Abstract
The design of a sample cell for high performance nuclear magnetic resonance (NMR) at elevated pressure is described. The cell has been optimized for the study of encapsulated proteins dissolved in low viscosity fluids but is suitable for more general NMR spectroscopy of biomolecules at elevated pressure. The NMR cell is comprised of an alumina toughened zirconia tube mounted on a self-sealing non-magnetic metallic valve. The cell has several advantages including relatively low cost, excellent NMR performance, high pressure tolerance, chemical inertness and a relatively large active volume. Also described is a low volume sample preparation device which allows for the preparation of samples under high hydrostatic pressure and their subsequent transfer to the NMR cell.
Keywords: high pressure NMR, encapsulated proteins, low viscosity solvent
INTRODUCTION
High pressure NMR spectroscopy has a long and rich history in physics, chemistry and biophysics. Historically, instrumental implementations of high pressure NMR can be grouped into two general types of apparatus. The original design pioneered by Benedek & Purcell1 and subsequently greatly refined by Jonas and coworkers2–7 was the so-called autoclave design where the entire NMR probe coil was placed under pressure. This design resulted in the highest attainable pressures but prevented the use of the most advanced probe technology. Accordingly the high pressure probe design was difficult to employ with biological systems where low sample concentrations and the demands of sophisticated multidimensional and multinuclear spectroscopy conspire to thwart optimal implementation.
An alternate approach to high pressure NMR focuses on applying hydrostatic pressure solely to the sample. The so-called high pressure NMR cell has taken many forms ranging from capillaries through larger volume tubes composed of single crystals of sapphire,8–10 plastics,11 glass12–14 and other composites.15 Many of these designs are suitable for use with state-of-the-art commercial probe technology. The capillary approach achieves the most impressive pressures but suffers somewhat from limited sample volume.13, 14 This is somewhat ameliorated by the emerging cold probe technology. At this time, the capillary approach is the only high pressure NMR cell able to attain the pressure required to undertake equilibrium studies of large scale macromolecular transitions such as protein unfolding.16–18 More modest pressures, in the 100–1000 bar range, are sufficient to probe low energy structural transitions that result in large system volume changes,19 such as those arising from electrostriction upon dissociation of ion pairs.9 Additionally, in favorable cases, pressure perturbations of protein equilibria can be studied far from the mid-point by employing hydrogen exchange.20, 21 In general, the larger volume high pressure NMR cells have found their greatest utility in studies in the sub-1 kbar pressure range.
More recently, elevated pressure has been employed to facilitate NMR-based studies of encapsulated proteins dissolved in low viscosity fluids.22 Historically, large, aggregation-prone, and membrane proteins have been difficult to examine by modern multinuclear and multidimensional NMR spectroscopy.23 A major limitation presented by these kinds of protein systems is that their slow molecular reorientation compromises many aspects of the more powerful solution NMR methods. Several approaches have emerged to deal with the various spectroscopic difficulties arising from slow molecular reorientation. One of these takes the approach of actively seeking to increase the effective rate of molecular reorientation by encapsulating the protein of interest within the protective shell of a reverse micelle, and dissolving the resulting particle in a low viscosity fluid.23
The preparation of solutions of encapsulated proteins dissolved in solvents of sufficiently low viscosity to obtain short molecular reorientation times has been restricted to the short chain alkanes,22 liquid carbon dioxide24 and recently supercritical xenon.25 In principle, the short chain alkanes offer the most promise for achieving solutions of lowest viscosity. Encapsulated proteins have been solublized at NMR concentrations in liquid propane with high structural fidelity using existing NMR cell technology.22 However, it is only with the development of the apparatus described here that it has become possible to undertake the preparation of solutions of encapsulated proteins dissolved in liquid ethane. Here we describe in some detail the design of a self-sealing NMR cell of relatively large active volume that is capable of withstanding 1 kbar pressures.
Previous work by us has produced several devices capable of handling high pressure samples in a variety of contexts within a standard 5mm triple-resonance NMR probe.11, 26 Each of these developments has limitations that prevented the reverse micelle method from being used to its full potential. One example used a 5mm or 3 mm o.d. single-crystal sapphire tube mated to a copper-beryllium valve.26 This particular NMR cell was capable of withstanding pressures to 1 kbar (5 mm o.d.) and 1.4 kbar (3 mm o.d.) for extended periods of time. However, despite these impressive capabilities there were several difficulties with the design of this cell.
An important limitation with this design is the requirement that a high pressure line remain connected while the cell was pressurized. This introduced significant practical issues with respect to the handling of the device not the least of which is the potential for damage to the NMR probe. In addition, the long section of tubing required to extend out the bore of the magnet demanded that the volume of the sample be relatively large compared to the active volume of the sapphire tube. An additional and more limiting characteristic of the cell was the material of the housing itself. It was found that ethane was capable of leeching paramagnetic copper from the housing wall thereby obliterating the NMR signal. To address these deficiencies the next-generation of apparatus was designed and built.
RESULTS
A. Optimized cell for high pressure NMR studies
For the present purposes, the NMR tube must be capable of withstanding pressures exceeding 700 bar, should be chemically inert and robust to significant handling, and must also possess excellent NMR characteristics. Ideally the tube should be produced at a modest cost. The single-crystal sapphire tubes used routinely in this lab, satisfy only two of these criteria. In addition, the significant cost of the single-crystal sapphire tube and its brittle character severely restricts its general use. As described by Flynn et al., plastics offer a potentially low cost solution.11 When machined from PEEK plastic this tube is capable of withstanding pressures to 500 bar. Unfortunately machined PEEK was found to be somewhat unreliable and had poor tolerance to ethane at elevated pressures. Use of precision injection molding, instead of machining, was unsuccessful for PEEK and other high strength plastics. Ceramics are an alternative material that have higher tensile strengths than plastics but do generally cost more.26, 27 These were examined in much greater detail as a viable alternative to sapphire tubes and form the central element of the current design.
Schematics of the high pressure NMR cell are shown in Figure 1. The high pressure tube has an outer diameter of slightly less than five millimeters and has a working sample volume of slightly more than 200 μL. This volume is approximately double that of the 5mm sapphire tube described previously.9 The tube is manufactured from alumina toughened zirconia (AZO), which is suitable for injection molding. Production of the tubes was carried out by Small Precision Tools Inc. using a proprietary injection molding procedure.
Figure 1.
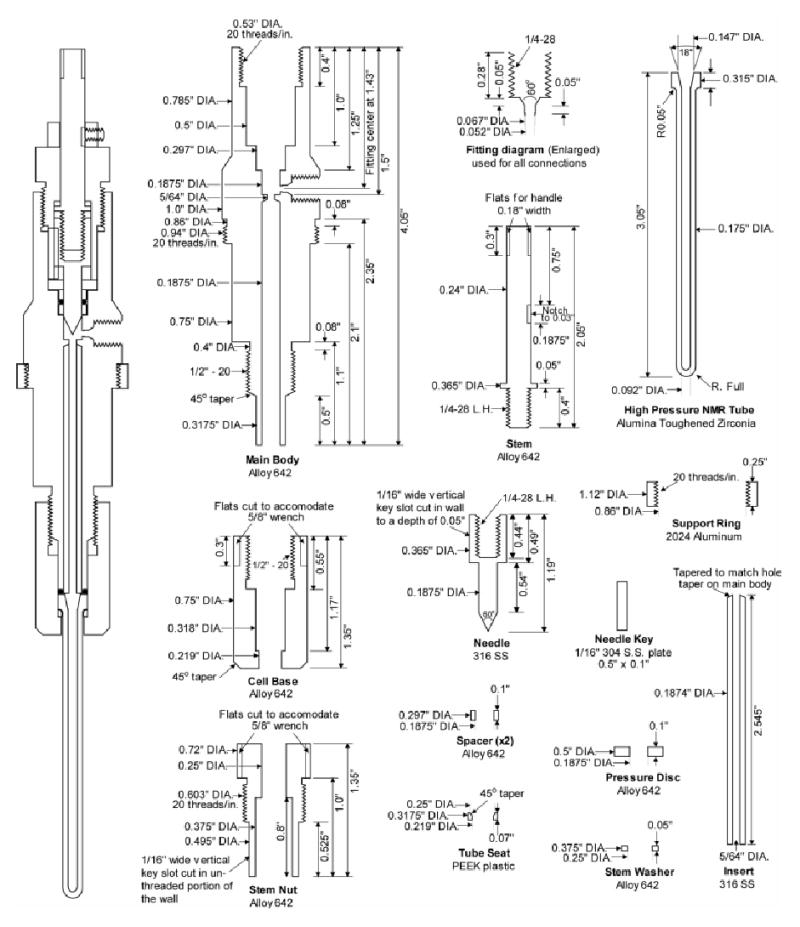
The fully assembled NMR cell is shown on the left-side of the figure. The AZO tube is shown in cross-section on the right. Also included is a diagram for the High Pressure Equipment Co. style AF1 fitting which is used for all machined connection points in the NMR cell and mixing chamber.
The performance of this tube was excellent. Tubes of this design and manufacture have been tested to pressures up to 960 bar for extended periods of time without failure. The calculated burst pressure is slightly more than 1 kbar, which is also the failure pressure for all the fittings used in the apparatus. The tubes were also capable of withstanding rapid pressurizations of at least 700 bar without rupture. This feature was especially important since samples prepared in a mixer must be rapidly transferred to a tube that is at significantly lower pressure. Compared to the brittle sapphire tubes, the AZO tubes can be handled quite roughly without losing structural integrity or pressure performance characteristics. Finally, the injection molding process allowed these tubes to be produced in significant numbers with the overall cost per tube being just a fraction of the cost of the sapphire tube. These tubes have been employed in the lab for routine collection of reverse micelle samples prepared in liquid ethane.28
Mating of the NMR cell to a non-magnetic support is a critical element of the basic method. The material comprising the cell has to not only be non-magnetic but must also be resistant to short chain alkane solvents and be capable of containing the high-pressure samples without failure. The NMR cell consists of 13 individually machined parts plus the tube, two orings, and one commercially available set screw collar. One major distinction between this NMR cell and previous designs is the integration of a high pressure valve into the cell. This eliminated the requirement for the high-pressure tube tether to the NMR cell, thus making the cell self-contained and easily inserted into the magnet.
The integrated high pressure valve shown is a traditional needle valve design. A threaded stem, held vertically stationary by a brass set screw collar (McMaster-Carr #6240K13) is turned to drive a stainless steel needle into place. The threads on the stem and needle are left-hand threads so that turning the handle clockwise results in closure of the valve. To keep the needle from rotating with the stem a key slot was cut into the stem nut and needle. This serves an additional purpose of keeping the needle oriented in the same position relative to the valve seat in the main body piece. The first time the valve is closed the needle and seat deform together and will not change over repeated use. To reduce the rotational wear on the stem nut the stem rotates against the stem washer. A light coating of grease on these mating surfaces allowed for smooth operation of the valve. Not shown in the figure is the handle that is attached to the stem during sample preparation, and removed prior to insertion into the magnet. This could be any set screw handle made for a 1/4” rod.
The needle was sealed in the valve by a single AS568A-901 viton o-ring (McMaster-Carr #9752K111). The o-ring was held between two spacers of appropriate dimensions such that when driven flush by the pressure disc the proper groove size would be maintained. The pressure disc was set by tightening the stem nut. The design allowed for fairly loose tolerances on dimensions with the exception of the spacers which must be precise to achieve maximal sealing characteristics.
The main-body piece has a single connection point for a high-pressure tube fitting. The dimensions of this connection point are consistent with the AF1 fitting style from High Pressure Equipment Co. (Fitting Gland #15-2AM1). A channel was cut into the main body directing sample flow from the connection point to the tube. A stainless steel sleeve was inserted into the channel to minimize contact of the sample with the Alloy 642 material. This extra precaution was meant to prevent any unforeseen sample reactivity with the NMR cell wall. Flats were cut into the main body above the support ring to fit a 7/8” wrench. The support ring itself was cut to match the diameter of the bore of the magnet. It is free to rotate and can be used to fine tune the vertical placement of the tube in the probe
The high pressure tube was sealed into the NMR cell using a single viton AS568A-008 oring (McMaster-Carr #1201T18). The o-ring also sealed the stainless steel sleeve to the main body. The placement of the o-ring is shown in the graphic of the assembled NMR cell (Figure 1). The tube sits on a PEEK tube seat cut to accommodate the curved structure of the base of the tube. This piece keeps the tube from slipping during assembly of the NMR cell. The sealing force on the o-ring was applied simply by tightening the base piece to the main body piece. Flats were cut into the base piece to fit a 5/8” wrench for this purpose. The design provides for rapid and reproducible assembly of the cell. The internal volume of the NMR cell with the valve closed is approximately 450μl.
The NMR cell was pressure tested by using a modified base piece that had a high pressure connection in place of the tube. This allowed a gauge to be attached to monitor the pressure loss over time. This tested both the robustness of the valve seal as well as the seal to the tube. The apparatus was pressurized to 960 bar using an Isco 65D syringe pump, and allowed to equilibrate for 30 minutes. The syringe pump pressure holding feature was shut off and the system allowed to relax. After 72 hours the system pressure loss was less then 7 bar demonstrating that the NMR cell seals could maintain pressure over long experiment times.
B. High pressure mixing apparatus for protein encapsulation in reverse micelles
With one important exception, the sample preparation apparatus is based largely on the original Palidini & Weber design for high pressure fluorescence spectroscopy.29 The cell as designed has been rigorously tested to its maximum of 1 kbar without catastrophic failure of any components. It was manufactured from a solid block of 2024 aluminum. Again the selection of materials was based on the non-reactivity, strength, and excellent machining characteristics. The volume of the mixing device was intentionally kept small (~1.25 mL) to minimize the waste of protein and deuterated propane and ethane. However, this entire volume is not useable since there is a certain volume lost by the placement of the windows and the reagent plate at the bottom of the cell. These will be discussed later as each component is described.
The preparation of the sample can be monitored visually through two observation windows built into the walls of the cell. Circular sapphire windows 0.5” in diameter by 0.125” thick obtained from Esco Products (Part #G105125) were sealed in the cell wall using a single viton As568A-014 o-ring (McMaster-Carr #1201T25). The plugs that provide support and sealing force to the window were held in place by an aluminum plate attached to the cell with four 3/4” × 1/4”-28 screws. The diameter of the through hole in the plug was 3/16”, while the hole in the cell was 1/4” to optimize the viewing angle. This 1/4” hole in the walls of the cell amounts to 160 μL of unusable sample volume.
Four solvent input lines are built into the mixing cell, each emanating from a corner. The corners were cut to a flat surface in which the holes for 1.03 kbar rated fittings from High Pressure Equipment Co. (Part #15-2AM1) could be machined. Solvent line channels 1/16” in diameter are cut from the bottom of each fitting to the central sample chamber. The high pressure 1/16” tubing is inserted along the solvent line channels until the ends are flush with the inside wall of the central chamber. Three solvent lines terminate at the top edge of the sample volume just below the leading face of the piston. The lines are 1.03 kbar rated tubing, 1/16” O.D x 0.006” I.D from High Pressure Equipment Co. (Part #15-9A1-006). The fourth line, the dip-line, terminates at the bottom of the useable sample volume space. This line employs 1.03 kbar rated tubing 1/16” O.D. x 0.03” I.D. from High Pressure Equipment Co. (Part #15-9A1-030).
One difficulty with preparing samples externally to the high pressure NMR tube is the transfer of the sample without significant loss of pressure. If the pressure falls below the threshold for encapsulation, the protein and surfactant begin to precipitate. Attempts were made to use an inert gas such as nitrogen to pre-pressurize the tube to minimize the pressure differential between the mixer and tube. Ironically, this proves to be ineffective since molecular nitrogen at the pressures used for encapsulation has a density that is greater than the liquid alkane solvents. This prevents the incoming reverse micelle solution from displacing the nitrogen from the NMR cell. The addition of a vent port was similarly ineffective as a means of displacing the inert gas while the transfer was taking place. Use of the less dense helium gas also failed as at the working pressures required it significantly distorted the phase behavior of the reverse micelle solutions such that appropriate preparations could not be obtained.
To overcome this challenge an approach was developed where the sample is physically pushed over by a piston driven by nitrogen gas at high pressure. The high-pressure line extending out the top was 1.03 kbar rated tubing with an I.D. of 0.03” to maximize air flow to the top of the piston. The indicated o-ring was a standard AS568A-906 Viton o-ring (McMaster-Carr #9752K116). This ring forms both a static seal during sample preparation and a dynamic seal as the piston was rammed into position. This ring is replaced after each sample preparation. The dimensions of the piston control the maximum volume of the cell, and can be adjusted accordingly to achieve target volumes up to around a maximum of two milliliters.
At the bottom of the sample chamber is a reagent plate. This piece is fabricated from 2024 aluminum or 316 stainless-steel. It can be removed from the mixer so that reagents can be measured directly onto the plate. This is especially important for accurate measurement of liquid surfactants. It performs a second function by acting as a stop for the piston, thereby preventing the stir-bar from being crushed by the force of the piston. The top of the reagent plate extends to just below the level of the dip tube. The volume of the reagent plate is typically 260 μL and represents wasted sample volume. Shallower plates can be used to minimize sample waste.
The chamber also had several circulation channels cut into the walls to allow for flow of thermally regulated water / glycol mix to control the cell temperature. The channel cuts are indicated on the figure. To cut a continuous channel several holes had to be drilled and were closed with threaded stainless steel plugs sealed with low pressure thread sealant. The circulation channel end points were terminated with Type 303 SS multi-barbed tube fitting 7/16”-20 threads (McMaster-Carr #5670K51). Rubber tubing terminated with quick disconnects are connected to these fitting allowing for easy switching between multiple mixing cells and a water bath.
C. Integration of apparatus
The component layout of the apparatus used to prepare reverse micelles is shown in Figure 3. The setup shown is for preparing reverse micelle samples using the injection method in which a small aliquot of concentrated protein sample is injected into a reverse micelle solution. In the context of this apparatus setup, the protein-water mix and the reverse micelle components are added to the mixing chamber first and the alkane solvents are then added on top. Agitation within the cell is provided by a Teflon micro stir bar.
Figure 3.
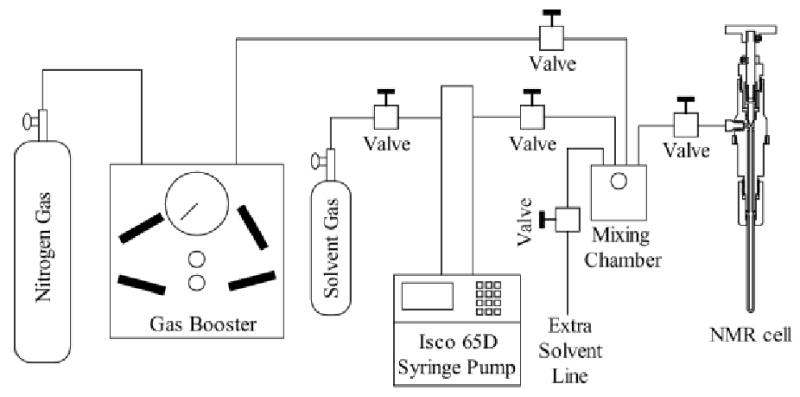
The complete apparatus used to prepare solutions of encapsulated proteins dissolved in low viscosity fluids under high pressure. The central element is the mixing cell that has been described in the text. The surfactants, water/protein solution, and any co-solvents are sealed in the mixing chamber. Pressurized alkane solvents are delivered to the chamber by the Isco 65D syringe pump. Once mixing is complete the mixer-cell piston, driven by high pressure nitrogen gas, pushes the sample into the NMR cell.
It is important to recognize that not all proteins can be concentrated to levels necessary for the injection method of sample preparation. Therefore, a second mixing chamber can be added to allow for a phase-transfer approach where a reverse micelle solution is produced in the first chamber and then pushed over and mildly agitated at a 1 to 1 volume ratio with a protein solution in the second mixing chamber. The advantage of this approach is that much more dilute protein solutions can be used in the preparation. The disadvantage is that the water loading parameter is no longer controlled. This approach will not be discussed further here.
The pressurized liquid alkanes are supplied to the mixing chamber by an Isco 65D syringe pump. This device has the capability of pressurizing fluid up to 1.38 kbar with a flow up to 30mL/min. The capacity of the syringe pump is 68 mL which is more than sufficient for our needs. Typically, the desired alkane gas is pressurized until just slightly above the liquefaction point before transfer. Upon opening the valve between the pump and mixing chamber the now liquefied alkane was transferred. By keeping an open system to the pump the pressure could be finely controlled until encapsulation of the protein ensued. For reverse micelle samples with a water loading of ten in ethane this pressure was typically between 550–620 bar.
The transfer of the sample is straightforward. After the sample is prepared the valve to the syringe pump is closed to isolate the mixing chamber. The nitrogen gas provided by a gas booster (High Pressure Equipment Company, Model GBS-30) is raised to 20 bar more than the sample encapsulation pressure. This is to assure that the piston immediately starts to move once the valve to the NMR cell is opened with the goal of minimizing the amount of material that precipitates due to the pressure drop. Once the high pressure nitrogen gas line is opened to the piston, the sample can be safely transferred. Opening the valve to the NMR cell causes the sample to be rammed into the NMR tube. The nitrogen gas line valve is then immediately closed, and the system again opened to the syringe pump to return to the encapsulation pressure. With the system open to the syringe pump the valve on the NMR cell is then closed. This last step is necessary since the displacement by the needle during valve closure is enough to raise the pressure in the tube by 20 bar. With the valve closed the external connection to the NMR cell can then be removed and it is ready for insertion into the NMR spectrometer magnet.
SUMMARY
We have described in detail an apparatus that will allow for the preparation of reverse micelles in liquid ethane in a safe and reproducible manner. The lack of such a device has severely limited the application and thus development of this potentially powerful technique for structural studies of macromolecules. The NMR cell described here makes use of a newly developed high pressure NMR tube with double the capacity of other high pressure tubes of similar dimensions but has comparable pressure tolerance. This greatly improves the quality of NMR data obtained and is especially significant since the effective protein concentration in reverse micelle samples tends to be on the order of only 100–200 μM. The high pressure valve incorporated into the NMR cell itself eliminates a problematic high pressure tube tether. Overall, the ease of assembly and capabilities of this NMR cell make it an essential component of the reverse micelle method as well as having general applications in high pressure NMR spectroscopy.
Also described here is a novel method for transferring samples into the NMR cell. Without this capability liquid ethane samples would necessarily have to be prepared by the addition of reagents to the tube then pumping ethane on top. We have not found this method to be very successful. Greater sample agitation can be achieved within the mixing cell and significantly increases the efficiency of protein encapsulation than could otherwise be achieved by mixing in the tube. In addition, preparation of samples in a mixing chamber allows a much greater range in sample composition to be prepared. Therefore, the incorporation of a volume displacement piston powered by high pressure nitrogen gas is a vital element to the success of this apparatus. The apparatus shown here will provide a platform for the expansion of the use of this technique for structural studies of macromolecules.
Figure 2.
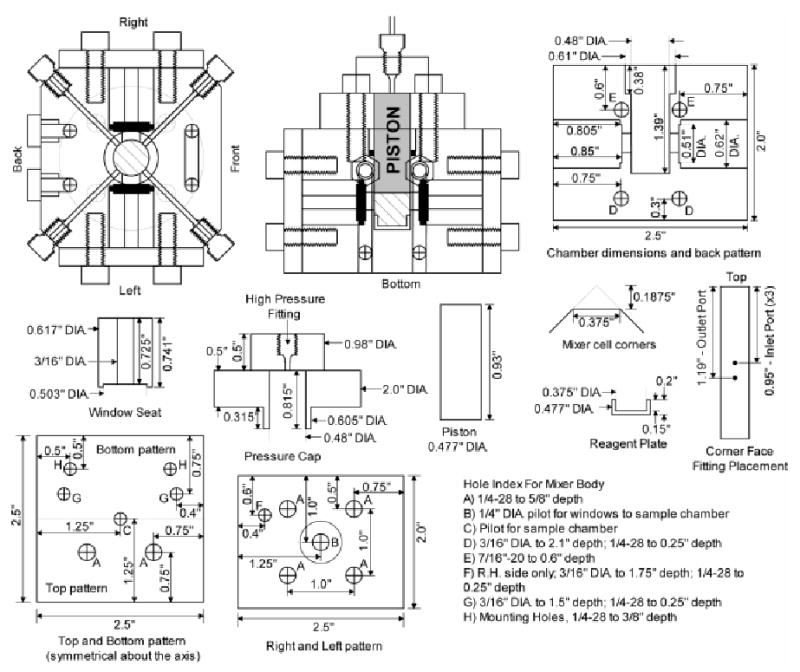
Two views of the mixing chamber are drawn to illustrate how the pieces fit in context. The left and right, and top and bottom walls of the mixer are combined into single diagrams. The left and right are identical except for hole F. The text section details the drill sizes and depths for the holes (A–H) indexed on the diagram. The top and bottom patterns are symmetrical about the horizontal axis indicated by a faint line. Holes that are not marked with specific dimensions are identical to holes of the same index letter. Additional parts detailed in the paper are also shown with corresponding dimensions.
Acknowledgments
This work was supported by NIH grant GM 62874 and by an NSF MRSEC Award (DMR00-79909).
References
- 1.Benedek GB, Purcell EM. J Chem Phys. 1954;22:2003. [Google Scholar]
- 2.Jonas J, Koziol P, Peng X, et al. J Magn Reson B. 1993;102:299. [Google Scholar]
- 3.Defries TH, Jonas J. J Magn Reson. 1979;35:111. [Google Scholar]
- 4.Vandervelde DG, Jonas J. J Magn Reson. 1987;71:480. [Google Scholar]
- 5.Koziol P, Reiner C, Jonas J. App Magn Reson. 1996;11:19. [Google Scholar]
- 6.Ballard L, Yu AM, Reiner C, et al. J Magn Reson. 1998;133:190. doi: 10.1006/jmre.1998.1463. [DOI] [PubMed] [Google Scholar]
- 7.Jonas J. Biochem Biophys Acta. 2002;1595:145. doi: 10.1016/s0167-4838(01)00341-7. [DOI] [PubMed] [Google Scholar]
- 8.Roe DC. J Magn Reson. 1985;63:388. [Google Scholar]
- 9.Urbauer JL, Ehrhardt MR, Bieber RJ, et al. J Am Chem Soc. 1996;118:11329. [Google Scholar]
- 10.Bai S, Taylor CM, Mayne CL, et al. Rev Sci Instr. 1996;67:240. [Google Scholar]
- 11.Flynn PF, Milton MJ, Babu CR, et al. J Biomol NMR. 2002;23:311. doi: 10.1023/a:1020229307552. [DOI] [PubMed] [Google Scholar]
- 12.Jonas J, Bull TE, Eckert CA. Rev Sci Instr. 1970;41:1240. [Google Scholar]
- 13.Yamada H, Nishikawa K, Honda M, et al. Rev Sci Instr. 2001;72:1463. [Google Scholar]
- 14.Yamada H. Rev Sci Instr. 1974;45:640. [Google Scholar]
- 15.Vanni H, Earl WL, Merbach AE. J Magn Reson. 1978;29:11. [Google Scholar]
- 16.Akasaka K, Yamada H. Methods Enzym. 2001;338:134. doi: 10.1016/s0076-6879(02)38218-1. [DOI] [PubMed] [Google Scholar]
- 17.Kamatari YO, Kitahara R, Yamada H, et al. Methods. 2004;34:133. doi: 10.1016/j.ymeth.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Lassalle MW, Yamada H, Akasaka K. J Mol Biol. 2000;298:293. doi: 10.1006/jmbi.2000.3659. [DOI] [PubMed] [Google Scholar]
- 19.Robinson CR, Sligar SG. Methods Enzym. 1995;259:395. doi: 10.1016/0076-6879(95)59054-4. [DOI] [PubMed] [Google Scholar]
- 20.Fuentes EJ, Wand AJ. Biochemistry. 1998;37:9877. doi: 10.1021/bi980894o. [DOI] [PubMed] [Google Scholar]
- 21.Kranz JK, Flynn PF, Fuentes EJ, et al. Biochemistry. 2002;41:2599. doi: 10.1021/bi011818f. [DOI] [PubMed] [Google Scholar]
- 22.Wand AJ, Ehrhardt MR, Flynn PF. Proc Natl Acad Sci USA. 1998;95:15299. doi: 10.1073/pnas.95.26.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wand AJ, Babu CR, Flynn PF, et al. Biol Magn Reson. 2003;20:121. [Google Scholar]
- 24.Gaemers S, Elsevier CJ, Bax A. Chem Phys Lett. 1999;301:138. [Google Scholar]
- 25.Meier M, Fink A, Brunner E. J Phys Chem B. 2005;109:3494. doi: 10.1021/jp044863g. [DOI] [PubMed] [Google Scholar]
- 26.Ehrhardt MR, Flynn PF, Wand AJ. J Biomol NMR. 1999;14:75. doi: 10.1023/a:1008354507250. [DOI] [PubMed] [Google Scholar]
- 27.Wu WJ, Vidugiris G, Mooberry ES, et al. J Magn Reson. 2003;164:84. doi: 10.1016/s1090-7807(03)00144-7. [DOI] [PubMed] [Google Scholar]
- 28.R. W. Peterson, B. G. Lefebvre, and A. J. Wand, J. Am. Chem. Soc., submitted (2005).
- 29.Paladini AA, Jr, Weber G. Biochemistry. 1981;20:2587. doi: 10.1021/bi00512a034. [DOI] [PubMed] [Google Scholar]


