Abstract
The involvement of p21-activated kinases (PAKs) in important cellular processes such as regulation of the actin skeleton morphology, transduction of signals controlling gene expression, and execution of programmed cell death has directed attention to the regulation of the activity of these kinases. Here we report that activation of PAK2 by p21 GTPases can be strongly potentiated by cellular tyrosine kinases. PAK2 became tyrosine phosphorylated in its N-terminal regulatory domain, where Y130 was identified as the major phosphoacceptor site. Tyrosine phosphorylation-mediated superactivation of PAK2 could be induced by overexpression of different Src kinases or by inhibiting cellular tyrosine phosphatases with pervanadate and could be blocked by the Src kinase inhibitor PP1 or by mutating the Y130 residue. Analysis of PAK2 mutants activated by amino acid changes in the autoinhibitory domain or the catalytic domain indicated that GTPase-induced conformational changes, rather than catalytic activation per se, rendered PAK2 a target for tyrosine phosphorylation. Thus, PAK activation represents a potentially important point of convergence of tyrosine kinase- and p21 GTPase-dependent signaling pathways.
The mammalian family of Saccharomyces cerevisiae Ste20-like kinases consists of six members known as p21-activated kinases 1 to 6 (PAK1 to -6). Phylogenetic analysis clusters the more recently identified PAK4, PAK5, and PAK6 as a distinct subfamily (PAK-II) separate from that of PAK1, PAK2, and PAK3 (PAK-I subfamily) (10, 15). The majority of the published data on PAK kinases still concern the members of the PAK-I subfamily, which serve important cellular functions involved in regulation of the actin cytoskeleton morphology, transduction of signals controlling gene expression, and execution of programmed cell death (for reviews, see references 3 and 11).
All PAKs contain a conserved serine/threonine kinase domain that comprises the C-terminal half and an N-terminally located p21-binding domain (PBD; also called the Cdc42/Rac-1 interactive binding [CRIB] domain) (24). PAK-I members also contain several characterized motifs for the interaction with signaling molecules (3, 11). These motifs include an N-terminal proline-rich region (PxxP motif), which can target PAKs to the plasma membrane and mediate interactions with transmembrane growth factor receptors via binding to the second SH3 domain of the adapter protein Nck (4, 22, 38), and another SH3-ligand motif that mediates binding to the alpha-PAK-interacting exchange factor (α-PIX) and β-PIX (also called COOL-1 and COOL-2, respectively) (5, 25). The cellular substrates of PAK that mediate the downstream effect of these kinases are still incompletely characterized but have been reported to include Bad and myosin heavy and light chains, as well as myosin regulatory light chain kinase, LIM kinase, Raf-1, p47phox, and MEK-1 (11).
The emerging roles of PAK kinases in regulation of multiple fundamental cellular processes have directed significant attention into understanding how the activity of these kinases is controlled. Biochemical and structural studies have revealed autoinhibition of the C-terminal catalytic domain by the N-terminal domain as the key mechanism in regulation of PAKs. Several layers of inhibition, involving dimerization and occupation of the catalytic cleft by contacts between the N- and C-terminal domains, keep the catalytic activity of PAK kinases in check (21; reviewed in reference 14). Autoinhibition of PAK1 has recently been shown to occur in trans, meaning that the inhibitory domain of one PAK1 molecule interacts with the kinase domain of another PAK1 molecule (30). Association of GTP-bound forms of Cdc42 or Rac1 with the PAK PBD/CRIB domain induces conformational changes in the N-terminal domain that no longer support its autoinhibitory function. Thus, the activity of PAKs can be induced by a variety of extracellular signals that control the cellular factors that regulate the GTP association state of Cdc42 or Rac1. As alternative or complementary mechanisms for p21-mediated PAK activation, binding of certain sphingolipids, such as sphingosine, and caspase-catalyzed proteolytic cleavage between the N- and C-terminal domains (in the case of PAK2) have also been shown to be able to overcome PAK autoinhibition (7, 19).
Autophosphorylation of serine and threonine residues is another key event in establishing and maintaining a catalytically active state of PAKs. Of the several serine residues that become phosphorylated upon PAK activation, Ser141 (numbered according to PAK2) has been reported to be particularly important (8). Similarly to that of many other kinases, phosphorylation of the activation loop in the kinase domain of PAK plays a dominant role the activation process (12, 37). The site of this phosphorylation is Thr402, which is the only threonine residue that becomes autophosphorylated in PAK (12, 23). An acidic T402E substitution of this residue is sufficient to render PAK catalytically active autonomously of the N-terminal regulatory region. It has been suggested that the earliest stages of PAK activation in cells may involve phosphorylation of this threonine residue by 3-phosphoinositide-dependent kinase-1 instead of by PAK autophosphorylation (17).
The catalytic activity of PAKs is accompanied by a shift in the electrophoretic mobility in sodium dodecyl sulfate (SDS)-polyacrylamide gels towards forms of higher apparent molecular weight (7, 17). An observation that focused our interest on PAK regulation was that pathogenicity factor Nef of human immunodeficiency virus type 1 specifically associated with a high-molecular-weight species of PAK2 which, despite its low relative abundance, accounted for most of the cellular PAK2 activity (31, 32). Our present studies, aimed at characterization of the mechanisms that govern generation of this highly active PAK2 species, have revealed a novel and potentially important feature of PAK regulation. Here we show that induction of PAK2 activity via p21 GTPases can be strongly potentiated by concurrent stimulation of cellular tyrosine kinase activity. Overexpression of various Src kinases or treatment of cells with the tyrosine phosphatase inhibitor pervanadate had little effect on PAK2 activity on their own but enhanced the ability of Cdc42 or Rac1 to stimulate PAK2 activity. This potentiation was mediated by phosphorylation of Y130 in the N-terminal regulatory domain. Together, the biochemical and genetic data from these studies support a model in which p21 GTPase binding-induced conformational changes render PAK2 a substrate for subsequent Src kinase-mediated tyrosine phosphorylation, leading to a robust enhancement of the catalytic activity of PAK2.
MATERIALS AND METHODS
Cell culture, transient transfections, and pervanadate treatment.
293T human embryonic kidney cells (American Type Culture Collection) were maintained under standard culture conditions. Transfections were performed using Lipofectamine transfection reagent (Gibco BRL) according to the manufacturer's instructions. Cells were used for experiments 48 h after transfection. Pervanadate was freshly made for every experiment by incubating 100 μl of 100 mM sodium orthovanadate with 9 μl of 30% H2O2 in a final volume of 200 μl for 15 min at room temperature and was kept on ice until use. Pervanadate was added to the transfected cell in a final concentration of 250 μM for 15 min, after which the cells were placed on ice, washed with ice cold phosphate-buffered saline, and lysed.
Plasmids and construction of PAK2 mutants.
The PAK2-encoding insert was cloned in a pEBB eukaryotic expression vector with either a MycHIS-HA (hemagglutinin) multiepitope (ME) tag (32) or a MycHIS (MH) tag. Since the ME tag used in our previous studies contains a HA tag with potential tyrosine phosphorylation sites, the MH-tagged proteins were used in most experiments. Unless otherwise indicated in the respective figure legend, the PAK2 constructs were MH tagged. All PAK2 mutants were made by overlap PCR, using a specific mutant primer pair and common outer primers containing restriction sites used for cloning. The expression plasmid for dominant active (V12) Cdc42V12 was kindly provided by B. Mayer.
As a substrate for the PAK2-kinase assays, we cloned three tandem copies of the 7-amino-acid peptide of the Rous sarcoma virus nucleocapsid (KKRKSGL) in frame with glutathione S-transferase (GST) into the bacterial expression vector pGEX-4T-1 (Amersham Biosciences). This peptide was shown by Tuazon et al. to contain the consensus substrate sequence for PAK2 (36). In contrast to myelin basic protein, this substrate did not promote p21-independent activation of PAK2 (data not shown).
Immunoprecipitations and in vitro kinase assays.
Immunoprecipitations using anti-Myc (9E10) antibodies and subsequent in vitro kinase assays (IVKA) were performed as described previously (32). When indicated, bacterially produced GST-substrate peptide fusion protein (100 μg/ml) was added to the IVKA reaction mixture. To visualize incorporation of 32P into the substrate peptide, a fraction of the kinase assay was analyzed using SDS-12% polyacrylamide gel electrophoresis (PAGE).
Tyrosine dephosphorylation using PTP-1B.
Protein tyrosine phosphatase 1B (PTP-1B) was expressed in bacteria as a GST fusion protein and purified from the lysed cells by using glutathione Sepharose beads (Amersham Biosciences). PAK2 was immunoprecipitated from cell extracts, and beads were washed in IVKA lysis buffer and equilibrated in dephosphorylation buffer (25 mM Tris [pH 7.6], 10% [vol/vol] glycerol, 1 mM dithiothreitol, 0.1 mM EDTA, 0.01% Nonidet P-40, including protease inhibitors). The beads were split in two identical samples and incubated with or without GST-PTP-1B (10 μg/ml) for 30 min at 35°C. After the incubation, the beads were washed in IVKA buffer and subjected to IVKA.
In vivo labeling and phosphoamino acid analysis.
Transfected cells were washed with phosphate-free medium (minimum essential medium without sodium phosphate) (Sigma) supplemented with 20 mM Ultraglutamine-1 (Biowhittaker) and starved in this medium for 1 h. [32P]orthophosphate (10 mCi/ml; Amersham Biosciences) was added to achieve a concentration of 0.5 mCi/ml, and cells were labeled for 3 h. After pervanadate treatment, the cells were washed with ice-cold phosphate-buffered saline and lysed in IVKA lysis buffer. Lysates were cleared by centrifugation (10 min, 16,000 × g) and immunoprecipitated with anti-Myc antibodies. After extensive washing with IVKA lysis buffer, the immunoprecipitates were boiled in SDS-PAGE sample buffer and separated by SDS-8% PAGE. Proteins were transferred to polyvinylidene difluoride membrane (Bio-Rad), and after exposure to X-ray film, the labeled PAK2 band was excised. The membrane piece was boiled in 6 M HCl at 110°C for 1 h. Hydrolyzed amino acids were processed as described previously (16), mixed with unlabeled phosphoamino acid standards, and separated on cellulose thin-layer chromatography plates (20 cm long by 20 cm wide; Merck) by electrophoreses, using a HTLE-7000 system for 30 min at 2,000 V in pH 1.9 buffer (formic acid [88%]/acetic acid/water ratio, 50:156:1,794) in the first dimension and for 20 min at 1,600 V in pH 3.5 buffer (acetic acid/pyridine/water ratio, 10:1:189) for the second dimension. Plates were stained with ninhydrin to visualize the unlabeled standards; labeled amino acids were visualized by autoradiography.
RESULTS
Synergistic regulation of PAK2 activity by Cdc42 and Src family tyrosine kinases.
As analyzed by IVKA and SDS-gel electrophoresis, activation of PAK2 by cotransfection of a dominant active variant of Cdc42 (Cdc42V12) into 293T cells resulted in increased PAK2 autokinase activity and the appearance of slower-migrating, differentially phosphorylated PAK2 species (Fig. 1A, two leftmost lanes). In agreement with the previous data of Renkema et al. (32), the Cdc42-induced high-molecular-weight PAK2 species exhibited a very high specific activity, as this band became intensely labeled in the kinase reaction whereas only the PAK2 species migrating below was abundant enough to be detected by immunoblotting. Similar results were obtained when a dominant active variant of Rac1 was used instead of Cdc42 (not shown).
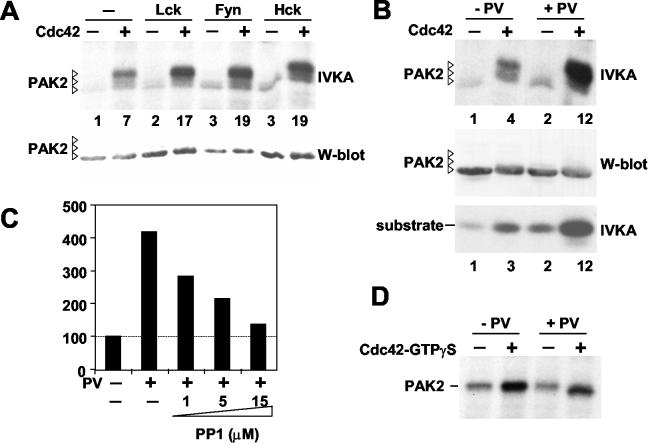
FIG. 1.
Potentiation of Cdc42-induced catalytic activation by overexpression of Src family kinases or by inhibition of cellular tyrosine kinase activity. (A) PAK2 was transfected into 293T cells in various combinations with or without different Src kinases and Cdc42V12, as indicated. PAK2 proteins were precipitated with an anti-Myc antibody and subjected to autophosphorylation in the presence of [32P]ATP. Half of the radiolabeled reaction mixtures were analyzed by anti-Myc Western blotting (lower panel), and the other half were analyzed by direct autoradiography of the gel (upper panel). PAK2 autophosphorylation in each lane was quantified by phosphorimager analysis of the gel and is indicated as a severalfold increase relative to the value obtained without cotransfection of Cdc42V12 and a Src kinase. (B) PAK2 expression, autokinase activity, and kinase activity towards the substrate peptide, with (+) or without (−) cotransfection of Cdc42v12, was examined as for panel A. Instead of Src kinase overexpression, however, tyrosine phosphorylation was induced by addition of pervanadate (PV) 15 min before lysis of the cells as indicated. PAK2 autophosphorylation and kinase activity in each lane was quantified by phosphorimager analysis of the gel and is indicated as a severalfold increase relative to the value obtained without activation. (C) Graph representation of phosphorimager-quantified autophosphorylation of PAK2 from cells cotransfected with PAK2 and Cdc42V12, analyzed as for panel B. The leftmost bar is normalized to 100% and represents PAK2 autokinase activity without pervanadate treatment (−). The four other bars indicate PAK2 activity in cells treated with pervanadate alone (+) or in combination with a 45-min pretreatment with Src kinase inhibitor PP1 (1, 5, or 15 μM). (D) The effect of pervanadate treatment of cells on PAK2 activity was examined as described above, except that PAK2 was transfected in the absence of Cdc42V12 or other activator proteins. Instead, PAK2 activity in half of the immunoprecipitated material was induced in vitro by addition of recombinant GTPγS-loaded Cdc42 into the IVKA reaction mixture as indicated.
To study possible involvement of tyrosine kinase signaling pathways in modulation of p21 GTPase-mediated PAK activation, we transfected cells with various combinations of PAK2, Cdc42V12, and different Src family tyrosine kinases, including Fyn, Hck, and Lck. As shown in Fig. 1A, overexpression of these kinases alone had only a marginal effect on PAK2 kinase activity and no effect on the mobility of PAK2 protein. Strikingly, however, overexpression of both Cdc42V12 and a Src kinase resulted in a significant potentiation of PAK2 autokinase activity.
To study whether endogenous tyrosine kinases could have a similar effect on PAK2 activation, we treated the Cdc42V12-transfected cells with pervanadate, a potent inhibitor of tyrosine phosphatases. This also resulted in a strong increase in PAK2 kinase activity (Fig. 1B), which was equally evident when assayed as autophosphorylation (top panel) or as kinase activity towards a peptide containing three copies of a PAK2-substrate consensus sequence (36) (bottom panel). As was observed by transfecting Src kinases, pervanadate stimulation had little effect unless the cells were cotransfected with an active p21 GTPase. Similar synergistic PAK2 activation by pervanadate was observed in COS-7 cells and in 3T3 cells transfected with either Cdc42V12 or Rac1V12 (data not shown). Pretreatment of cells before the pervanadate stimulation with PP1, a pharmacological inhibitor of Src kinases, abolished the potentiation of Cdc42-induced PAK2 activation in a dose-dependent manner (Fig. 1C). We therefore concluded that the endogenous tyrosine kinase involved in the synergistic activation of PAK2 was likely to be a Src kinase family member also.
When PAK2 was immunoprecipitated from pervanadate-treated cells that had not been transfected with Cdc42V12 and was only subsequently activated in vitro by using GTPγS-loaded recombinant Cdc42, no difference in the extent of activation of PAK2 derived from pervanadate-treated or control cells was observed (Fig. 1D). This suggested that induction of cellular tyrosine phosphorylation could not prime PAK2 for subsequent activation by p21 GTPases and that the reverse order of events was the case.
PAK2 becomes directly tyrosine phosphorylated.
We next wanted to determine whether the synergy of Src kinases and p21 GTPases involved direct tyrosine phosphorylation of PAK2. Probing anti-PAK2 immunoprecipitates from differentially transfected and stimulated cells with anti-phosphotyrosine antibodies gave no specific signal, indicating that, regardless of whether Cdc42V12 was transfected or not, the bulk of PAK2 did not become tyrosine phosphorylated even upon pervanadate treatment (data not shown). However, considering that the slower-migrating, superactivated species of PAK2 was not abundant enough to be detected by anti-PAK2 immunoblotting (Fig. 1), anti-phosphotyrosine Western blotting would not be expected to be a suitable strategy for examining the phosphorylation status of this subpopulation of PAK2. Therefore, a more sensitive experimental strategy was adopted. PAK2, radiolabeled by IVKA, was eluted from the immunobeads and reprecipitated using an anti-phosphotyrosine antibody (PY20) or an anti-PAK (anti-Myc-tag) antibody as a positive control. As shown in the upper panel of Fig. 2, cotransfection of Cdc42V12 together with pervanadate treatment resulted in a PAK2 subpopulation that could be readily reimmunoprecipitated with the anti-pTyr antibody. By contrast, cotransfection of Cdc42V12 alone did not give rise to a PAK2 subpopulation that could be detected by the anti-pTyr antibody. Most notably, pervanadate treatment of cells not transfected with Cdc42V12 failed to induce significant tyrosine phosphorylation of PAK2. Similar results were also obtained with a different monoclonal anti-pTyr antibody (4G10; data not shown). In anti-pTyr precipitates from pervanadate-treated cells, an additional upper band was also seen. We have previously noted this protein to be phosphorylated in anti-PAK2 IVKA assays. And we believe it to be PIX or a PIX-associated protein, as its presence was dependent on an intact PIX-binding motif in PAK2 (32). Indeed, when a PAK2 mutant with a disrupted PIX-binding motif was used, this protein was not seen (data not shown). Notably, this had no effect on the ability of the anti-pTyr antibodies to reimmunoprecipitate PAK2, indicating that PIX or another PIX-associated phosphoprotein, such as G-protein-coupled receptor kinase-interacting target (GIT) or paxillin, did not help to mediate precipitation of PAK2 by the anti-pTyr antibodies.
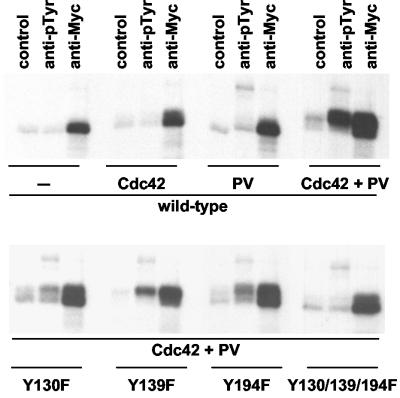
FIG. 2.
P21-activated PAK2 becomes a target for Y130 phosphorylation. 293T cells were transfected with wild-type PAK2 (upper panel) or PAK2 constructs with individual point mutations or a triple mutation disrupting the three potential N-terminal tyrosine phosphorylation sites (lower panel) and were cotransfected with Cdc42V12 and treated with pervanadate (PV) as indicated. After immunoprecipitation and IVKA, the labeled PAK2 proteins were eluted and split into three identical fractions that were subsequently reimmunoprecipitated with empty beads (control), anti-phosphotyrosine antibodies (anti-pTyr), or anti-PAK antibodies (anti-Myc).
To determine which tyrosine residues in PAK2 were the targets for phosphorylation, a mutagenesis approach was undertaken. Since human PAK2 contains 11 tyrosine residues, we first determined in which part of the protein the target tyrosine residue(s) was located by making use of the ability of PAK2 to be cleaved by caspase 3 between residues 212 and 213 (35). Reimmunoprecipitations of the caspase 3 cleavage fragments of PAK2 indicated that only the N-terminal half of PAK2 was tyrosine phosphorylated (data not shown). Therefore, we mutated the three tyrosine residues found in the N-terminal half of PAK2 into phenylalanines to create the mutants Y130F, Y139F, and Y194F and used the reimmunoprecipitation assay to test their capacity to become tyrosine phosphorylated (Fig. 2, bottom panel). Comparison of the abilities of the anti-Myc and anti-pTyr antibodies to precipitate the mutant proteins revealed that the Y130F mutation alone almost completely abolished tyrosine phosphorylation. By contrast, the Y139F could still be recognized by the anti-pTyr antibody. Also, the Y194F mutation appeared to decrease the relative ability of anti-pTyr (compared to anti-Myc) to precipitate this protein, suggesting that Y194 may be an additional phosphoacceptor site. As expected, when all three mutations were combined into the same molecule (Y130/139/194F), the anti-pTyr precipitation was completely lost.
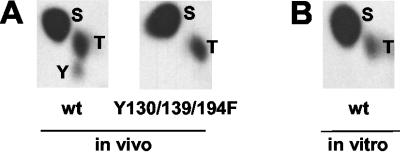
In agreement with the reimmunoprecipitation findings, phosphoamino acid analysis of cells labeled in vivo with [32P]orthophosphate revealed a phosphotyrosine signal from the wild-type PAK2-expressing cells but not the Y130/139/194F-expressing cells cotransfected with Cdc42V12 and stimulated with pervanadate (Fig. 3A). No 32P-labeled phosphotyrosine was detected from wild-type PAK2 immunoprecipitated from similarly activated unlabeled cells and subsequently radiolabeled in vitro by IVKA (Fig. 3B), indicating that tyrosine phosphorylation of PAK2 takes place in the cells, rather than during the IVKA assay due to a coprecipitating kinase. From these results, we concluded that Cdc42V12-activated PAK2 is susceptible to direct tyrosine phosphorylation and that Y130 in its regulatory domain is the main target residue.
FIG. 3.
Phosphoamino acid analysis of PAK2. (A) Analysis of wild-type PAK2 and PAK2-Y130/139/194F from cells that were cotransfected with Cdc42v12 and treated with pervanadate and labeled in vivo with [32P]orthophosphate. (B) Analysis of wild-type PAK2 labeled in vitro by IVKA reaction with [32P]ATP after immunoprecipitation from cells that were cotransfected with Cdc42v12 and treated with pervanadate.
Tyrosine phosphorylation increases the intrinsic catalytic activity of PAK2.
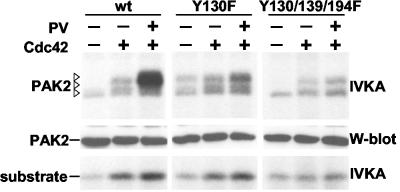
To directly study the role of Y130 in the tyrosine phosphorylation-mediated activation of PAK2, we compared the ability of Cdc42V12 and pervanadate to synergistically activate wild-type PAK2, PAK2-Y130F, and PAK2-Y130/139/194F. As is evident in Fig. 4, unlike its effect on that of the wild type, pervanadate had little additional effect on kinase activity of Y130F and Y130/139/194F mutant proteins. Thus, we concluded that Y130 in the N-terminal regulatory domain is not only the predominant site for tyrosine phosphorylation but is also the critical target residue for Src kinase-mediated potentiation of PAK2 kinase activity.
FIG. 4.
PAK2-Y130 is critical for synergistic activation by Cdc42 and pervanadate treatment. 293T cells were transfected with wild-type PAK2, PAK2-Y130F, or PAK2-Y130/139/194F and were cotransfected with Cdc42V12 and treated with pervanadate (PV) as indicated. Protein expression and autokinase activity and kinase activity towards the substrate peptide was examined as for Fig. 1B.
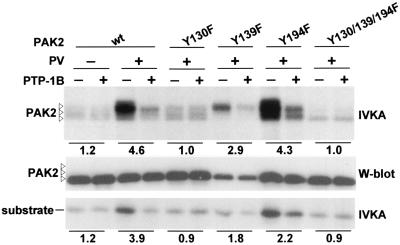
To further characterize the functional role of PAK2 Y130 phosphorylation, we tested the effect of in vitro tyrosine dephosphorylation on PAK2 kinase activity. To this end, Cdc42V12-pervanadate-activated PAK2 was treated with the tyrosine phosphatase PTP-1B before subjecting it to IVKA (Fig. 5). This treatment potently reduced PAK2 activity, decreasing its autophosphorylation by 4.6-fold and its activity towards the substrate peptide 3.9-fold, thus virtually abolishing the superactivating effect of pervanadate. By contrast, the activity of PAK2 induced by Cdc42V12 alone without pervanadate stimulation was not sensitive to PTP-1B, indicating that the inhibition by this treatment was indeed specifically due to removal of the phosphotyrosine modification.
FIG. 5.
Tyrosine phosphorylation increases the specific activity of PAK2. PAK2 and tyrosine mutants were immunoprecipitated from cells that were cotransfected with Cdc42V12 and treated or not treated with pervanadate (PV) as indicated. Half of the immunocomplexes were incubated with PTP-1B, as indicated, to remove phosphate from tyrosine residues before they were subjected to IVKA. Numbers indicate the severalfold decrease (compared to the untreated sample) in autophosphorylation or kinase activity towards the substrate peptide, as determined by phosphorimager analysis of the gels.
When the different tyrosine mutants of PAK2 activated by Cdc42V12 plus pervanadate were treated with PTP-1B, a significant reduction in the catalytic activity of Y139F and Y194F (2.9-fold and 4.3-fold, respectively) was observed, whereas the Y130F and the Y130/139/139F mutants were completely insensitive to PTP-1B treatment. Although tyrosine 194, in addition to the major phosphoacceptor site Y130, was also found to be phosphorylated (Fig. 2), modification of Y194 did not seem to be involved in positive regulation of PAK2 by Src kinases. In fact, the activity of PAK-Y194F in Cdc42V12 plus pervanadate-stimulated cells was slightly higher than that of wild-type PAK2. In conclusion, these data further confirm the critical role of Y130 in tyrosine kinase-mediated regulation of PAK2 activity and provide a direct demonstration that this regulation is achieved via an increased intrinsic catalytic activity of PAK2.
Tyrosine phosphorylation is dependent on p21-induced conformational changes in PAK2.
Activation of PAKs by Cdc42 involves disruption of the autoinhibitory interactions between the N-terminal regulatory domain and the C-terminal kinase domain (14, 21, 30). Since PAK2 tyrosine phosphorylation was dependent on prior activation by Cdc42, we considered the possibility that p21 binding-induced changes in the PAK2 structure would lead to exposure of potential sites for tyrosine phosphorylation. To test this possibility, we examined a panel of previously characterized function-modifying PAK2 mutations for their effects on tyrosine phosphorylation of PAK2.
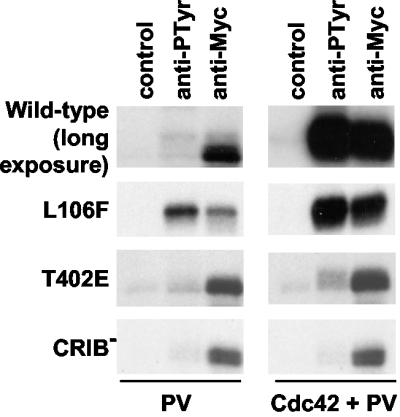
Mutations H82L and H85L, which inactivate the PBD/CRIB domain, result in a PAK2 protein (PAK2-CRIB−) that has some constitutive activity but is resistant to further activation by Cdc42V12. In agreement with our previous conclusions regarding the requirement of prior action by a p21 GTPase for PAK2 tyrosine phosphorylation, PAK2-CRIB− failed to become tyrosine phosphorylated in pervanadate-treated cells even when cotransfected with Cdc42V12 (Fig. 6). A very different picture was seen when the PAK2-L106F mutant was tested. The L106F mutation mimics the action of p21 GTPases by partially abolishing the autoinhibition exerted by the N-terminal domain and results in a PAK2 mutant which has an increased preference for an open conformation and significant constitutive activity. Similarly to that of Cdc42-stimulated wild-type PAK2, however, the fraction of total cellular PAK2-L106F present in the shifted conformation remained too small to be readily detected by Western blotting (data not shown). Unlike wild-type PAK2, PAK2-L106F became intensely phosphorylated on tyrosine residues in pervanadate-treated cells even in the absence of Cdc42V12 (Fig. 6). Identical results were obtained when another conformationally activating mutant (PAK-D125R) was used (data not shown). To confirm that the open conformation of PAK2-L106F, rather than its increased catalytic activity per se, was important for its targeting to tyrosine phosphorylation, we tested another type of constitutively active PAK2 mutant. PAK2-T402E is catalytically active because of an acidic substitution involving the T402 autophosphorylation site in its kinase domain. This mutant is also poorly responsive to activated p21 GTPases, as indicated by only a small further increase in kinase activity in Cdc42V12-transfected cells (Fig. 6 and results not shown). Despite its constitutive activity, PAK2-T402E did not become tyrosine phosphorylated upon pervanadate treatment when transfected alone, and unlike wild-type PAK2 and PAK2-L106F, it did not become significantly tyrosine phosphorylated in pervanadate-stimulated cells even when cotransfected with Cdc42V12 (Fig. 6). Of note, despite an intact PBD/CRIB motif, PAK2-T402E resembled PAK-CRIB− in that it failed to undergo the characteristic shift in mobility indicative of an altered conformation and/or autophosphorylation status when cotransfected with Cdc42V12.
FIG. 6.
PAK2 tyrosine phosphorylation is dependent on Cdc42-induced conformational changes. Different PAK2 mutants carrying the ME tag were transfected with or without cotransfection of Cdc42V12 as indicated and treated with pervanadate (PV). After primary immunoprecipitation and IVKA, samples were eluted and reimmunoprecipitated, using empty beads (control), anti-phosphotyrosine antibodies (anti-PTyr), or anti-PAK antibodies (anti-Myc). The autoradiograph showing results obtained with wild-type PAK2 was exposed six times longer then the other panels.
Thus, collectively these data suggest a model in which conformational changes associated with p21-mediated activation target PAK2 for subsequent tyrosine phosphorylation, which in turn leads to robust enhancement of the catalytic activity of PAK2.
DISCUSSION
In this study, we describe a new signaling mechanism that regulates PAK kinase activity. Activation of PAK2 by p21 GTPases was found to be significantly potentiated by overexpression of Src family tyrosine kinases or treatment of cells with pervanadate, a potent inhibitor of cellular tyrosine phosphatases. This effect of pervanadate could be abolished by the presence of the Src kinase inhibitor PP1. The enhancement of PAK2 activity by Src kinases involved tyrosine phosphorylation of PAK2 itself, which occurred in the N-terminal regulatory domain, where Y130 was identified as the major phosphoacceptor site. The positive effect of tyrosine phosphorylation on the catalytic activity of PAK2 was direct, since specific removal of these phosphates using the tyrosine phosphatase PTP-1B also abolished the increased activity of PAK2.
Perhaps the most interesting aspect of this phosphotyrosine-based regulation was its strict dependence on prior activation of PAK2 by p21 GTPases. Wild-type PAK2 could only become phosphorylated on tyrosine residues when an active Cdc42 or Rac1 was cotransfected, whereas a mutant form of PAK2 unable to interact with p21 GTPases (PAK2-CRIB−) did not undergo tyrosine phosphorylation, even when such an activator was provided. The major acceptor site for tyrosine phosphorylation in PAK2 was mapped to residue Y130. The crystal structure of PAK1 (21) shows that this residue would be hidden when PAK2 is in its autoinhibited conformation. Binding of a GTP-loaded p21 GTPase results in an open, catalytically active conformation of PAK2 in which Y130 would become exposed and available for phosphorylation by Src kinases. In direct support of such conformational regulation of PAK2 tyrosine phosphorylation, we observed that PAK2 mutants that were constitutively active (because of amino acid changes that oppose the closed conformation of PAK2 by interfering with the autoinhibitory contacts between its N- and C-terminal domains [L106F and D125R]) could be tyrosine phosphorylated independently of p21 GTPases. By contrast, this was not the case with PAK2-T402E, which is constitutively active because of a mutation in its catalytic apparatus. In addition to such activation-associated changes in PAK structure, it is of course possible that other concurrent effects of Cdc42/Rac1 binding on PAK2, such as membrane recruitment (33) and breakdown of dimers (30), also contribute to the observed p21 GTPase-dependent Src kinase action on PAK2.
While the concept of Cdc42/Rac-dependent PAK tyrosine phosphorylation leading to subsequent catalytic superactivation is novel, previous studies have implicated certain non-Src tyrosine kinases in PAK regulation. Traugh and colleagues have reported that overexpression of c-Abl can result in phosphorylation of a significant fraction of cellular PAK2 (34). However, in contrast to the effect of Src kinases reported here, phosphorylation by c-Abl was not dependent on cotransfection of an active p21 GTPase, and instead of potentiating PAK2 activity, it caused a sixfold decrease in the specific activity of PAK2. By contrast, tyrosine phosphorylation by the Etk/Bmx kinase (2) or by v-ErbB (28) has been reported to be associated with an increased activity of PAK1. Although the transforming action of v-ErbB itself appeared to be Rho dependent (6, 28), in neither of these cases did PAK tyrosine phosphorylation require activated Cdc42 or Rac, which led the authors of the latter study to suggest that tyrosine phosphorylation represents a p21 GTPase-independent mechanism of PAK activation (28). The sites of tyrosine phosphorylation of PAK by c-Abl, v-ErbB, or Etk/Bmx were not mapped in any of these studies, but it would seem logical to assume that these sites are different from that of the PAK2 Y130 (or those of the corresponding residues in PAK1 and PAK3).
The coordinated regulation of PAK2 by p21 GTPases and tyrosine phosphorylation bears intriguing resemblance to regulation of Raf-1, another important and more extensively studied upstream regulator of cellular serine/threonine kinase cascades. Raf-1 is synergistically activated by binding to the p21 GTPase protein Ras and by tyrosine phosphorylation by Src (reviewed in reference 29). In this case also, prior action by Ras is required for the stimulatory phosphorylations to occur at Ser338 and Tyr341 of Raf-1 (27). The role of Ras in this process is to translocate Raf-1 to the membrane where these modifications take place (20, 26), but notably, a cysteine-rich domain adjacent to the Ras binding domain of Raf-1 has also been implicated in autoinhibition by a physical interaction with the catalytic region of this kinase, in direct analogy with PAK regulation (9). Moreover, similar to the multistep process of PAK2 activation described here, only a small proportion of total cellular Raf-1 becomes activated by stimuli that activate Ras (13), and therefore, despite the importance of Tyr341 phosphorylation for maximal Raf-1 activity, only a small subpopulation of Raf-1 molecules becomes tyrosine phosphorylated by Src, which may explain why this modification has not been observed in all studies (see references 1 and 18, for example). Thus, although a number of differences in PAK and Raf-1 regulation are also apparent, the complex molecular mechanisms that govern the catalytic activities of these kinases may have more in common than has been previously appreciated.
In conclusion, the p21 GTPase-dependent tyrosine phosphorylation of the Y130 residue, leading to superactivation of PAK2, represents a novel point of convergence for signal transduction pathways mediated via small G proteins and Src family tyrosine kinases. This mechanism may be important in providing additional plasticity and complexity to the regulation of cellular activities of PAK and thus allowing a cell to respond appropriately to different extracellular stimuli and could also have relevance for development of novel therapeutic strategies based on protein kinase inhibitors.
Acknowledgments
Kati Takaluoma and Marko Pesu are kindly acknowledged for their help in phosphoamino acid analysis experiments. We are grateful to Marika Mäkelä and Kristina Lehtinen for their expert technical assistance.
This work was supported by grants to K.S. from the Academy of Finland (Project 53913) and the Medical Research Fund of Tampere University (Project 9B074) and by a fellowship for G.H.R. from the Academy of Finland (Project 49299).
REFERENCES
- 1.Baccarini, M., D. M. Sabatini, H. App, U. R. Rapp, and E. R. Stanley. 1990. Colony stimulating factor-1 (CSF-1) stimulates temperature dependent phosphorylation and activation of the RAF-1 proto-oncogene product. EMBO J. 9:3649-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagheri-Yarmand, R., M. Mandal, A. H. Taludker, R. A. Wang, R. K. Vadlamudi, H. J. Kung, and R. Kumar. 2001. Etk/bmx tyrosine kinase activates pak1 and regulates tumorigenicity of breast cancer cells. J. Biol. Chem. 276:29403-29409. [DOI] [PubMed] [Google Scholar]
- 3.Bagrodia, S., and R. A. Cerione. 1999. PAK to the future. Trends Cell Biol. 9:350-355. [DOI] [PubMed] [Google Scholar]
- 4.Bagrodia, S., S. J. Taylor, C. L. Creasy, J. Chernoff, and R. A. Cerione. 1995. Identification of a mouse p21Cdc42/Rac activated kinase. J. Biol. Chem. 270:22731-22737. [DOI] [PubMed] [Google Scholar]
- 5.Bagrodia, S., S. J. Taylor, K. A. Jordon, L. Van Aelst, and R. A. Cerione. 1998. A novel regulator of p21-activated kinases. J. Biol. Chem. 273:23633-23636. [DOI] [PubMed] [Google Scholar]
- 6.Boerner, J. L., A. J. Danielsen, M. J. McManus, and N. J. Maihle. 2001. Activation of Rho is required for ligand-independent oncogenic signaling by a mutant EGF receptor. J. Biol. Chem. 276:3691-3695. [DOI] [PubMed] [Google Scholar]
- 7.Bokoch, G. M., A. M. Reilly, R. H. Daniels, C. C. King, A. Olivera, S. Spiegel, and U. G. Knaus. 1998. A GTPase-independent mechanism of p21-activated kinase activation. Regulation by sphingosine and other biologically active lipids. J. Biol. Chem. 273:8137-8144. [DOI] [PubMed] [Google Scholar]
- 8.Chong, C., L. Tan, L. Lim, and E. Manser. 2001. The mechanism of PAK activation. Auto-phosphorylation events in both regulatory and kinase domains control activity. J. Biol. Chem. 276:17347-17353. [DOI] [PubMed] [Google Scholar]
- 9.Cutler, R. E., Jr., R. M. Stephens, M. R. Saracino, and D. K. Morrison. 1998. Autoregulation of the Raf-1 serine/threonine kinase. Proc. Natl. Acad. Sci. USA 95:9214-9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dan, I., N. M. Watanabe, and A. Kusumi. 2001. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11:220-230. [DOI] [PubMed] [Google Scholar]
- 11.Daniels, R. H., and G. M. Bokoch. 1999. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem. Sci. 24:350-355. [DOI] [PubMed] [Google Scholar]
- 12.Gatti, A., Z. Huang, P. T. Tuazon, and J. A. Traugh. 1999. Multisite autophosphorylation of p21-activated protein kinase gamma-PAK as a function of activation. J. Biol. Chem. 274:8022-8028. [DOI] [PubMed] [Google Scholar]
- 13.Hallberg, B., S. I. Rayter, and J. Downward. 1994. Interaction of Ras and Raf in intact mammalian cells upon extracellular stimulation. J. Biol. Chem. 269:3913-3916. [PubMed] [Google Scholar]
- 14.Hoffman, G. R., and R. A. Cerione. 2000. Flipping the switch: the structural basis for signaling through the CRIB motif. Cell 102:403-406. [DOI] [PubMed] [Google Scholar]
- 15.Jaffer, Z. M., and J. Chernoff. 2002. p21-activated kinases: three more join the Pak. Int. J. Biochem. Cell Biol. 34:713-717. [DOI] [PubMed] [Google Scholar]
- 16.Kamps, M. P., and B. M. Sefton. 1989. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractioned proteins. Anal. Biochem. 176:22-27. [DOI] [PubMed] [Google Scholar]
- 17.King, C. C., E. M. Gardiner, F. T. Zenke, B. P. Bohl, A. C. Newton, B. A. Hemmings, and G. M. Bokoch. 2000. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1). J. Biol. Chem. 275:41201-41209. [DOI] [PubMed] [Google Scholar]
- 18.Kovacina, K. S., K. Yonezawa, D. L. Brautigan, N. K. Tonks, U. R. Rapp, and R. A. Roth. 1990. Insulin activates the kinase activity of the Raf-1 proto-oncogene by increasing its serine phosphorylation. J. Biol. Chem. 265:12115-12118. [PubMed] [Google Scholar]
- 19.Lee, N., H. MacDonald, C. Reinhard, R. Halenbeck, A. Roulston, T. Shi, and L. T. Williams. 1997. Activation of hPAK65 by caspase cleavage induces some of the morphological and biochemical changes of apoptosis. Proc. Natl. Acad. Sci. USA 94:13642-13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leevers, S. J., H. F. Paterson, and C. J. Marshall. 1994. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature 369:411-414. [DOI] [PubMed] [Google Scholar]
- 21.Lei, M., W. Lu, W. Meng, M. C. Parrini, M. J. Eck, B. J. Mayer, and S. C. Harrison. 2000. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell 102:387-397. [DOI] [PubMed] [Google Scholar]
- 22.Lu, W., S. Katz, R. Gupta, and B. J. Mayer. 1997. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr. Biol. 7:85-94. [DOI] [PubMed] [Google Scholar]
- 23.Manser, E., H.-Y. Huang, T.-H. Loo, X.-Q. Chen, J.-M. Dong, T. Leung, and L. Lim. 1997. Expression of constitutively active α-PAK reveals effects of the kinase on actin and focal complexes. Mol. Cell. Biol. 17:1129-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manser, E., T. Leung, H. Salihuddin, Z. S. Zhao, and L. Lim. 1994. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367:40-46. [DOI] [PubMed] [Google Scholar]
- 25.Manser, E., T. H. Loo, C. G. Koh, Z. S. Zhao, X. Q. Chen, L. Tan, I. Tan, T. Leung, and L. Lim. 1998. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell 1:183-192. [DOI] [PubMed] [Google Scholar]
- 26.Marais, R., Y. Light, H. F. Paterson, and C. J. Marshall. 1995. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 14:3136-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mason, C. S., C. J. Springer, R. G. Cooper, G. Superti-Furga, C. J. Marshall, and R. Marais. 1999. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 18:2137-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McManus, M. J., J. L. Boerner, A. J. Danielsen, Z. Wang, F. Matsumura, and N. J. Maihle. 2000. An oncogenic epidermal growth factor receptor signals via a p21-activated kinase-caldesmon-myosin phosphotyrosine complex. J. Biol. Chem. 275:35328-35334. [DOI] [PubMed] [Google Scholar]
- 29.Morrison, D. K., and R. E. Cutler. 1997. The complexity of Raf-1 regulation. Curr. Opin. Cell Biol. 9:174-179. [DOI] [PubMed] [Google Scholar]
- 30.Parrini, M. C., M. Lei, S. C. Harrison, and B. J. Mayer. 2002. PAK1 kinase homodimers are autoinhibited in trans and dissociated upon activation by Cdc42 and Rac1. Mol. Cell 9:73-83. [DOI] [PubMed] [Google Scholar]
- 31.Renkema, G. H., A. Manninen, D. A. Mann, M. Harris, and K. Saksela. 1999. Identification of the Nef-associated kinase as p21-activated kinase 2. Curr. Biol. 9:1407-1410. [DOI] [PubMed] [Google Scholar]
- 32.Renkema, G. H., A. Manninen, and K. Saksela. 2001. Human immunodeficiency virus type-1 Nef selectively associates with a catalytically active subpopulation of p21-activated kinase 2 (PAK2) independently of PAK2 binding to Nck or β-PIX. J. Virol. 75:2154-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roig, J., Z. Huang, C. Lytle, and J. A. Traugh. 2000. p21-activated protein kinase gamma-PAK is translocated and activated in response to hyperosmolarity. J. Biol. Chem. 275:16933-16940. [DOI] [PubMed] [Google Scholar]
- 34.Roig, J., P. T. Tuazon, P. A. Zipfel, A. M. Pendergast, and J. A. Traugh. 2000. Functional interaction between c-Abl and the p21-activated protein kinase gamma-PAK. Proc. Natl. Acad. Sci. USA 97:14346-14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudel, T., and G. M. Bokoch. 1997. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science 276:1571-1574. [DOI] [PubMed] [Google Scholar]
- 36.Tuazon, P. T., W. C. Spanos, E. L. Gump, C. A. Monnig, and J. A. Traugh. 1997. Determinants for substrate phosphorylation by p21-activated protein kinase (gamma-PAK). Biochemistry 36:16059-16064. [DOI] [PubMed] [Google Scholar]
- 37.Zenke, F. T., C. C. King, B. P. Bohl, and G. M. Bokoch. 1999. Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J. Biol. Chem. 274:32565-32573. [DOI] [PubMed] [Google Scholar]
- 38.Zhao, Z.-S., E. Manser, and L. Lim. 2000. Interaction between PAK and Nck: a template for nck targets and role of PAK autophosphorylation. Mol. Cell. Biol. 20:3906-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]