rosenfeld, T. M. Willson, C. K. Glass, and M. V. Milburn. 1998. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-γ. Nature 395:137-143. [DOI] [PubMed] [Google Scholar]
- 41.Perissi, V., L. M. Staszewski, E. M. McInerney, R. Kurokawa, A. Krones, D. W. Rose, M. H. Lambert, M. V. Milburn, C. K. Glass, and M. G. Rosenfeld. 1999. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 13:3198-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pissios, P., I. Tzameli, P. Kushner, and D. D. Moore. 2000. Dynamic stabilization of nuclear receptor ligand binding domains by hormone or corepressor binding. Mol. Cell 6:245-253. [DOI] [PubMed] [Google Scholar]
- 43.Qi, J.-S., V. Desai-Yajnik, M. E. Greene, B. M. Raaka, and H. H. Samuels. 1995. The ligand binding domains of the thyroid hormone/retinoid receptor gene subfamily function in vivo to mediate heterodimerization, gene silencing, and transactivation. Mol. Cell. Biol. 15:1817-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renaud, J.-P., N. Rochel, M. Ruff, V. Vivat, P. Chambon, H. Gronemeyer, and D. Moras. 1995. Crystal structure of the RAR-γ ligand-binding domain bound to all-trans retinoic acid. Nature 378:681-689. [DOI] [PubMed] [Google Scholar]
- 45.Repa, J. J., S. D. Turley, J. A. Lobaccaro, J. Medina, L. Li, K. Lustig, B. Shan, R. A. Heyman, J. M. Dietschy, and D. J. Mangelsdorf. 2000. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289:1524-1529. [DOI] [PubMed] [Google Scholar]
- 46.Rogatsky, I., K. A. Zarember, and K. R. Yamamoto. 2001. Factor recruitment and TIF2/GRIP1 corepressor activity at a collagenase-3 response element that mediates regulation by phorbol esters and hormones. EMBO J. 20:6071-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy, B., R. Taneja, and P. Chambon. 1995. Synergistic activation of retinoic acid (RA)-responsive genes and induction of embryonal carcinoma cell differentiation by an RA receptor alpha (RAR alpha)-, RAR beta-, or RAR gamma-selective ligand in combination with a retinoid X receptor-specific ligand. Mol. Cell. Biol. 15:6481-6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schulman, I. G., C. Li, J. W. Schwabe, and R. M. Evans. 1997. The phantom ligand effect: allosteric control of transcription by the retinoid X receptor. Genes Dev. 11:299-308. [DOI] [PubMed] [Google Scholar]
- 49.Shiau, A. K., D. Barstad, P. M. Loria, L. Cheng, P. J. Kushner, D. A. Agard, and G. L. Greene. 1998. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95:927-937. [DOI] [PubMed] [Google Scholar]
- 50.Umesono, K., K. K. Murakami, C. C. Thompson, and R. M. Evans. 1991. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D receptors. Cell 65:1255-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner, R. L., J. W. Apriletti, M. E. McGrath, B. L. West, J. D. Baxter, and R. J. Fletterick. 1995. A structural role for hormone in the thyroid hormone receptor. Nature 378:690-697. [DOI] [PubMed] [Google Scholar]
- 52.Wan, Y. J., D. An, Y. Cai, J. J. Repa, T. Hung-Po Chen, M. Flores, C. Postic, M. A. Magnuson, J. Chen, K. R. Chien, S. French, D. J. Mangelsdorf, and H. M. Sucov. 2000. Hepatocyte-specific mutation establishes retinoid X receptor alpha as a heterodimeric integrator of multiple physiological processes in the liver. Mol. Cell. Biol. 20:4436-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, J., M. G. Guenther, R. W. Carthew, and M. A. Lazar. 1998. Proteasomal regulation of nuclear receptor corepressor-mediated repression. Genes Dev. 12:1775-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, J., I. Zamir, and M. A. Lazar. 1997. Differential recognition of liganded and unliganded thyroid hormone receptor by retinoid X receptor regulates transcriptional repression. Mol. Cell. Biol. 17:6887-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]