Abstract
ATRIAL FIBRILLATION (AF) IS A COMMON CONTRIBUTOR to cardiovascular morbidity and mortality. Two generally acceptable strategies exist for long-term AF management, with ongoing studies comparing the overall mortality associated with each. One strategy aims to maintain sinus rhythm, with antiarrhythmic agents if necessary, thereby preserving physiological cardiac electrical function but exposing the patient to the potential side effects of potent drugs. The second approach is to control the ventricular rate and prevent thromboembolic complications with anticoagulants, leaving the patient with AF. Both beta-blocking agents and calcium antagonists are more effective than digoxin in achieving rate control. Several nonpharmacological therapies including catheter ablation, implantable devices and surgical interventions show promise for rate control and maintenance of sinus rhythm. This paper provides an overview of new developments in pharmacological and nonpharmacological therapy. Key features of recently published clinical guidelines, including a unified classification scheme for AF and issues relating to rate control and maintenance of sinus rhythm, are considered. In addition, preliminary results from the recently presented AFFIRM study, the largest AF trial to date, are summarized. Finally, we discuss recent insights into the basic mechanisms underlying AF that have potentially significant clinical implications.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia found in clinical practice; it is characterized by rapid ineffective atrial activity with irregularly irregular ventricular contractions. The resulting hemodynamic alterations may cause a variety of clinical manifestations. Potential complications include stroke, congestive heart failure (CHF) and tachycardia-induced cardiomyopathy. In contrast to most other arrhythmias, for which effective nonpharmacological therapies are presently available, AF management remains problematic and controversial. Over the past several years, significant progress has been made in understanding the underlying pathophysiology and treatment options for this complex arrhythmia. This article focuses on new insights into AF mechanisms, reviews recent advances in pharmacological and nonpharmacological therapy, and examines the salient results from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial and recently published clinical guidelines.
Epidemiology and risk factors
Much of our knowledge regarding the incidence of AF is derived from the Framingham Heart Study.1 Although AF affects fewer than 1% of individuals in their fifties, up to 11% of 80 year olds suffer from this arrhythmia, with a total incidence of 2.2 million cases per year in the United States alone.2 AF often accompanies left atrial enlargement and mitral valve calcification in patients over 60 years of age and commonly complicates cardiac surgery and acute myocardial infarction.3,4,5 Multivariate analysis has identified increasing age, heart failure, smoking, diabetes, hypertension, male sex, left ventricular hypertrophy, myocardial infarction and valvular heart disease as risk factors.1
AF may cause systemic thromboembolic complications, decreased exercise capacity, impaired ventricular function, reduced quality of life and significant health care costs.6,7 Over a 2-year period, patients with AF require an average of 14 office visits, 12 outpatient visits, 2 admissions to hospital and one emergency department visit.6 In 1999, Catherwood and colleagues7 reported average AF-related costs of US$9300–18 900 per quality-adjusted life-year. After adjusting for underlying cardiac conditions, AF is associated with a 1.5-fold to 1.9-fold increase in risk of mortality in both men and women across a wide spectrum of ages.8
Mechanisms of AF
The traditional view of AF mechanisms is that the arrhythmia results from multiple re-entrant wavelets that move randomly throughout the atria.9 Re-entry is promoted by decreased atrial refractory periods, slowed conduction and an increased mass of cardiac tissue.10,11 Recently, it has been shown that atrial tachyarrhythmias, including AF, alter atrial electrical properties thus promoting multiple-circuit re-entrant AF.12,13 This “electrical remodelling” encompasses a variety of changes including alterations in sarcolemmal ion channel gene expression, cellular size and content, in addition to changes in connexins that couple cells electrically.14 The most important ionic changes involve, but are not limited to, reductions in L-type calcium current.14,15,16,17,18 The net effect of these modifications is to decrease the atrial refractory period and possibly interfere with atrial conduction in a spatially heterogeneous way (i.e., the magnitude of the changes varies in different locations, increasing electrical heterogeneity and promoting fibrillation),19,20 thereby providing a substrate for multicircuit re-entry and facilitating reinitiation of AF, should it end.21 Moreover, sustained AF causes important reductions in cellular contractility, resulting in a tachycardia-induced atrial cardiomyopathy that may be responsible for delayed thromboembolic events as contractility recovers after cardioversion.22
Although these observations provide a potential mechanistic basis for the self-perpetuating nature of AF, they do not elucidate AF initiation. Experimental CHF promotes AF by causing interstitial fibrosis that interferes with atrial conduction and promotes wavelet re-entry,23 as well as by causing ionic transport alterations that promote atrial ectopic impulse formation.24 Renin-angiotensin activation appears to play an important role in CHF-related atrial arrhythmogenic remodelling, which can be attenuated by treatment with an angiotensin-converting-enzyme (ACE) inhibitor.25 Recent experimental data have broadened our thinking about AF from a monolithic notion of multiple-circuit re-entry by adding an appreciation of the potentially important roles of ectopic activity and single-circuit generators. Relations between present and potential AF therapies and the underlying pathophysiology are depicted in Fig. 1.14,21
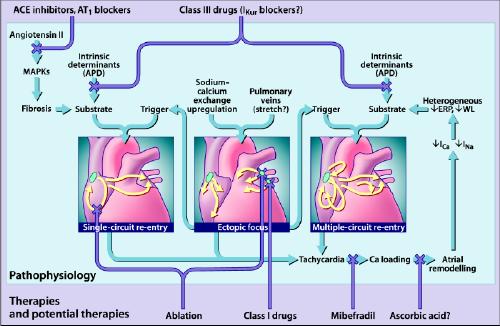
Fig. 1: The primary mechanisms believed to underlie atrial fibrillation (AF) (for detailed discussions, see references 14 and 81). AF may be maintained by rapidly discharging atrial ectopic foci, by a single, rapidly firing atrial re-entry circuit or by multiple functional re-entry circuits. Re-entry mechanisms require both a favourable substrate that can support re-entry and a trigger mechanism to initiate re-entry. The triggers are generally provided by atrial ectopic activity, often originating in the pulmonary veins. The substrate can be produced by angiotensin activation of mitogen-activated protein kinases (MAPKs) that cause tissue fibrosis, or by atrial tachycardia itself, which causes Ca2+ overloading followed by altered atrial electrical properties (“atrial remodelling”), including spatially heterogeneous reductions in effective refractory period (ERP) that promote multiple-circuit re-entry. Ectopic foci can promote single-circuit re-entry by providing triggers for re-entry induction and can promote multiple-circuit re-entry both by causing tachycardias that cause atrial remodelling and by providing triggers for re-entry induction. Single-circuit re-entry can lead to multiple-circuit re-entry by causing atrial remodelling. Both single-circuit re-entry and multiple-circuit re-entry can promote ectopic activity, perhaps by causing Ca2+-loading that favours the occurrence of triggered activity. Thus, the 3 mechanisms shown are not independent but can each play a role in the occurrence of the other. The Xs show potential sites of therapeutic intervention, which can be individualized to the pathophysiology in each patient. Targeted tissue destruction (ablation) can be used to destroy key points in a re-entry circuit or ectopic foci. Angiotensin antagonists (angiotensin-converting-enzyme [ACE] inhibitors or angiotensin-1 receptor blockers [AT1]) may be able to prevent arrhythmogenic fibrosis. The Ca2+ antagonist mibefradil, or possibly even ascorbic acid, may be effective in preventing atrial remodelling. Class III drugs, possibly even atrial-selective agents that target atrial-specific channels like the ultra-rapid potassium current (IKur), may be used to prolong action potential duration (APD) and prevent re-entry. WL = wavelength. Photo: Lianne Friesen and Nicholas Woolridge
Clinical manifestations
The clinical presentation of AF is highly variable, ranging from the complete absence of symptoms to heart failure and hemodynamic collapse. Symptoms result from the irregular and often rapid ventricular response, as well as from ensuing autonomic reflex changes and loss of atrial systole. In the Canadian Registry of Atrial Fibrillation (CARAF), only 21% of patients were asymptomatic on presentation.26 Among the 79% of patients with symptoms, palpitations occurred in 50%, chest pain and fatigue in more than 25% and dizziness, presyncope or syncope in about 25%. In the Canadian Trial of Atrial Fibrillation (CTAF), women had a significantly more impaired quality of life than men.27 The most feared complication of AF is stroke, which is often caused by thromboembolism from clotting in the relatively static blood pool of the fibrillating atrium, particularly in the left atrial appendage. AF increases the risk of stroke about 5 times28 and is the single factor most commonly associated with stroke in those over 75 years of age.29 Risk factors for stroke in patients with AF include advanced age, diabetes, hypertension, previous cerebrovascular accident and left ventricular dysfunction.30
Clinical evaluation
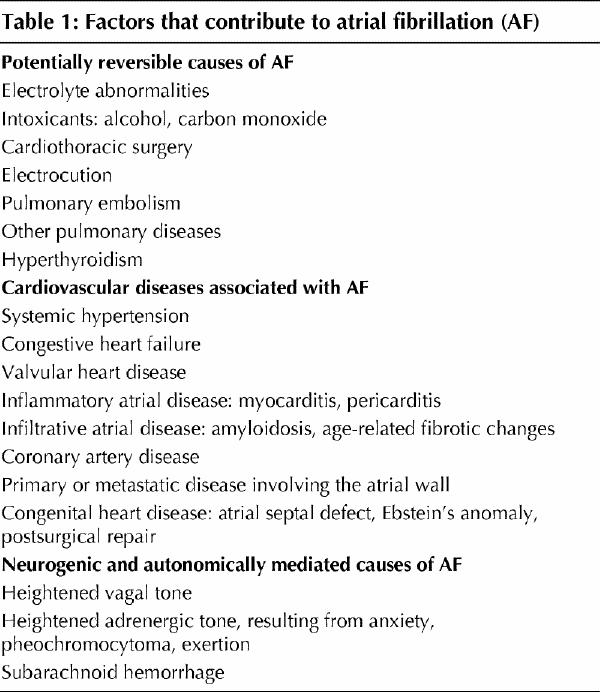
The initial evaluation of a patient with AF begins with a thorough history focused on identifying precipitants (Table 1), defining associated cardiac and extracardiac factors, and characterizing the pattern of arrhythmia (e.g., symptoms, duration, paroxysmal v. persistent, first episode v. recurrent). Physical examination typically reveals an irregularly irregular pulse, irregular jugular venous pulsations with absent A waves and variations in the intensity of the first heart sound.31 Associated valvular disease, primary or secondary (i.e., tachycardia-induced) cardiomyopathies, or heart failure may also be identified. The definitive diagnosis of AF requires at least one ECG lead documenting the arrhythmia from a rhythm strip, standard 12-lead ECG, Holter monitoring, or transtelephonic or telemetric recording. If episodes are infrequent, an external or internally implanted event recorder32 may allow the patient to transmit the stored ECG to a recording facility when symptoms occur.
Table 1
Acute management
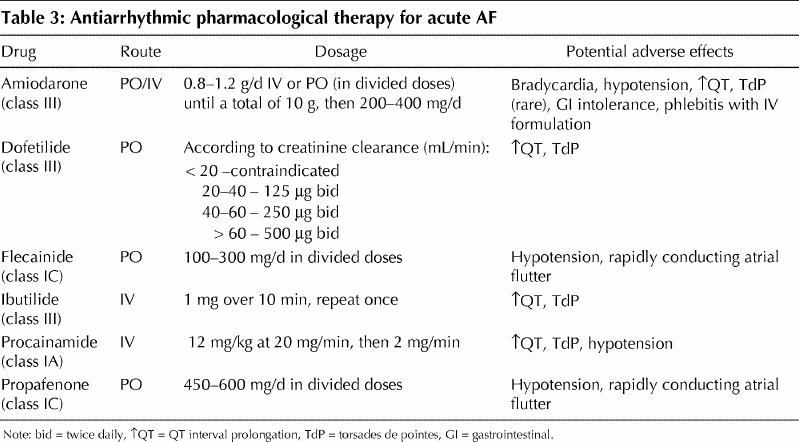
The initial aggressiveness with which AF is treated should be determined by the severity of the symptoms manifested. Hypotension with signs of hypoperfusion, severe heart failure or intractable ischemic chest pain are indications for urgent synchronized direct-current cardioversion. An initial energy of 200 J or greater is recommended, preferably with a biphasic waveform. In the absence of hemodynamic instability, patients with AF lasting longer than 48 hours or of unknown duration should be anticoagulated with a therapeutic international normalized ratio (INR) of 2–3 for at least 3–4 weeks before and after cardioversion. Screening for left atrial thrombus by transesophageal echocardiography is an acceptable alternative to routine pre-anticoagulation. In most cases, however, initial treatment focuses on controlling the rapid ventricular response. Although intravenous digoxin was the traditional drug of choice for rate control, beta-blocking agents and calcium antagonists are more effective (Table 2).33 Digoxin remains a drug of choice in patients with left ventricular dysfunction and a potentially useful adjunct therapy to beta-blockers or calcium antagonists.34 Recommended doses and potential adverse effects of antiarrhythmic agents with proven efficacy in the pharmacological conversion of AF are summarized in Table 3.
Table 2
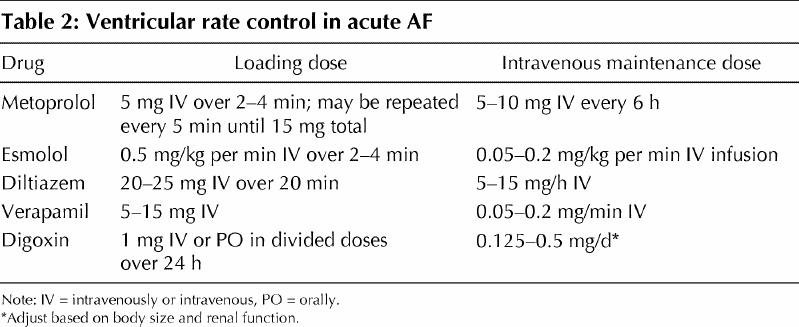
Table 3
Antiarrhythmic maintenance therapy
Maintenance of sinus rhythm often produces a better symptomatic result than a rate control strategy; however, this often requires the long-term use of antiarrhythmic drugs that have an unknown effect on overall mortality. The relative mortality risk of sinus rhythm maintenance versus rate control strategies for AF is presently under active investigation.35 Patients who do not receive antiarrhythmic agents have a 1-year AF recurrence rate of about 75%.36 With antiarrhythmic drugs, sinus rhythm may be maintained in 50%–65% of cases.36,37,38,39 The choice of antiarrhythmic agent should be guided by the presence or absence of structural heart disease, tolerability, ease of administration and side effect profile. Class IA, IC and III agents have proven efficacy in maintaining sinus rhythm. The major limitation hindering the use of antiarrhythmic drugs is the risk of arrhythmia promotion, or “proarrhythmia,” which may be lethal. Whereas the noncardiac side effects of amiodarone (e.g., pulmonary fibrosis, thyroid dysfunction, hepatitis and neurotoxicity) are well-known, cardiovascular toxicity, including ventricular proarrhythmia, is uncommon,40,41 and large-scale studies42,43 in patients post myocardial infarction with left ventricular dysfunction have confirmed its safety. The Canadian Trial of Atrial Fibrillation (CTAF)38 compared low-dose amiodarone (200 mg/day) to conventional antiarrhythmic therapy with either sotalol or propafenone and provided compelling evidence of amiodarone's superiority in maintaining sinus rhythm (Fig. 2). After a mean follow-up of 16 months, recurrence rates were significantly lower with amiodarone (35% v. 63%, p < 0.001). There was a trend toward a higher rate of discontinuation of therapy in patients assigned to amiodarone. Sotalol and propafenone demonstrated comparable efficacy and tolerability.
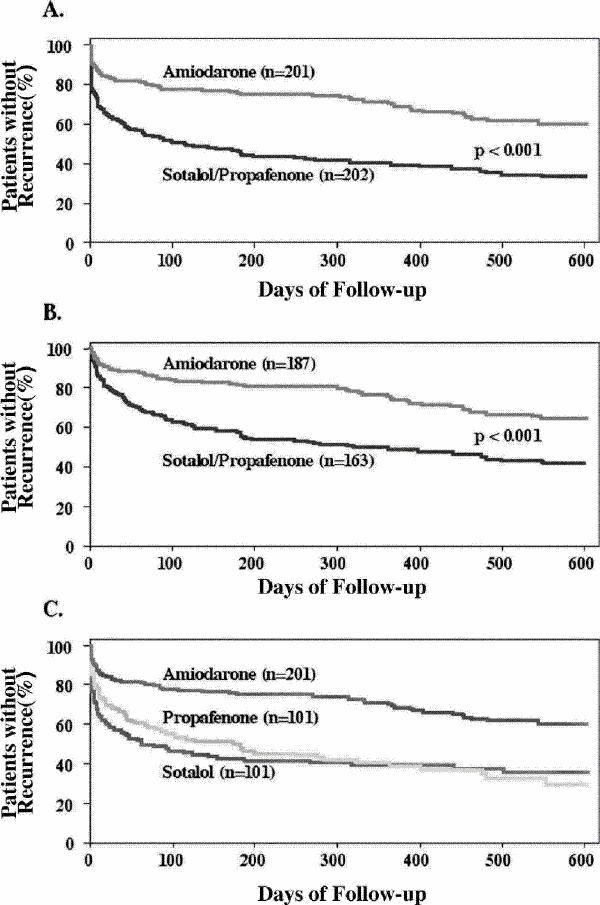
Fig. 2: Panel A shows estimates of the proportion of patients with no recurrence of atrial fibrillation in the group taking amiodarone and in the group assigned to sotalol or propafenone (hazard ratio for recurrence among patients in the amiodarone group 0.43 [95% confidence interval 0.32–0.57]); panel B shows the estimates for the 350 patients (187 in the amiodarone group and 163 in the group assigned to sotalol or propafenone) who were in sinus rhythm 21 days after randomization (hazard ratio 0.45, 95% confidence interval 0.32–0.63); and panel C shows the estimates for the patients who received amiodarone, those who received sotalol and those who received propafenone. Follow-up began 21 days after randomization (designated day 0). Reprinted with permission from the Massachusetts Medical Society (N Engl J Med 2000;342:913-20).38
Despite the many antiarrhythmic agents currently available, AF remains a challenge to medical therapy. With no new class I agents on the horizon, their use is likely to continue declining. However, class IC agents such as propafenone and flecainide are entrenched in clinical practice and are useful for restoring and maintaining sinus rhythm in patients with structurally normal hearts. The future focus is likely to remain on compounds that act predominantly by prolonging repolarization. Dofetilide is a class III agent that acts by blocking the delayed rectifier current (IKr) and prolonging action potential duration. Azimilide blocks both IKr and IKs (i.e., both rapidly and slowly activating components of the delayed rectifier potassium current) and differs from dofetilide in that it does not exhibit the phenomenon of reverse rate dependence (i.e., greater drug-induced prolongation of the action potential at slower heart rates).44,45 Multifaceted molecules resembling sotalol (e.g., ersentilide) and amiodarone (e.g., dronedarone) are currently under investigation. Dronedarone is a derivative of amiodarone without iodine that may have fewer thyroid-interacting properties than its parent compound.46
Anticoagulation
Several large-scale randomized trials have demonstrated the benefits of anticoagulation in preventing strokes among patients with paroxysmal and permanent AF.47,48,49,50 The choice of antithrombotic therapy should be tailored to the individual's risk factors (Table 4). In high-risk patients, adjusted-dose warfarin is about twice as effective as ASA and reduces the absolute rate of primary events by 1.2% per year.50 The INR must be closely monitored, because the risk of thromboembolic stroke increases with values below 2.0, whereas major hemorrhages increase with INR values that are greater than 3.0.51 In patients with nonvalvular AF and one or more risk factors for stroke, warfarin is associated with improved quality- adjusted survival and cost savings.52 It must be emphasized that anticoagulation therapy tends to be underused for patients with AF, exposing individuals unnecessarily to an increased risk of stroke.53,54
Table 4

Nonpharmacological interventions
Nonpharmacological therapies have revolutionized the treatment of atrioventricular (AV) re-entrant arrhythmias and atrial flutter.55 Limitations of pharmacological therapy for AF, including drug intolerance, proarrhythmic potential and incomplete efficacy, have motivated the development of a variety of nonpharmacological approaches. Surgery for AF was introduced in 1985 with the “corridor” procedure,56 whereby atrial free walls were surgically isolated from the septum through a series of sutures. In the remaining corridor, a conduction pathway between the sinus and AV nodes was maintained. However, 5 years after surgery, new atrial arrhythmias consisting of atrial flutter and atrial tachycardia appeared in 27% of patients and sinus node dysfunction necessitated pacing therapy in 13%.57 Subsequently, Cox and colleagues58 developed the “Maze” procedure, creating lines of conduction block to isolate portions of the atria into areas too small to sustain re-entry. The simplified Maze-III procedure involves excision of the left and right atrial appendages, isolation of pulmonary veins and several additional incisions to prevent atrial re-entry. It is now the surgical procedure of choice for medically refractory AF, but it requires an open thoracotomy, has a 2%–3% perioperative mortality rate and is complicated by significant sinus node dysfunction. Consideration for a concomitant Maze procedure should be given to patients with symptomatic AF who require cardiac surgery for ischemic, valvular or congenital heart disease.
Several catheter-based interventions for AF have emerged. In patients with intractable AF, ablation of the AV junction with permanent pacemaker implantation is an established, effective option that ensures rate control, alleviates symptoms and improves quality of life.59 This therapy may be particularly useful when a rapid ventricular rate results in tachycardia-induced cardiomyopathy despite appropriate medications.31,60,61 AV junction ablation and permanent pacing do not adversely affect long-term survival.62 In patients who strongly object to pacemaker implantation but suffer from uncontrollable symptoms, AV node modification may be considered. AV node modification is based on the principle that ventricular response rate in AF is determined primarily by posterior inputs to the AV node with inherently shorter refractory periods.63 Modifying the AV node by eliminating the posterior input may control the ventricular rate at rest and during exercise without the need for permanent pacing.64 The procedure, however, has a 16% incidence of AV block, 10% recurrence of rapid AF and a less marked improvement in quality of life compared with complete AV nodal ablation.65 Thus, AV nodal modification without pacemaker implantation is only rarely used in patients with rapid ventricular rates during AF.
In a landmark study by Häissaguerre and colleagues,66 94% of ectopic triggers for AF were found to arise from sleeves of myocardial tissue around the pulmonary veins. Ablation of these foci prevented AF recurrence in 62% of patients. This curative approach has a number of limitations. The myocardial architecture of pulmonary veins is complex and highly variable.67 Recurrence rates exceed 65% in some studies, with an 8.3% incidence of pulmonary vein stenosis.68 Success rates vary widely across studies and are highly dependent on the definition employed. The technique is in rapid evolution. Rather than identifying and ablating individual foci within pulmonary veins, the os of one or more pulmonary veins may be isolated electrically, thereby preventing the trigger from reaching the left atrium.69 This approach may be associated with less recurrence and a lower risk of pulmonary vein stenosis.
Another area of active investigation is the application of device therapy to maintain sinus rhythm. Atrial pacing may prevent AF recurrence in patients with sick-sinus syndrome, perhaps because bradycardia increases the heterogeneity of refractory periods and promotes re-entry.70,71 Implantable atrial defibrillators that detect AF and deliver low-energy synchronized shocks successfully convert most AF episodes with a single shock.72 As AF rarely produces loss of consciousness, shock-induced discomfort is a prime concern. New waveforms, lead configurations and preventive algorithms may expand the indications for these devices. Finally, biatrial pacing, which synchronizes atrial activity and may thereby make re-entry less likely, may be a useful approach,73 although its efficacy is still a matter of controversy.
Clinical guidelines
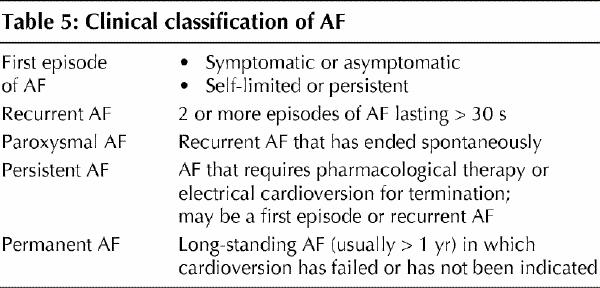
The American College of Cardiology, the American Heart Association and the European Society of Cardiology recently established joint guidelines for AF management.31 Although it is beyond the scope of this article to review all the recommendations, some key features are considered here. When an episode of AF is first detected, it should be described according to whether it is symptomatic or not, or self-limited or not. AF is considered to be recurrent if 2 or more episodes have been documented (Table 5). If AF terminates spontaneously, it is considered to be “paroxysmal.” Non-self-terminating AF is considered to be “persistent,” regardless of whether cardioversion is performed pharmacologically or electrically. If cardioversion is not indicated or attempted and the patient remains in AF, the arrhythmia is designated “permanent.”
Table 5
In most patients with persistent or permanent AF, the heart rate may be controlled with either beta-blockers or calcium channel antagonists and should be measured both at rest and during exercise. The rate of AF is considered controlled when the ventricular response is between 60 and 80 beats per minute (bpm) at rest and between 90 and 115 bpm during moderate exercise.74,75 Selection of pharmacological agents to maintain sinus rhythm should be based predominantly on safety. In patients undergoing cardioversion, anticoagulation should be administered for AF that has been present for more than 48 hours regardless of the method used to restore sinus rhythm.
Immediate electrical cardioversion is indicated in clinically unstable patients with a rapid ventricular response. In hemodynamically stable patients with ventricular pre-excitation (Wolff-Parkinson-White [WPW] syndrome), procainamide or ibutilide may be used to restore sinus rhythm. Digoxin, diltiazem and verapamil are generally contraindicated in WPW patients with AF because of the risk of accelerating the ventricular response.
AFFIRM trial
Preliminary results from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial were reported in March 2002 at the American College of Cardiology Annual Scientific Session.76 In this trial, 4060 patients with AF were randomly allocated to rhythm control or rate control strategies. All patients had to have had more than 6 hours of AF and be either over 65 years of age or under 65 years with at least one risk factor for stroke. After an average follow-up of 3.5 years, no difference in total mortality was observed, although a trend toward increased mortality late in follow-up with rhythm control was noted. Most strokes occurred in patients who either stopped warfarin or had subtherapeutic INRs. A greater number of admissions to hospital, torsades de pointes episodes and bradycardic cardiac arrests occurred in the rhythm control arm. No differences in quality of life and functional capacity were noted. Overall, AFFIRM does not provide any evidence for the superiority of rhythm control and points out some potentially important limitations. Definitive conclusions will require careful analysis of the complete published results.
Potential future developments
Recent insights into the basic mechanisms of AF may allow for improved therapeutic approaches in the future. The importance of electrical remodelling indicates the value of early AF cardioversion and treatment of associated atrial tachyarrhythmias in preventing permanent AF. An improved understanding of the processes leading to the development of the AF substrate may lead to better strategies for AF prevention. For example, enalapril attenuates experimental CHF-induced arrhythmogenic atrial structural remodelling,25 and ACE inhibitor therapy prevents AF in patients post myocardial infarction with left ventricular dysfunction.77 Recent work shows that oxidative stress may play a role in atrial remodelling, and there is preliminary evidence that oral vitamin C (ascorbic acid) may prevent electrical remodelling and postoperative AF.78 The L- and T-type calcium channel blocker mibefradil strongly suppresses atrial tachycardia remodelling, indicating the feasibility of pharmacological prevention.79 The discovery of an atrial-specific repolarizing current (the ultrarapid delayed rectifier, IKur) in humans has opened up interesting possibilities for developing class III drugs effective in AF without the risks of ventricular proarrhythmia.80 New methods for safely and effectively ablating atrial arrhythmogenic foci to prevent AF are being developed actively, as are improved device-based approaches. The future will likely bring a more multifaceted and mechanistically based approach to AF therapy that is tailored to the atrial arrhythmogenic factors present in each patient.81
Acknowledgments
The authors' research was supported by the Canadian Institutes of Health Research and the Quebec Heart and Stroke Foundation.
Footnotes
This article has been peer reviewed.
Contributors: Paul Khairy wrote the first and final drafts of the paper. Stanley Nattel helped to prepare the first draft by contributing ideas and suggestions. He reworked the first and final drafts into the present version.
Competing interests: None declared.
Correspondence to: Dr. Stanley Nattel, Research Center, Montreal Heart Institute, 5000 Bélanger St. E, Montreal QC H1T 1C8; fax 514 376-5241; nattel@icm.umontreal.ca
References
- 1.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840-4. [PubMed]
- 2.Ryder KM, Benjamin EJ. Epidemiology and significance of atrial fibrillation. Am J Cardiol 1999;84:131R-8R. [DOI] [PubMed]
- 3.Kerr CR. Atrial fibrillation: extending basic science to clinical frontiers. Can J Cardiol 1997;13:1059-61. [PubMed]
- 4.Goldberg RJ, Yarzebski J, Lessard D, Wu J, Gore JM. Recent trends in the incidence rates of and death rates from atrial fibrillation complicating initial acute myocardial infarction: a community-wide perspective. Am Heart J 2002; 143: 519-27. [DOI] [PubMed]
- 5.Sra J, Dhala A, Blanck Z, Deshpande S, Cooley R, Akhtar M. Atrial fibrillation: epidemiology, mechanisms, and management. Curr Probl Cardiol 2000;25:405-524. [PubMed]
- 6.Maglio C, Ayers G, Tidball EW, Akhtar M. Health care utilization and cost of care in patients with symptomatic atrial fibrillation. Circulation 1996;94 (Suppl):1-169.8964107
- 7.Catherwood E, Fitzpatrick WD, Greenberg ML, Holzberger PT, Malenka DJ, Gerling BR, et al. Cost-effectiveness of cardioversion and antiarrhythmic therapy in nonvalvular atrial fibrillation. Ann Intern Med 1999;130:625-36. [DOI] [PubMed]
- 8.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946-52. [DOI] [PubMed]
- 9.Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J 1959;58:59-70. [DOI] [PubMed]
- 10.Allessie MA, Boyden PA, Camm AJ, Kleber AG, Lab MJ, Legato MJ, et al. Pathophysiology and prevention of atrial fibrillation. Circulation 2001; 103:769-77. [DOI] [PubMed]
- 11.Allessie MA, Konings K, Kirchhof CJ, Wijffels M. Electrophysiologic mechanisms of perpetuation of atrial fibrillation. Am J Cardiol 1996;77:10A-23A. [DOI] [PubMed]
- 12.Wijffels MC, Kirchhof CJ, Dorland R, Power J, Allessie MA. Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats: roles of neurohumoral changes, ischemia, atrial stretch, and high rate of electrical activation. Circulation 1997;96:3710-20. [DOI] [PubMed]
- 13.Allessie MA. Atrial fibrillation-induced electrical remodeling in humans: What is the next step? Cardiovasc Res 1999;44:10-2. [DOI] [PubMed]
- 14.Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002; 415 (6868): 219-26. [DOI] [PubMed]
- 15.Nattel S, Li D. Ionic remodeling in the heart: pathophysiological significance and new therapeutic opportunities for atrial fibrillation. Circ Res 2000;87:440-7. [DOI] [PubMed]
- 16.Courtemanche M, Ramirez RJ, Nattel S. Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: insights from a mathematical model. Cardiovasc Res 1999;42:477-89. [DOI] [PubMed]
- 17.Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res 1997;81:512-25. [DOI] [PubMed]
- 18.van der Velden HM, Ausma J, Rook MB, Hellemons AJ, van Veen TA, Allessie MA, et al. Gap junctional remodeling in relation to stabilization of atrial fibrillation in the goat. Cardiovasc Res 2000;46:476-86. [DOI] [PubMed]
- 19.Gaspo R, Bosch RF, Talajic M, Nattel S. Functional mechanisms underlying tachycardia-induced sustained atrial fibrillation in a chronic dog model. Circulation 1997;96:4027-35. [DOI] [PubMed]
- 20.Fareh S, Villemaire C, Nattel S. Importance of refractoriness heterogeneity in the enhanced vulnerability to atrial fibrillation induction caused by tachycardia-induced atrial electrical remodeling. Circulation 1998;98:2202-9. [DOI] [PubMed]
- 21.Nattel S, Li D, Yue L. Basic mechanisms of atrial fibrillation — very new insights into very old ideas. Annu Rev Physiol 2000;62:51-77. [DOI] [PubMed]
- 22.Sun H, Gaspo R, Leblanc N, Nattel S. Cellular mechanisms of atrial contractile dysfunction caused by sustained atrial tachycardia. Circulation 1998; 98:719-27. [DOI] [PubMed]
- 23.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation 1999;100:87-95. [DOI] [PubMed]
- 24.Li D, Melnyk P, Feng J, Wang Z, Petrecca K, Shrier A, et al. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation 2000;101:2631-8. [DOI] [PubMed]
- 25.Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, et al. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation 2001;104:2608-14. [DOI] [PubMed]
- 26.Kerr CR, Boone J, Connolly SJ, Dorian P, Green M, Klein G, et al. The Canadian Registry of Atrial Fibrillation: a noninterventional follow-up of patients after the first diagnosis of atrial fibrillation. Am J Cardiol 1998;82:82N-5. [DOI] [PubMed]
- 27.Paquette M, Roy D, Talajic M, Newman D, Couturier A, Yang C, et al. Role of gender and personality on quality-of-life impairment in intermittent atrial fibrillation. Am J Cardiol 2000;86:764-8. [DOI] [PubMed]
- 28.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8. [DOI] [PubMed]
- 29.Hart RG, Halperin JL. Atrial fibrillation and stroke: concepts and controversies. Stroke 2001;32:803-8. [DOI] [PubMed]
- 30.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154:1449-57. [PubMed]
- 31.Fuster V, Ryden LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol 2001;38:1231-66. [DOI] [PubMed]
- 32.Fogel RI, Evans JJ, Prystowsky EN. Utility and cost of event recorders in the diagnosis of palpitations, presyncope, and syncope. Am J Cardiol 1997;79:207-8. [DOI] [PubMed]
- 33.Schreck DM, Rivera AR, Tricarico VJ. Emergency management of atrial fibrillation and flutter: intravenous diltiazem versus intravenous digoxin. Ann Emerg Med 1997;29:135-40. [DOI] [PubMed]
- 34.Wattanasuwan N, Khan IA, Mehta NJ, Arora P, Singh N, Vasavada BC, et al. Acute ventricular rate control in atrial fibrillation: IV combination of diltiazem and digoxin vs. IV diltiazem alone. Chest 2001;119:502-6. [DOI] [PubMed]
- 35.Wyse DG. The AFFIRM trial: main trial and substudies—what can we expect? J Interv Card Electrophysiol 2000;(Suppl 4)1:171-6. [DOI] [PubMed]
- 36.Coplen SE, Antman EM, Berlin JA, Hewitt P, Chalmers TC. Efficacy and safety of quinidine therapy for maintenance of sinus rhythm after cardioversion. A meta-analysis of randomized control trials. Circulation 1990;82:1106-16. [DOI] [PubMed]
- 37.Pritchett EL, Page RL, Connolly SJ, Marcello SR, Schnell DJ, Wilkinson WE. Antiarrhythmic effects of azimilide in atrial fibrillation: efficacy and dose-response. Azimilide Supraventricular Arrhythmia Program 3 (SVA-3) Investigators. J Am Coll Cardiol 2000;36:794-802. [DOI] [PubMed]
- 38.Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med 2000;342:913-20. [DOI] [PubMed]
- 39.Chun JG, Brodsky MA, Allen BJ. Modern concepts of atrial fibrillation. Herz 1993;18:67-75. [PubMed]
- 40.Ceremuzynski L, Kleczar E, Krzeminska-Pakula M, Kuch J, Nartowicz E, Smielak-Korombel J, et al. Effect of amiodarone on mortality after myocardial infarction: a double-blind, placebo-controlled, pilot study. J Am Coll Cardiol 1992;20:1056-62. [DOI] [PubMed]
- 41.Teo KK, Yusuf S, Furberg CD. Effects of prophylactic antiarrhythmic drug therapy in acute myocardial infarction. An overview of results from randomized controlled trials. JAMA 1993;270:1589-95. [PubMed]
- 42.Cairns JA, Connolly SJ, Roberts R, Gent M. Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial Investigators. Lancet 1997;349:675-82. [DOI] [PubMed]
- 43.Julian DG, Camm AJ, Frangin G, Janse MJ, Munoz A, Schwartz PJ, et al. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. European Myocardial Infarct Amiodarone Trial Investigators. Lancet 1997;349:667-74. [DOI] [PubMed]
- 44.Nattel S, Liu L, St Georges D. Effects of the novel antiarrhythmic agent azimilide on experimental atrial fibrillation and atrial electrophysiologic properties. Cardiovasc Res 1998;37:627-35. [DOI] [PubMed]
- 45.Connolly SJ, Schnell DJ, Page RL, Wilkinson WE, Marcello SR, Pritchett EL. Dose-response relations of azimilide in the management of symptomatic, recurrent, atrial fibrillation. Am J Cardiol 2001;88:974-9. [DOI] [PubMed]
- 46.Sun W, Sarma JS, Singh BN. Electrophysiological effects of dronedarone (SR33589), a noniodinated benzofuran derivative, in the rabbit heart: comparison with amiodarone. Circulation 1999;100:2276-81. [DOI] [PubMed]
- 47.Jagasia DH, Williams B, Ezekowitz MD. Clinical implication of antiembolic trials in atrial fibrillation and role of transesophageal echocardiography in atrial fibrillation. Curr Opin Cardiol 2000;15:58-63. [DOI] [PubMed]
- 48.Hart RG, Pearce LA, McBride R, Rothbart RM, Asinger RW. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I-III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke 1999;30:1223-9. [DOI] [PubMed]
- 49.Patients with nonvalvular atrial fibrillation at low risk of stroke during treatment with aspirin: Stroke Prevention in Atrial Fibrillation III Study. The SPAF III Writing Committee for the Stroke Prevention in Atrial Fibrillation Investigators. JAMA 1998;279:1273-7. [PubMed]
- 50.Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet 1994;343:687-91. [PubMed]
- 51.Kottkamp H, Hindricks G, Breithardt G. Role of anticoagulant therapy in atrial fibrillation. J Cardiovasc Electrophysiol 1998;9:S86-96. [PubMed]
- 52.Gage BF, Cardinalli AB, Albers GW, Owens DK. Cost-effectiveness of warfarin and aspirin for prophylaxis of stroke in patients with nonvalvular atrial fibrillation. JAMA 1995;274:1839-45. [PubMed]
- 53.Cohen N, Almoznino-Sarafian D, Alon I, Gorelik O, Koopfer M, Chachashvily S, et al. Warfarin for stroke prevention still underused in atrial fibrillation: patterns of omission. Stroke 2000;31:1217-22. [DOI] [PubMed]
- 54.Caro JJ, Flegel KM, Orejuela ME, Kelley HE, Speckman JL, Migliaccio-Walle K. Anticoagulant prophylaxis against stroke in atrial fibrillation: effectiveness in actual practice. CMAJ 1999;161:493-7. [PMC free article] [PubMed]
- 55.Calkins H, Yong P, Miller JM, Olshansky B, Carlson M, Saul JP, et al. Catheter ablation of accessory pathways, atrioventricular nodal reentrant tachycardia, and the atrioventricular junction: final results of a prospective, multicenter clinical trial. The Atakr Multicenter Investigators Group. Circulation 1999;99:262-70. [DOI] [PubMed]
- 56.Leitch JW, Klein G, Yee R, Guiraudon G. Sinus node-atrioventricular node isolation: long-term results with the “corridor” operation for atrial fibrillation. J Am Coll Cardiol 1991;17:970-5. [DOI] [PubMed]
- 57.Van Hemel NM, Defauw JJ, Guiraudon GM, Kelder JC, Jessurun ER, Ernst JM. Long-term follow-up of corridor operation for lone atrial fibrillation: evidence for progression of disease? J Cardiovasc Electrophysiol 1997;8:967-73. [DOI] [PubMed]
- 58.Cox JL, Ad N, Palazzo T, Fitzpatrick S, Suyderhoud JP, DeGroot KW, et al. Current status of the Maze procedure for the treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg 2000;12:15-9. [DOI] [PubMed]
- 59.Brignole M, Menozzi C, Gianfranchi L, Musso G, Mureddu R, Bottoni N, et al. Assessment of atrioventricular junction ablation and VVIR pacemaker versus pharmacological treatment in patients with heart failure and chronic atrial fibrillation: a randomized, controlled study. Circulation 1998;98:953-60. [DOI] [PubMed]
- 60.Brignole M, Menozzi C. Control of rapid heart rate in patients with atrial fibrillation: drugs or ablation? Pacing Clin Electrophysiol 1996;19:348-56. [DOI] [PubMed]
- 61.Fitzpatrick AP, Kourouyan HD, Siu A, Lee RJ, Lesh MD, Epstein LM, et al. Quality of life and outcomes after radiofrequency His-bundle catheter ablation and permanent pacemaker implantation: impact of treatment in paroxysmal and established atrial fibrillation. Am Heart J 1996;131:499-507. [DOI] [PubMed]
- 62.Ozcan C, Jahangir A, Friedman PA, Patel PJ, Munger TM, Rea RF, et al. Long-term survival after ablation of the atrioventricular node and implantation of a permanent pacemaker in patients with atrial fibrillation. N Engl J Med 2001; 344:1043-51. [DOI] [PubMed]
- 63.Feld GK. Radiofrequency catheter ablation versus modification of the AV node for control of rapid ventricular response in atrial fibrillation. J Cardiovasc Electrophysiol 1995;6:217-28. [DOI] [PubMed]
- 64.Markowitz SM, Stein KM, Lerman BB. Mechanism of ventricular rate control after radiofrequency modification of atrioventricular conduction in patients with atrial fibrillation. Circulation 1996;94:2856-64. [DOI] [PubMed]
- 65.Morady F, Hasse C, Strickberger SA, Man KC, Daoud E, Bogun F, et al. Long-term follow-up after radiofrequency modification of the atrioventricular node in patients with atrial fibrillation. J Am Coll Cardiol 1997;29:113-21. [DOI] [PubMed]
- 66.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [DOI] [PubMed]
- 67.Ho SY, Cabrera JA, Tran VH, Farre J, Anderson RH, Sanchez-Quintana D. Architecture of the pulmonary veins: relevance to radiofrequency ablation. Heart 2001;86:265-70. [DOI] [PMC free article] [PubMed]
- 68.Gerstenfeld EP, Guerra P, Sparks PB, Hattori K, Lesh MD. Clinical outcome after radiofrequency catheter ablation of focal atrial fibrillation triggers. J Cardiovasc Electrophysiol 2001;12:900-8. [DOI] [PubMed]
- 69.Guerra PG, Lesh MD. The role of nonpharmacologic therapies for the treatment of atrial fibrillation. J Cardiovasc Electrophysiol 1999;10:450-60. [DOI] [PubMed]
- 70.Attuel P, Pellerin D, Mugica J, Coumel P. DDD pacing: an effective treatment modality for recurrent atrial arrhythmias. Pacing Clin Electrophysiol 1988;11:1647-54. [DOI] [PubMed]
- 71.Stangl K, Seitz K, Wirtzfeld A, Alt E, Blomer H. Differences between atrial single chamber pacing (AAI) and ventricular single chamber pacing (VVI) with respect to prognosis and antiarrhythmic effect in patients with sick sinus syndrome. Pacing Clin Electrophysiol 1990;13:2080-5. [DOI] [PubMed]
- 72.Daoud EG, Timmermans C, Fellows C, Hoyt R, Lemery R, Dawson K, et al. Initial clinical experience with ambulatory use of an implantable atrial defibrillator for conversion of atrial fibrillation. Metrix Investigators. Circulation 2000;102:1407-13. [DOI] [PubMed]
- 73.Daubert C, Gras D, Berder V, Leclercq C, Mabo P. Permanent atrial resynchronization by synchronous bi-atrial pacing in the preventive treatment of atrial flutter associated with high degree interatrial block. Arch Mal Coeur Vaiss 1994;87:1535-46. [PubMed]
- 74.Rawles JM. What is meant by a “controlled” ventricular rate in atrial fibrillation? Br Heart J 1990; 63:157-61. [DOI] [PMC free article] [PubMed]
- 75.Resnekov L, McDonald L. Electroversion of lone atrial fibrillation and flutter including haemodynamic studies at rest and on exercise. Br Heart J 1971; 33: 339-50. [DOI] [PMC free article] [PubMed]
- 76.Wyse DG. Rate versus rhythm control in management of atrial fibrillation (AFFIRM), ACC Annual Scientific Session 2002, Late-Breaking Clinical Trials II; 2002 Mar 19; Atlanta (GA).
- 77.Pedersen OD, Bagger H, Kober L, Torp-Pedersen C. Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation 1999;100:376-80. [DOI] [PubMed]
- 78.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res 2001;89:E32-8. [DOI] [PubMed]
- 79.Fareh S, Bénardeau A, Thibault B, Nattel S. The T-type Ca2+ channel blocker mibefradil prevents the development of a substrate for atrial fibrillation by tachycardia-induced atrial remodelling in dogs. Circulation 1999;100:2191-7. [DOI] [PubMed]
- 80.Feng J, Wible B, Li GR, Wang Z, Nattel S. Antisense oligonucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier potassium current in cultured adult human atrial myocytes. Circ Res 1997; 80: 572-9. [DOI] [PubMed]
- 81.Nattel S. Therapeutic implications of atrial fibrillation mechanisms: Can mechanistic insights be used to improve AF management? Cardiovasc Res 2002; 54:347-60. [DOI] [PubMed]


