Abstract
Epidermal gene therapy may benefit a variety of inherited skin disorders and certain systemic diseases. Both in vivo and ex vivo approaches of gene transfer have been used to target human epidermal stem cells and achieve long term transgene expression in immunodeficient mouse/human chimera models. Immunological responses however, especially in situations where a neoantigen is expressed, are likely to curtail expression and thereby limit the therapy. In vivo gene transfer to skin has been shown to induce transgene-specific immune responses. Ex vivo gene transfer approaches where keratinocytes are transduced in culture and transplanted back to patient however, may avoid signals provided to the immune system by in vivo administration of vectors. In the current study, we have developed a stable epidermal graft platform in immunocompetent mice to analyze host responses in ex vivo epidermal gene therapy. Using green fluorescent protein (GFP) as a neoantigen and an ex vivo retrovirus-mediated gene transfer to mouse primary epidermal cultures depleted of antigen presenting cells (APCs), we show induction of GFP-specific immune responses leading to the clearance of transduced cells. Similar approach in immunocompetent mice tolerant to GFP resulted in permanent engraftment of transduced cells and continued GFP expression. Activation of transgene-specific immune responses in ex vivo gene transfer targeted to keratinocytes requires cross-presentation of transgene product to APCs, a process that is most amenable to immune modulation. This model may be used to explore strategies to divert transgene-specific immune responses to less destructive or tolerogenic ones.
Keywords: gene therapy, epidermis, immune response, keratinocytes
INTRODUCTION
Transfer of new genetic material to epidermis offers an opportunity for corrective gene therapy for a variety of inherited skin and systemic deficiency disorders for which the genetic basis has been identified (1,2). Significant progress has been made in using integrating vectors to target epithelial stem cells to achieve long-term transgene expression in both in vivo and ex vivo approaches to epidermal gene therapy (3–6). Despite these advances, the role of immunological responses in limiting the durability of transgene expression is often ignored as most studies are carried out in immune-deficient mice (5,7,8). The de novo expression of a protein in patients with null mutations in the target gene is likely to result in immune responses and clearance of the transduced cells. Moreover, in protocols that require regulated expression of a transgene, transactivators have been found to be immunogenic, resulting in mobilization of immune responses and loss of transduced cells (9).
Previously, we demonstrated a direct correlation between the presence of transgene-specific immune responses and the duration of transgene expression, using a method for in vivo retrovirus-mediated transduction of skin in immunocompetent mice. In this study transgene expression was persistent in immunocompetent mice that were tolerant to the transgene product but transient in non-tolerant mice (4). A primary role for T cell-mediated clearance of keratinocytes expressing a neoantigen was demonstrated. Moreover using β-galactosidase as a model antigen, either CD4+ T cells or CD8+ T cells were sufficient to mediate clearance of transduced cells (10). Direct injection of retroviral particles likely resulted in the delivery of transgene product into the classical endogenous MHC class I pathway either by direct transduction and expression of transgene in APCs (11), or by phagocytosis of antigen-filled retroviral particles by APCs resulting in T cell priming and activation (12,13).
In theory, host responses in ex vivo gene transfer approaches in which keratinocytes are transduced in culture and transplanted back to patients, is likely to be different than host responses to in vivo gene transfer. Activation signals provided to immune system following in vivo administration of viral particles or any vector system may be avoided in ex vivo gene transfer. Furthermore, restriction of gene expression to keratinocytes which lack co-stimulatory signals required for T-cell activation, may induce T cell ignorance or tolerance (14–16).
Loss of transgene expression following grafting of transduced cultured keratinocytes to immune-competent animals has been shown, however the mechanism of transgene loss was not studied and could have been attributed to poor engraftment of transduced cells (17,18). To date, implantation of cultured sheets of mouse keratinocytes to immunocompetent mice has often resulted in short-term grafts as a result of infection, graft contracture and host re-epithelialization (19,20). Unstable grafts have been associated with gene silencing in transduced keratinocytes, a phenomenon likely to complicate assessment of immune-mediated transgene loss (21). In the present study, we have adopted a model in which murine keratinocytes are transduced in culture and grafted onto immune-competent mice to establish stable skin grafts. This model allows us to delineate host responses to keratinocyte-derived antigens in ex vivo approaches to epidermal gene therapy.
METHODS
Animals and vectors
FVB mice were from Taconic laboratories (James Town, NY, USA). FVB-GadGFP and FVB-GFPNagy transgenic lines were purchased from the Jackson Laboratories (Bar Harbor, MA, USA). All strains of mice used in this study were between 7 to 9 week of age at the time of gene transfer or transplantation. LZRS-based retroviral vectors encoding GFP under the control of 5’long terminal repeat (LZRS-GFP) were pseudotyped with vesicular somatitis virus envelope protein and used to transduced mouse epidermal cells(22). GFP expression in transduce cultures was determined by fluorescent microscopy and flow cytometry. Viral stocks used for ex vivo gene transfer had titers of 2–5x106 transducing units/ml. Animal studies were performed in accordance with the institutional guidelines set forth by the Institute of comparative medicine at Columbia University.
Ex vivo transduction and skin reconstitution
Epidermal cells were prepared from newborn mouse skin (FVB) using standard procedures (23). Epidermal cells were seeded on collagen coated plates in keratinocyte-serum free media (Invitrogen, Grand Island, NY, USA) containing 0.3mM Calcium for 6 hours to allow attachment, then media was changed to 0.05 mM Calcium to allow optimum condition for cell proliferation. Three days later keratinocytes were transduced with pseudotyped retroviral vectors LZRS-GFP at various multiplicity of infections (MOIs). Cells were harvested 3 days post-transduction and transduction efficiency was determined by fluorescent activated cell sorter analysis on a Becton Dickinson FACscan (BD Immunocytometry ststem, San Diago, CA). In all experiments cultures with transduction efficiency of more than 60% were used. For transplantation, 2X106 transduced epidermal cells were mixed with 4x106 newborn dermal fibroblasts (isolated from FVB mice) and implanted as a slurry (150 μl volume) onto the fascia but under a silicon chamber implanted onto the back of an anesthetized mouse (24). After one week, chambers were removed and wounds were allowed to heal. A well differentiated skin appeared 7–10 days thereafter.
Flow cytometric staining and depletion of APCs from epidermal preparation
Epidermal cells were harvested from 1–2 day-old mice by trypsinization and either cultured on collagen-coated plates for 3 days or negatively selected for APCs by incubating cells with anti-murine MHC class II magnetic microbeads according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA, USA). For flow cytometric analysis freshly isolated, cultured or APC-depleted epidermal cells were incubated with rat anti-mouse I-A/I-E antibody (BD Pharmingen, San Diego, CA, USA) or isotype control for 30 min at 4°C followed by 30 min staining with FITC conjugated goat anti-rat antibody. Cells were washed extensively and analyzed on a FACSCaliber flow cytometer (BD Immunocytometry ststem, San Diago, CA) and analyzed using CellQuest Pro software. All results were repeated at least three times.
Detection of transgene expression in skin
To assess GFP expression in transduced skin of live animals, animals were anesthetized and placed under a fluorescent stereoscope (Bio 2M, Zeiss Inc., Thornwood, NY, USA) equipped with a mercury lamp and wide band filter set for GFP. Images were captured using a Nikon Coolpix 995 (Nikon Instrument Inc. Melville, NY) and processed using Adobe Photoshop. For detection of GFP expression in tissue sections, samples were fixed in cold 4% paraformaldehyde for 30 min prior to embedding in OCT. Frozen sections were dried and rehydrated, mounted in Vectashield mounting media with DAPI (Vector Laboratories, Inc. Burlingame, CA, USA), examined and photographed with an epifluorescent Eclipse 800 microscope (Nikon Instrument Inc. Melville, NY) equipped with SPOT camera and image analyzing software.
Immunofluorescent analysis
Skin tissues were fixed in 4% paraformaldehyde for 30 min, rinsed in PBS, embedded in OCT media and cryopreserved. Sections were rinsed with PBS, dried and fixed with cold acetone for 2 min. Fixed tissue sections were blocked in 5% non-fat milk in PBS and stained with 1 μg/ml of rat anti-mouse CD4 or anti-mouse CD8a monoclonal antibodies (BD-Pharmingen, San Diego, CA) for 60 min. Antibodies were detected using the Alexa 594-conjugated goat anti-rat antibody (Molecular probes, Eugene, OR).
Analysis of humoral responses in transduced mice
Antibody responses to GFP were determined by ELISA using recombinant GFP (BD Biosciences Clontech, Palo Alto, CA, USA). Microtiter plates (Costar, Corning, NY, USA) were coated with 100 ng of GFP in 100 μl carbonate buffer for 16 h at 4°C. The wells were washed and blocked with 5% non-fat dry milk in Tris-buffered Saline (TBS) for 1 hr at room temperature. Diluted serum samples were added to the blocking solution and incubated for 2 h. As a standard control, serial dilutions of monoclonal anti-GFP antibody (Zymed, San Francisco, CA, USA) were used. The wells were washed extensively, refilled with 100 μl of 1:10000 diluted horseradish peroxidase-conjugated goat anti-mouse IgG (Sigma, St. Louis, MO, USA) and bound antibodies were detected using tetramethyl-benzidine as substrate (Sigma).
RNA and DNA analysis
For detection of low levels of contaminating APCs in culture, total RNA was isolated from cultured cells, epidermal APCs and freshly isolated epidermal cells depleted of APCs using Trizol reagent (Gibco/BRL, Grand Island, NY). RNA samples (0.2 ug) were analyzed by RT-PCR (OneStep RT-PCR kit, Qiagen, Hilden, Germany) using the following primers specific to cd11c gene, a marker for dendritic cells: 5’-ACGGTGCTG AGTTCGGACAC-3’ and 5’-GAACTGATGCTACCCGAGCC-3’ under the following conditions: 95° C for 3 min; 32 cycles of 95° C for 45 sec, 58° C for 45 sec, 72° C for 1 min; 72° C for 10 min.
For detection of transgenic DNA in grafts, grafted tissue was dissected from the surrounding tissue and genomic DNA was isolated using DNeasy Tissue Kit (Qiagen, Hilden, Germany), and analyzed by PCR to detect transgene sequences. For amplification of GFP the following primers were used: 5’-GACCACATGAA GCAGCACGAC-3’ and 5’-GTCACGAACTCCAGCA GGACC-3’. PCR was performed under the following conditions: 95° C for 3 min; 30 cycles of 95° C for 45 sec, 55° C for 45 sec, 72° C for 1 min; 72° C for 10 min. Phosphoglycerate kinase (pgk-1) primers (5’-GGCTGACTTTA TCCTCCGTGTTC-3’ and 5’-ATGAGATGATTA TTGGTGGTGGA-3’ were used as controls to ensure RNA and DNA integrity. PCR products were analyzed by 1 % agarose electrophoresis with ethidium bromide staining.
RESULTS
Development of stable epidermal grafts in immunocompetent mice
The current ex vivo model of cutaneous gene therapy which is based on grafting of genetically modified cultured human keratinocytes onto immunodeficient mice is not suitable to study immunological responses to transgene product. To develop a stable graft platform in immunocompetent mice we examined a hair reconstitution assay that was initially developed to analyze hair formation in nude mice to work in immunocompetent mice. In this assay, newborn mouse epidermal cells are mixed with freshly isolated mouse dermal fibroblasts, and implanted as a slurry under a transplantation chamber on the fascia but under a transplantation chamber to regenerate a well-differentiated epidermis (24). To examine whether this procedure results in permanent skin grafts in immune-competent mice, GFP-expressing cells isolated from FVB-GFPNagy mice were used to reconstitute skin on the back of FVB-GadGFP mice (tolerant to GFP) as described in Materials and Methods. GFP expression in the former transgenic line is controlled by actin promoter and therefore expressed in all nucleated cell types (25), while in the latter expression is limited to hippocampal and cortical GABAergic interneurons in brain (26). Gad-GFP mice do not express GFP in skin and are tolerant to GFP. Transplantation chambers were removed at 1 week post-grafting and a week later, reconstituted tissues were completely healed, inflammation subsided and signs of new hair growth appeared. Skin grafts were observed repeatedly by fluorescent stereoscopy for any sign of GFP loss. Figure 1 shows a representative reconstituted skin at 5 and 40 weeks post-grafting in GFP-tolerant mice (Fig. 1A-B) with all cells in the grafted area expressing GFP (Fig. 1D, E,G,H). There was a sharp separation between GFP-expressing grafted keratinocytes and non-GFP expressing host cells indicating a lack of invasion by host keratinocytes (arrowheads in Fig. 1D and E). Furthermore, no reduction in the size of the grafts was observed from 5 to 40 weeks post grafting. These data demonstrated a lack of mechanical displacement of grafted cells by the host keratinocytes at least for 40 weeks, providing a stable epidermal graft platform in immunocompetent mice.
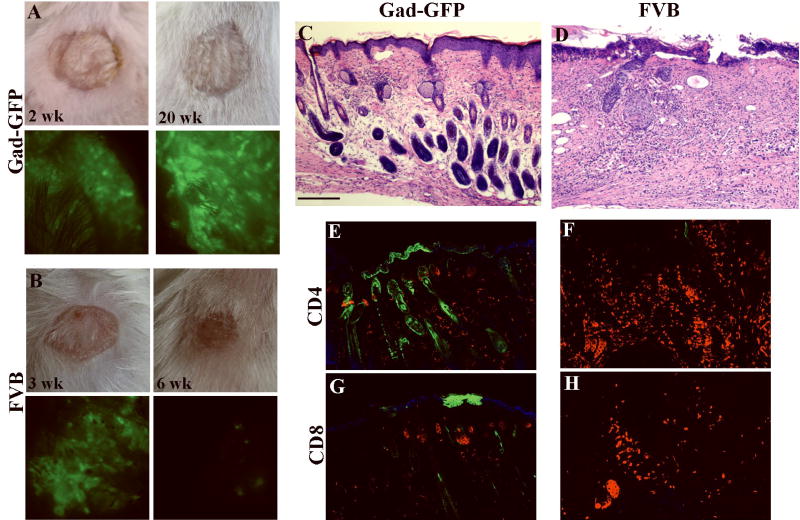
Figure 1. Murine cells grafted to immuno-competent mice form a stable graft with persistent long-term transgene expression.
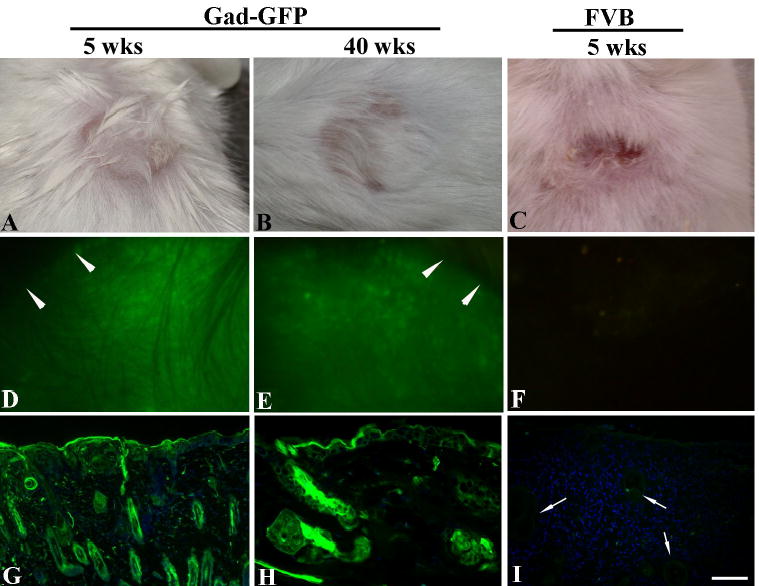
Epidermal and dermal cells were isolated from newborn GFP-Nagy transgenic mice, and used to reconstitute skin on Gad-GFP mice (GFP-tolerant) (A-B) or FVB mice (C). Grafts were observed repeatedly by fluorescent stereoscopy for any sign of GFP loss up to 40 weeks post grafting. Light and fluorescent images of grafted mice at 5 weeks (A,C,D,F) and at 40 weeks (B and E) post-grafting. Arrowheads denote sharp separation between grafted skin and recipient skin. The lower panels are tissue sections obtained from a representative mouse at 5 wk (G, I) and 40 wk (H) post-grafting. GFP-positive cells are in green. Arrows in I point to epidermal cysts in the dermis of the rejected skin graft. Sections were counterstained by Dapi (blue nuclear staining). Bar=100 μm for G and I, and 50 μm for H.
GFP as a model antigen in FVB mice
GFP has been used as a model tumor antigen in BALB/c mice, however in C57Bl/6 mice GFP-expressing tumor cells were minimally immunogenic (27,28). To verify the use of GFP as a model antigen in FVB mice, freshly isolated Nagy-GFP epidermal and FVB dermal fibroblasts were reconstituted on the back of normal FVB mice and grafts were analyzed as described above. The fate of GFP expressing reconstituted skin was significantly different than that observed for GFP-tolerant mice. By 4 weeks post grafting, GFP expression and graft size in normal FVB mice started to decrease and grafts appeared crusty. By 5–6 weeks post-grafting, GFP expression in the reconstituted skin was completely lost (Fig. 1F and I) and grafts were replaced by a dry scab (Fig. 1C). Rejection of GFP expressing skin cells in FVB mice but not in Gad-GFP (GFP-tolerant) mice indicated GFP-specific immune-mediated loss of reconstituted skin.
Retrovirus-mediated transduction of APC-depleted mouse epidermal cultures
The loss of skin grafts reconstituted from freshly isolated GFP-expressing epidermal cells in FVB mice is likely due to direct antigen presentation by GFP-expressing professional antigen presenting cells (APCs) that may be present in the grafted cell slurry. Ex vivo epidermal gene therapy however, involves gene transfer to cultured epidermal cells that may be depleted of APCs (20). Epidermis consists of a heterogeneous cell population including Langerhans cells that are the only professional APCs in epidermis under normal conditions (29). Flow cytometric analysis of freshly isolated newborn mouse epidermal cells with anti-Ia/Ie antibody confirmed the presence of 3–4% class II positive cells in epidermal preparations (Fig. 2A)(30). Examination of epidermal cells maintained in culture for three days indicated a significant depletion of MHC class II positive cells comparable to that observed following negative selection with anti-murine MHC class II magnetic microbeads (Fig. 2A, lower panels).
Figure 2. Fate of ex vivo transduced keratinocytes grafted to immunocompetent mice.
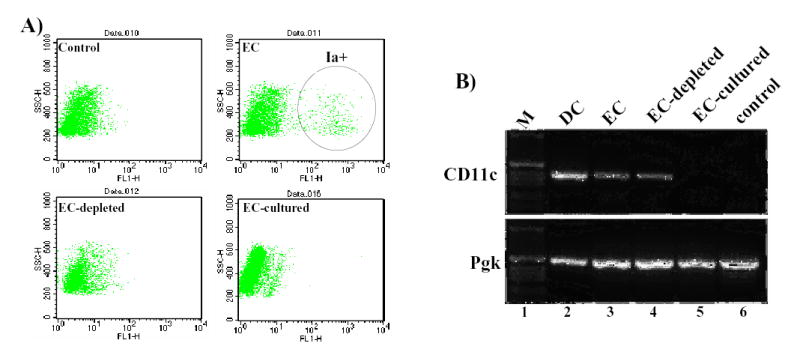
(A) Class II expression on mouse epidermal cells as assessed by flow cytometry. Analysis was performed on freshly isolated newborn epidermal cells after staining with isotype matched control antibody (control); anti mouse Ia antibody (EC); anti-mouse Ia antibody following treatment with anti-MHC classII magnetic beads (EC-depleted); and anti-mouse Ia antibody following 3 days of culture (EC-cultured). (B) RT-PCR analysis of APC contamination in epidermal cultures. Total RNA samples from epidermal dendritic cells islolated with Ia-specific immunobeads (DC), freshly isolated epidermal cells (EC), anti-Ia immunobead-depleted cells (EC-depleted), cells cultured for 7 days (EC-cultured) and immortalized mouse keratinocytes by RT-PCR using a set of primers specific for CD11c (upper panel) or phosphoglycerate kinase-1 (lower panel).
To examine the possibility of low levels of contaminating APCs that may not be detected by FACS analysis, total RNA was isolated from immunobead-depleted epidermal cells or cultured epidermal cells and analyzed by RT-PCR using a set of primers specific for amplification of CD11c, a dendritic cell-specific marker. Phosphoglycerate kinase (Pgk-1) primers were used as controls to ensure RNA integrity. As shown in Figure 2B, CD11c transcript was detected in both freshly isolated and even after negative selection with anti-Ia immunobeads (lanes 3 and 4), but not in epidermal cells maintained in culture for 7 days (lane 1). Dendritic cells positively selected by anti-Ia immunobeads served as positive controls and immortalized mouse cells served as negative controls (lane 6). These data indicated that APCs are efficiently depleted from epidermal cells cultured for several days. A recent study have also shown a progressive loss of CD86 (another marker for APCs) from mouse epidermal cells cultured for 1, 4 and 7 days with a complete loss of CD86 signal on day 7 (31).
Based on these results in the following experiments, to limit transgene expression to keratinocytes, cells recovered from mouse epidermis were cultured for 3 days prior to gene transfer and for 4 additional days following transduction.
To optimize gene transfer to mouse keratinocytes, epidermal cultures were transduced with a VSV-G-pseudotyped retroviral vector encoding GFP (LZRS-GFP) at MOIs ranging from 0.5 to 10. Three days later cells were harvested and GFP expression was examined by flow cytometry. As shown in Figure 3, analysis of transduced cultures indicated more than 85% of cells were transduced at MOI of 2.5. Higher concentration of virus did not result in a further increase the percentage of transduced cells or the level of GFP expression (indicated by median fluorescent intensity).
Figure 3. Optimization of retrovirus transduction of mouse epidermal cultures.
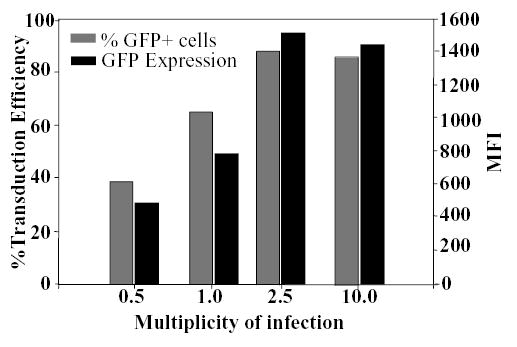
Cultured murine epidermal cells were transduced with LZRS-GFP at MOIs indicated at the bottom of the figure. Three days post-transduction, GFP expression was determined by FACS. The percent GFP positive cells in each population indicated transduction efficiency (gray bars) and the mean fluorescent intensity (MFI) indicated the levels of GFP expression (black bars).
Loss of ex vivo transduced keratinocytes expressing a neoantigen in normal mice
To determine the fate of ex vivo transduced keratinocytes expressing a neoantigen in immunocompetent mice, GFP-transduced keratinocytes were mixed with syngeneic mouse fibroblasts (FVB strain) and reconstituted skin on the back of adult FVB or Gad-GFP (FVB background) mice. Grafts were examined weekly for surface GFP expression and signs of acute or chronic graft rejection. As shown in Fig. 4, transduced cells formed well-differentiated GFP-expressing skin grafts as early as two weeks post grafting. No significant difference in the appearance of grafted skin was noted between FVB and transgenic GFP-tolerant mice (Gad-GFP) for the first three weeks (Fig. 4A ,B). Shortly after however, clinical signs of graft rejection, including inflammation, hair loss and a progressive loss in GFP-positive cells were noted in FVB mice. At 6 weeks post grafting, expression of GFP was completely lost in the skin reconstituted on the back of FVB mice (n=15; Fig. 4B). However, GFP-tolerant mice accepted the grafts and expressed GFP indefinitely (n=12; Fig 4A), thereby verifying the presence of transduced stem cells in the graft.
Figure 4. GFP expression in transduced cells grafted onto immunocompetent mice.
Cultured epidermal cells were transduced with LZRS-GFP at MOI of 2 and grafted onto FVB or GFP-tolerant FVB mice (Gad-GFP). Gross appearance of grafts and surface GFP expression at indicated time points are shown in Gad-GFP mice (A) and FVB mice (B). Surface GFP is lost in FVB mice at 6 weeks post-grafting (B). Hematoxylin/eosin histological sections (C-D) and immunostaining with anti-CD4 or anti-CD8 antibodies (red fluorescent staining) of grafted skin taken at 6 weeks post grafting from Gad-GFP (C, E, G) or FVB (D, F, H). GFP expressing cells appeared as green and were present in epidermis, sebaceous glands and hair follicles formed by the grafted cells in Gad-GFP mice (E and G). No GFP positive cells were present in the LZRS-GFP-transduced skin of FVB mice (F and H). Tissue sections were counterstained with DAPI (blue nuclear staining in J and L). Bar=100 μm for C-D, and 70 μm for E-H.
At six weeks post-grafting, grafted skin of a representative mouse from each group was harvested and tissue sections were prepared. Histological examination of skin reconstituted from LZRS-GFP in GFP-tolerant mice indicated a well-differentiated skin with GFP-expressing keratinocytes in hair follicle, epidermis and sebaceous glands (Fig 4C, E). In FVB mice however, no GFP-expressing cell was detected (Fig. 4F, H) and grafted tissues were acutely inflamed, as noted by significant cellular infiltrates and the loss of normal tissue organization (Fig. 4D). The phenotype of infiltrating lymphocytes was assessed by immunofluorescent staining for CD4+ or CD8+ cells. Analysis of lymphocytic infiltrates demonstrated CD4+ and CD8+ cells invading both dermal and epidermal compartments of skin (Fig. 4 F, H). In GFP-tolerant mice, there was no sign of inflammation and the numbers of CD4 and CD8 positive cells in skin were comparable to that of the normal skin (Fig 4 E,G)(10).
To confirm that the loss of GFP expression in the transduced reconstituted skin was mediated by loss of transduced cells, skin grafts of FVB mice were harvested at 3, 5 and 6 weeks post-grafting. Genomic DNA was isolated and analyzed by PCR using primers specific to GFP or Pgk-1 as a control. As shown in Figure 5, GFP DNA was readily detected in transduced skin at 3 wks post grafting (lanes 1). However, analysis of LZRS-transduced grafts at later time demonstrated a decreased level of vector DNA in 5 weeks post grafting followed by a complete lost a week later (Lanes 2–3) correlated with the loss of GFP expression in the grafted skin. Therefore, the loss of GFP in tissue is due to the clearance of transduced keratinocytes.
Figure 5. Loss of surface GFP correlates with the loss of transduced cells.
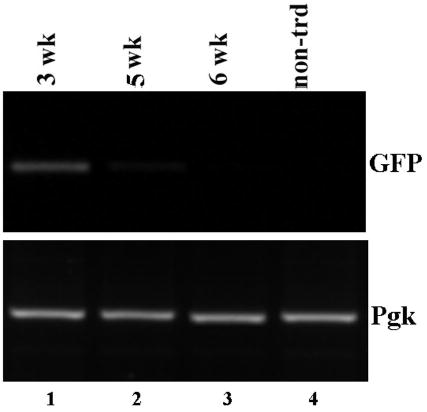
PCR analysis of genomic DNA isolated from skin grafts reconstituted from transduced keratinocytes. Skin tissues were harvested at 3, 5 and 6 weeks post-grafting (lanes 1–3), genomic DNA were isolated and analyzed by PCR using primers specific to GFP (top panel) or phosphoglycerate kinase (lower panels). DNA isolated from non-transduced skin (lane 4) was used as a control.
The loss of transgene and rejection of grafted keratinocytes in normal mice and long-term gene expression in GFP-tolerant mice suggested immune-mediated clearance of transduced keratinocytes. To confirm the induction of GFP-specific immune responses in ex vivo-transduced mice, sera were collected from transduced mice at 3 and 6 weeks post-grafting and were analyzed for the presence of anti-GFP immunoglobulin G (IgG) by ELISA. Analysis of sera indicated substantial quantities of GFP-specific antibodies as early as 3 weeks post grafting while no anti-GFP IgG were detected in the sera collected from GFP-tolerant mice (Fig. 6). These data indicated elicitation of transgene-specific immune responses to keratinocyte-derived transgene product in an ex vivo model of gene therapy.
Figure 6. Immune activation in FVB mice grafted with transduced keratinocytes.
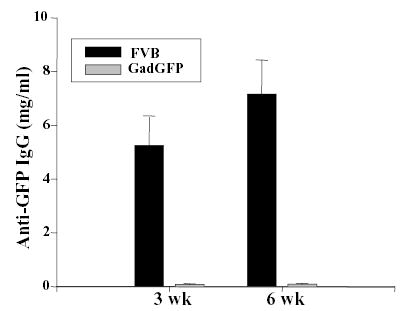
FVB or Gad-GFP mice were grafted with keratinocytes transduced with LZRS-GFP. Sera was collected at 3 or 6 weeks post-grafting and assessed for the presence of anti-GFP IgG by ELISA. The concentration of anti-GFP IgG is expressed based on the concentration of monoclonal anti-GFP antibody used as a standard in the ELISA. Error bar indicates standard deviation for each group (n = 10).
DISCUSSION
In cutaneous gene therapy, immunological responses especially in situations where a neoantigen is expressed, are likely to curtail transgene expression. Understanding the molecular and cellular mechanism underlying activation of immune responses in cutaneous gene therapy will result in more effective and compelling clinical trials.
In the present study, we developed an ex vivo model of long-term gene transfer to mouse epidermis in FVB mice to study host responses in ex vivo approaches to epidermal gene therapy. Long-term GFP expression (more than 20 weeks) following implantation of transduced keratinocytes onto mice tolerant to GFP indicated efficient retrovirus-mediated transduction of mouse epidermal stem cells in culture and their stable engraftment in vivo without a requirement for their enrichment. In non-tolerant mice however, intracellular antigenic transgene products expressed in keratinocytes were effectively presented to T cells resulting in activation of transgene-specific immune responses, and clearance of transduced cells. The immune-mediated loss of transduced cells in FVB mice was indicated by a dense CD4 and CD8 positive cell infiltration within the graft bed, high titers of anti-GFP IgG in sera and the loss of transgene DNA in the reconstituted skin. None of these observations were made in Gad-GFP mice that are immunocompetent but tolerant to GFP.
The possibility of transduction of APCs and antigen presentation to T cells by direct pathway was minimized by depletion of APCs from epidermal cultures prior to skin reconstitution in mice. Epidermal cells were cultured for a total of 7 days before transplantation when no dendritic cell was detected in culture by either FACS analysis or a more sensitive RT-PCR (Figure 2)(20,31). While the kinetics of graft loss in mice transplanted with epidermal cells isolated from Nagy-GFP mice (containing GFP-expressing APCs) is similar to that of mice grafted with cultured epidermal cells transduced with GFP (depleted of APCs), the type of immune mediators is likely to be different. Analysis of anti-GFP IgG isotypes in the sera of grafted mice indicated prevalence of IgG1 in mice grafted with transduced cells while in mice grafted with epidermal cells isolated from Nagy-GFP, high titers of both IgG1 and IgG2a were detected in sera (data not shown). Noteworthy, the levels of GFP expression in transduced and transgenic cells were comparable (data not shown) and is unlikely to have contributed to the observed differences.
In the absence of direct antigen presentation by APCs , induction of immune responses is thought to require transfer of transgene product from keratinocyte to recipient APCs and processing of antigen into the MHC class I pathway for stimulation of cellular immunity, a process called cross-presentation (32,33). The normal outcome of cross-presentation in an unperturbed immune system is thought to be tolerance (34). However, cross-presentation may be redirected to a productive and subsequently destructive immune response by expression of proinflammatory signals and upregulation of costimulatory molecules on APCs (35,36). In our study, the initial stages of ketratinocyte transplantation are accompanied by inflammatory responses and tissue remodeling. The release of proinflammatory cytokines and apoptotic or necrotic cellular material that are produced along with transgene product would provide proinflammatory signals necessary for the induction of transgene-specific immunity to keratinocyte-derived neoantigen (37,38).
It is worth noting that in the presence of significant tissue-specific inflammation, keratinocytes may function as primary APCs by upregulating the expression of MHC-class II molecules and costimulatory molecule including ICAM-1 and CD80 (31,39). Whether antigen is presented by activated keratinocytes or transferred to dendritic cells, activation of cell-mediated immunity requires upregulation of costimulatory signals during antigen presentation. Obviously more experiments are required to delineate the exact mechanism of T cell priming by keratinocyte-derived antigens in this model. While slurry grafting may not be the method to approach a clinical protocol, all grafting procedures are associated with inflammation, wound healing response and keratinocyte activation which may be crucial in determining the outcome of host response to keratinocyte-derived antigens.
In conclusion, we described a model for ex vivo gene transfer to mouse epidermis resulting in long-term transgene expression in immunocompetent mice tolerant to transgene product. Using this model we showed that expression of a neoantigen in keratinocytes induces potent GFP-specific immunity, resulting in clearance of transduced cells. As keratinocytes are the major targets of ex vivo cutaneous gene therapy, induction of cellular immunity in response to antigenic transgene expression in keratinocytes is likely dependent upon cross-priming of T cells (38,40). A clear understanding of the mechanism of uptake and presentation of antigenic transgene products in gene therapy protocols could result in development of effective strategies for diverting destructive immune responses to tolerogenic ones and achieving an effective clinical cutaneous gene therapy.
Acknowledgments
We wish to thank Dr Lorne Taichman and Richard Kalish for helpful discussions. This research was supported by Grants K01-AR02100 and R01-AR050525 from National Institute of Health to SG.
References
- 1.Uitto J, Pulkkinen L. The genodermatoses: candidate diseases for gene therapy. Hum Gene Ther. 2000;11:2267–2275. doi: 10.1089/104303400750035807. [DOI] [PubMed] [Google Scholar]
- 2.Khavari PA, Rollman O, Vahlquist A. Cutaneous gene transfer for skin and systemic diseases. J Intern Med. 2002;252:1–10. doi: 10.1046/j.1365-2796.2002.00995.x. [DOI] [PubMed] [Google Scholar]
- 3.Kolodka TM, Garlick JA, Taichman LB. Evidence for keratinocyte stem cells in vitro: long term engraftment and persistence of transgene expression from retrovirus-transduced keratinocytes. Proc Natl Acad Sci USA. 1998;95:4356–4361. doi: 10.1073/pnas.95.8.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghazizadeh S, Harrington R, Taichman LB. In vivo transduction of mouse epidermis with recombinant retroviral vectors: implications for cutaneous gene therapy. Gene Ther. 1999;6:1267–1275. doi: 10.1038/sj.gt.3300956. [DOI] [PubMed] [Google Scholar]
- 5.Ghazizadeh S, Taichman LB. Organization of Stem Cells and Their Progeny in Human Epidermis. J Invest Dermatol. 2005;124:367–372. doi: 10.1111/j.0022-202X.2004.23599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Rio M, Larcher F, Serrano F, et al. A Preclinical Model for the Analysis of Genetically Modified Human Skin In Vivo. Hum Gene Ther. 2002;13:959–968. doi: 10.1089/10430340252939069. [DOI] [PubMed] [Google Scholar]
- 7.Robbins PB, Lin Q, Goodnough JB, Tian H, Chen X, Khavari PA. In vivo restoration of laminin 5 beta 3 expression and function in junctional epidermolysis bullosa. Proc Natl Acad Sci U S A. 2001;98:5193–5198. doi: 10.1073/pnas.091484998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baek SC, Lin Q, Robbins PB, Fan H, Khavari PA. Sustainable systemic delivery via a single injection of lentivirus into human skin tissue. Hum Gene Ther. 2001;12:1551–1558. doi: 10.1089/10430340152480276. [DOI] [PubMed] [Google Scholar]
- 9.Favre D, Blouin V, Provost N, et al. Lack of an Immune Response against the Tetracycline-Dependent Transactivator Correlates with Long-Term Doxycycline-Regulated Transgene Expression in Nonhuman Primates after Intramuscular Injection of Recombinant Adeno-Associated Virus. J Virol. 2002;76:11605–11611. doi: 10.1128/JVI.76.22.11605-11611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghazizadeh S, Kalish RS, Taichman LB. Immune-mediated loss of transgene expression in skin: implications for cutaneous gene therapy. Mol Ther. 2003;7:296–303. doi: 10.1016/s1525-0016(03)00013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song ES, Lee V, Surh CD, et al. Antigen presentation in retroviral vector-mediated gene transfer in vivo. Proc Natl Acad Sci USA. 1997;94:1943–1948. doi: 10.1073/pnas.94.5.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varmus H. Retroviruses. Science. 1988;240:1427–1435. doi: 10.1126/science.3287617. [DOI] [PubMed] [Google Scholar]
- 13.Cullen BR. Journey to the center of the cell. Cell. 2001;105:697–700. doi: 10.1016/s0092-8674(01)00392-0. [DOI] [PubMed] [Google Scholar]
- 14.Hammond EJ, Ng RL, Stanley MA, Munro AJ. Prolonged survival of cultured keratinocyte allografts in the nonimmunosuppressed mouse. Transplant. 1987;44:106–112. doi: 10.1097/00007890-198707000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Tinois E, Cobbold S, Faure M, Yeoman H, Stanley M. Cultured keratinocyte grafts are recognized, but not rejected by CD8+ T cells in vivo. Eur J Immunol. 1989;19:1031–1035. doi: 10.1002/eji.1830190612. [DOI] [PubMed] [Google Scholar]
- 16.Doan T, Herd K, Street M, et al. Human papillomavirus type 16 E7 oncoprotein expressed in peripheral epithelium tolerizes E7-directed cytotoxic T-lymphocyte precursors restricted through human (and mouse) major histocompatibility complex class I alleles. J Virol. 1999;73:6166–6170. doi: 10.1128/jvi.73.7.6166-6170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan JR, Barrandon Y, Green H, Mulligan RC. Expression of an exogenous growth hormone gene by transplantable human epidermal cells. Science. 1987;237:1476–1479. doi: 10.1126/science.3629250. [DOI] [PubMed] [Google Scholar]
- 18.Ng RLH, Woodward B, Bevan S, Green C, Martin R. Retroviral marking identifies grafted autologous keratinocytes in porcine wounds receiving cultured epithelium. J Invest Dermatol. 1997;108:457–462. doi: 10.1111/1523-1747.ep12289716. [DOI] [PubMed] [Google Scholar]
- 19.Kawai K, Ikarashi Y, Tomiyama K, Matsumoto Y, Fujiwara M. Rejection of cultured keratinocyte allografts in presensitized mice. Transplant. 1993;56:265–269. doi: 10.1097/00007890-199308000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Rouabhia M, Germain L, Belanger F, Auger FA. Cultured epithelium allografts: Langerhans cell and Thy-1+ dendritic epidermal cell depletion effects on allograft rejection. Transplant. 1993;56:259–264. [PubMed] [Google Scholar]
- 21.Ghazizadeh S, Taichman LB. Virus-mediated gene transfer for cutaneous gene therapy. Hum Gene Ther. 2000;11:2247–2251. doi: 10.1089/104303400750035771. [DOI] [PubMed] [Google Scholar]
- 22.Ghazizadeh S, Doumeng C, Taichman LB. Durable and stratum-specific gene expression in epidermis. Gene Ther. 2002;9:1278–1285. doi: 10.1038/sj.gt.3301800. [DOI] [PubMed] [Google Scholar]
- 23.Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- 24.Lichti U, Weinberg WC, Goodman L, et al. In vivo regulation of murine hair growth: insights from grafting defined cell populations onto nude mice. J Invest Dermatol. 1993;101:124S–129S. doi: 10.1111/1523-1747.ep12363165. [DOI] [PubMed] [Google Scholar]
- 25.Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech Dev. 1998;76:79–90. doi: 10.1016/s0925-4773(98)00093-8. [DOI] [PubMed] [Google Scholar]
- 26.Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gambotto A, Dworacki G, Cicinnati V, et al. Immunogenicity of enhanced green fluorescent protein (EGFP) in BALB/c mice: identification of an H2-Kd-restricted CTL epitope. Gene Ther. 2000;7:2036–2040. doi: 10.1038/sj.gt.3301335. [DOI] [PubMed] [Google Scholar]
- 28.Skelton D, Satake N, Kohn DB. The enhanced green fluorescent protein (eGFP) is minimally immunogenic in C57BL/6 mice. Gene Ther. 2001;8:1813–1814. doi: 10.1038/sj.gt.3301586. [DOI] [PubMed] [Google Scholar]
- 29.Caughman SW, Sharrow SO, Shimada S, et al. Ia+ murine epidermal Langerhans cells are deficient in surface expression of the class I major histocompatibility complex. Proc Natl Acad Sci U S A. 1986;83:7438–7442. doi: 10.1073/pnas.83.19.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krueger GG, Emam M. Biology of Langerhans cells: analysis by experiments to deplete Langerhans cells from human skin. J Invest Dermatol. 1984;82:613–617. doi: 10.1111/1523-1747.ep12261453. [DOI] [PubMed] [Google Scholar]
- 31.Lei J, Cheng J, Li Y, Li S, Zhang L. CD80, but not CD86, express on cultured murine keratinocyte stem cells. Transplantation Proc. 2005;37:289–291. doi: 10.1016/j.transproceed.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 32.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 33.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic Cells Acquire Antigens from Live Cells for Cross-Presentation to CTL. J Immunol. 2001;166:3717–3723. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 34.Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol. 2001;1:126–134. doi: 10.1038/35100512. [DOI] [PubMed] [Google Scholar]
- 35.Matzinger P, Anderson CC. Immunity or tolerance: Opposite outcomes of microchimerism from skin grafts. Nat Med. 2001;7:80–87. doi: 10.1038/83393. [DOI] [PubMed] [Google Scholar]
- 36.Frazer IH, De Kluyver R, Leggatt GR, et al. Tolerance or immunity to a tumor antigen expressed in somatic cells can be determined by systemic proinflammatory signals at the time of first antigen exposure. J Immunol. 2001;167:6180–6187. doi: 10.4049/jimmunol.167.11.6180. [DOI] [PubMed] [Google Scholar]
- 37.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 38.Heath WR, Belz GT, Behrens GM, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 39.Fan L, Busser BW, Lifsted TQ, Lo D, Laufer TM. Antigen presentation by keratinocytes directs autoimmune skin disease. Proc Natl Acad Sci USA. 2003;100:3386–3391. doi: 10.1073/pnas.0437899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larrengina AT, Falo LD., Jr Changing paradigms in cutaneous immunology: adapting with dendritic cells. J Invest Dermatol. 2005;124:1–12. doi: 10.1111/j.1523-1747.2004.23554.x. [DOI] [PubMed] [Google Scholar]