Abstract
Little information is available about the precise mechanisms and determinants of freeze resistance in baker's yeast, Saccharomyces cerevisiae. Genomewide gene expression analysis and Northern analysis of different freeze-resistant and freeze-sensitive strains have now revealed a correlation between freeze resistance and the aquaporin genes AQY1 and AQY2. Deletion of these genes in a laboratory strain rendered yeast cells more sensitive to freezing, while overexpression of the respective genes, as well as heterologous expression of the human aquaporin gene hAQP1, improved freeze tolerance. These findings support a role for plasma membrane water transport activity in determination of freeze tolerance in yeast. This appears to be the first clear physiological function identified for microbial aquaporins. We suggest that a rapid, osmotically driven efflux of water during the freezing process reduces intracellular ice crystal formation and resulting cell damage. Aquaporin overexpression also improved maintenance of the viability of industrial yeast strains, both in cell suspensions and in small doughs stored frozen or submitted to freeze-thaw cycles. Furthermore, an aquaporin overexpression transformant could be selected based on its improved freeze-thaw resistance without the need for a selectable marker gene. Since aquaporin overexpression does not seem to affect the growth and fermentation characteristics of yeast, these results open new perspectives for the successful development of freeze-resistant baker's yeast strains for use in frozen dough applications.
Bread making is one of the oldest food-manufacturing processes and involves the fermentative capacity of the yeast Saccharomyces cerevisiae for the leavening of the dough. Special types of dough, such as sweet or sour dough, present specific challenges to the leavening activity of the yeast, and specific strains with better performance under such conditions have been selected. However, no appropriate strains of yeast are available yet for use in frozen doughs, an important recent development in the bakery industry (2, 33).
The use of frozen doughs is steadily increasing in all industrialized countries because it offers great convenience, automation, and economy of scale. However, significant reduction of the leavening capacity during freeze storage is a serious drawback. Minimizing this loss requires specialized equipment for cold and rapid mixing of the dough which is not available to artisanal bakers. Moreover, these optimized production conditions still cannot completely overcome the drop in leavening activity during long-term storage.
Conditions for production of baker's yeast have been optimized in the past decades and nowadays allow yeast with a very high stress resistance to be produced. Active dry yeast, for instance, is guaranteed to maintain its activity during shelf storage at room temperature for 2 years. However, the preparation of frozen doughs presents an unusual challenge. Although marketed baker's yeast is highly stress resistant, it rapidly loses this stress resistance upon the initiation of fermentation during the preparation of the dough. Moreover, a short prefermentation period before freezing of the dough is required to obtain an appropriate texture in the bread. Hence, fermentation-induced loss of stress resistance is a central obstacle to the production of frozen doughs (28, 35). The rapid loss of stress resistance in the yeast is due to activation of signal transduction pathways by the nutrients in the flour. In particular, activation of the Ras-cyclic AMP (cAMP)-protein kinase A pathway by sucrose and glucose causes rapid loss of stress resistance due to mobilization of trehalose, repression of heat shock proteins, and disappearance of other, unknown stress protection factors (42, 43). Neither the addition of more yeast or of protective additives nor the optimization of dough production conditions has resulted in a satisfying solution for the loss of rising capacity in frozen doughs.
Yeast strains with improved freeze tolerance have been isolated from natural sources, selected from culture collections, or obtained by mutagenesis, hybridization, or protoplast fusion of natural and commercial strains (1, 8, 12, 28, 29). Upon characterization of those strains, several correlations have been reported between freeze resistance and cellular factors such as trehalose content (11, 16, 36, 45), heat shock protein levels (14), the lipid composition of the cell membrane (27), respiratory capacity (31), and accumulation of charged amino acids (39). However, to date no single factor has been identified which allows reduction or enhancement of freeze tolerance in baker's yeast by genetic modification of specific target genes in a consistent and predictable way.
Yeast mutants deficient in “fermentation-induced loss of stress resistance” (fil mutants) have been isolated, and components of the cAMP-protein kinase A pathway, such as the putative glucose-sensing G-protein-coupled receptor Gpr1 (17) and adenylate cyclase (46), have been shown to be affected in these mutants. Recently, fil mutant AT25, derived from the industrial strain S47, which is in commercial use worldwide, has been isolated (41a). We have now performed genomewide expression analysis with this strain and its parent strain S47, as well as with several freeze-resistant and freeze-sensitive derivatives of AT25 and S47, respectively. This has led to the identification of aquaporins as determinants of freeze resistance.
Aquaporins belong to the major intrinsic protein (MIP) family of membrane proteins. Members of this family are channel proteins with six transmembrane domains. They are involved in the transport of water and/or small neutral solutes such as glycerol (30). S. cerevisiae contains four genes encoding members of the MIP family (30): the osmoregulated glycerol facilitator Fps1 (24, 40), its homologue Yfl054c, with putative glycerol transport function, and the two aquaporin water channels, Aqy1 and Aqy2. In most laboratory strains, industrial strains, and natural isolates, the AQY2 open reading frame (ORF) is split into two overlapping ORFs (YLL052c-YLL053c) as a consequence of an 11-bp deletion (AQY2-2). Only in strains with the Σ1278b background is an intact, nondisrupted ORF found, encoding a functional Aqy2 water channel (AQY2-1) (20). For the AQY1 gene also, functional (AQY1-1) and nonfunctional (AQY1-2) alleles have been identified (20). Both yeast aquaporins are localized at the plasma membrane (26; F. Sidoux-Walter and S. Hohmann, unpublished data). Aqy1 has been shown to mediate water transport upon expression in Xenopus laevis oocytes (3), while Aqy2 has been shown by stopped-flow analysis to mediate water transport in yeast cell-derived vesicles (26). While mammalian and plant aquaporins have important functions in water homeostasis and osmoregulation of individual cells and whole organisms, no well-defined phenotype indicative of a physiological function for yeast or other microbial aquaporins has been described yet. In Escherichia coli, for instance, a requirement for the water channel AqpZ during rapid growth and osmotic adaptation has been suggested, but so far without any direct evidence (5). In baker's yeast, a possible role during yeast spore formation and germination has been attributed to Aqy1, whereas Aqy2 has been suggested to play a role in water retrieval after hyperosmotic shock. However, these suggestions were based only on the results of expression analyses (18). The precise physiological functions of the yeast aquaporins and apparently of other microbial aquaporins as well have remained unknown so far (15).
Here we demonstrate a novel phenotype for yeast strains with a modification of aquaporin expression. Deletion reduces the freeze tolerance of the cells, while overexpression enhances it. We also show that the freeze tolerance of industrial strains can be improved by aquaporin overexpression without affecting growth and fermentation characteristics, making the aquaporin genes promising tools for improvement of freeze tolerance in commercial baker's yeast.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
The strains and plasmids used in this study are listed in Table 1. Cells were routinely grown in molasses medium [0.5% (wt/vol) yeast extract, 0.5% (wt/vol) molasses (Lesaffre Développement, Lille, France), 0.05% (wt/vol) (NH4)2HPO4 (pH 5.0 to 5.5)] or in YP (1% [wt/vol] yeast extract, 2% [wt/vol] Bacto Peptone) with either 2% glucose (YPD), 2% galactose (YPGal), or 0.5% molasses (YPM) at 30°C in an orbital shaker.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Industrial strains | ||
| S47 | Polyploid, aneuploid, prototrophic | Lesaffre Développement |
| AT25 | Polyploid, aneuploid, prototrophic | 41a |
| SS1 | Polyploid, aneuploid, prototrophic | This study |
| HAT36, -43, -44 | Polyploid, aneuploid, prototrophic | This study |
| S47/HXT7p | S47/pYX012 HXT7p KanMX | This study |
| S47/HXT7pA1-1 | S47/pYX012 HXT7p KanMX AQY1-1 | This study |
| S47/HXT7pA2-1 | S47/pYX012 HXT7p KanMX AQY2-1 | This study |
| AT25/HXT7p | AT25/pYX012 HXT7p KanMX | This study |
| AT25/HXT7pA1-1 | AT25/pYX012 HXT7p KanMX AQY1-1 | This study |
| AT25/HXT7pA2-1 | AT25/pYX012 HXT7p KanMX AQY2-1 | This study |
| AT25/TPIIp | AT25/pYX012 KanMX | This study |
| AT25/TPIIpA2-1 | AT25/pYX012 KanMX AQY2-1 | This study |
| Laboratory strains | ||
| BY4743 | MATa/MATα his3DI leu2D0 ura3D0 | 4 |
| 10560-6B | MATα leu2::hisG trp1::hisG his3::hisG ura3-52 | G. Fink |
| YSH 1170 | 10560-6B aqy1::KanMX4 | 26 |
| YSH 1171 | 10560-6B aqy2::HIS3 | 26 |
| YSH 1172 | 10560-6B aqy1::KanMX4 aqy2::HIS3 | V. Laizé |
| Plasmids | ||
| pUG6 | Containing loxP-KanMX-loxP cassette | 7 |
| pYX012 | Integrative plasmid with TPI promoter, URA3 marker | Novagen |
| pYX012 KanMX | pYX012 URA3::loxP-KanMX-loxP | This study |
| pYX012 KanMX/AQY2-1 | AQY2-1 cloned into pYX012 KanMX | This study |
| pYX012 HXT7p KanMX | pYX012 KanMX TPI1p replaced by HXT7p | This study |
| pYX012 HXT7p KanMX AQY1-1 | AQY1-1 cloned into pYX012 HXT7p KanMX | This study |
| pYX012 HXT7p KanMX AQY2-1 | AQY2-1 cloned into pYX012 HXT7p KanMX | This study |
| pYX242/AQY2-1 | AQY2-1 cloned into pYX242 | 26 |
| pYeDP | 2μm plasmid with GAL10-CYC1 promoter, URA3 marker | 32 |
| pYeDP hAQP1 | hAQP1 (CHIP28) in pYeDP | 19 |
| pYeDP hAQP1-A73M | hAQP1-A73M (CHIP28-A73M) in pYeDP | R. Bill |
| pYeDP KanMX | 2μm plasmid with GAL10-CYC1 promoter, KanMX marker | This study |
| pYeDP hAQP1 KanMX | KanMX in pYeDP hAQP1 | This study |
AT25 was obtained via UV mutagenesis of the production strain S47 (Lesaffre Développement), followed by screening for survival after multiple freeze-thaw cycles of small doughs prepared with UV-mutagenized S47 cells, and was subsequently characterized as a fil mutant (deficient in fermentation-induced loss of stress resistance). In addition to its higher freeze tolerance, the commercially important properties of mutant AT25 are similar to or better than those of the parent strain S47 (41a).
Strains S47 and AT25 were sporulated, and mutual mating of freeze-resistant spores of AT25 and freeze-sensitive spores of S47 resulted in resistant strains HAT36, HAT43, and HAT44 and the sensitive strain SS1. The idea behind this was to concentrate possible positive alleles for freeze resistance in the HAT strains and to diminish their number in strain SS1. The integrative plasmid pYX012 (Novagen) was modified with a dominant marker gene for use in prototrophic strains by cloning the EcoRV/PvuII fragment containing the loxP-KanMX4-loxP cassette from pUG6 (7) in the URA3 marker, resulting in plasmid pYX012 KanMX. The aquaporin ORFs AQY1-1 and AQY2-2 were PCR amplified using genomic DNA of strain 10560-6B (G. R. Fink, Cambridge, Mass.) and W303-1A (44), respectively, and cloned into pYX012 KanMX downstream of the TPI promoter. Likewise, AQY2-1 was subcloned from pYX242/AQY2-1 (26). Integration of pYX012 KanMX/AQY1-1, AQY2-1, and AQY2-2 at the TPI locus resulted in Geneticin-resistant strains of 10560-6B (Σ1278b background), BY4743 (S288C background) (4), and AT25 overexpressing AQY1-1, AQY2-1, and AQY2-2, respectively. The empty plasmid pYX012 KanMX was routinely inserted as a control. The TPI1 promoter of pYX012 KanMX was also replaced by the truncated HXT7 promoter (10), resulting in plasmid pYX012 HXT7p KanMX. Subsequently, the aquaporin-encoding genes AQY1-1 and AQY2-1 were PCR amplified and cloned downstream of this strong, constitutive promoter. Correct cloning was verified by sequence analysis. Integration of NdeI-linearized plasmids at the URA3 locus resulted in Geneticin-resistant strains of AT25 and S47, overexpressing AQY1-1 and AQY2-1, respectively. The empty plasmid pYX012 HXT7p KanMX was routinely inserted as a control. Selection for Geneticin resistance was carried out with media supplemented with 150 mg of G418 sulfate (Life Technologies)/liter. All strains were checked by PCR on genomic DNA. For use in industrial strains, the loxP-KanMX-loxP cassette from pUG6 (7) was inserted into plasmids pYeDP (32) and pYeDP hAQP1 (19) at the EcoRV restriction site.
RNA isolation, microarray analysis, and Northern analysis.
Strains were grown for 2.5 days until stationary phase in YPD or YPM at 30°C in an orbital shaker. Cells were collected and resuspended in YP. After 30 min of incubation at 30°C, glucose was added to a final concentration of 100 mM. Culture samples for total RNA isolation were taken 30 min after the resuspension in YP as well as 30 min after the addition of glucose and were immediately added to ice-cold water. The cells were washed once with ice-cold water and stored at −70°C. Total RNA was isolated using the RNApure reagent (GeneHunter Corporation) according to the manufacturer's instructions. Microarray analysis was performed using microarrays containing 6,144 yeast ORFs on nylon membranes (Yeast Genefilters Microarrays; Research Genetics) according to the manufacturer's instructions. Probes were prepared by reverse transcription-PCR in the presence of [α-33P]dCTP by using total RNA as a template. Microarray imaging results (Fuji BAS-1000 with MacBAS, version 2.5, software) were compared using Pathways 2.0 software (Research Genetics). Data were normalized against all data points. This genomewide expression analysis was used as a screening method for candidate genes involved in freeze resistance; therefore, each hybridization was performed only once. The reliability and reproducibility of the technique in our hands has been tested extensively as described previously (34). It should be noted that the set of genes present on the membranes is incomplete: genes YPR131C through YPR204W and a number of smaller ORFs were not represented on the Yeast Genefilters Microarrays. For Northern analysis, total RNA was separated in denaturing agarose gels and transferred to nylon membranes. Generally, probes used for hybridization were α-32P-labeled fragments generated with Highprime (Boehringer Mannheim) by using PCR-amplified ORFs as templates. For AQY1 and AQY2, the C-terminal parts of the ORFs and part of the terminator sequence were amplified and labeled. Actin was used as a loading standard. Signals were quantified using a phosphorimager (Fuji BAS-1000 with MacBAS, version 2.5, software) and expressed as percentages of the actin messenger level. For the Northern analysis, independent isolations of total RNA were used.
RGC after freezing.
Strains were grown for 2.5 days until stationary phase in YPD or YPGal at 30°C in an orbital shaker. Equal amounts of cells (corresponding to 1 ml of culture with an optical density at 600 nm [OD600] of 20 [about 25 mg {wet weight}/ml] for laboratory strains and an OD600 of 15 [about 20 mg {wet weight}/ml] for industrial strains) were collected and resuspended in 1 ml of YP. After incubation at 30°C for 30 min, glucose was added to final concentrations of 100 mM for industrial strains and 200 mM for laboratory strains. Half of the cell suspension was immediately cooled on ice (nonfermenting cells), and the other half was incubated at 30°C for either 30 min (industrial strains) or 40 min (laboratory strains) and then cooled on ice (fermenting cells). After being harvested and resuspended in precooled YP, the cell suspensions were again divided: two aliquots were kept on ice, and another two aliquots were frozen. After freezing in an ethanol bath at −30°C for 1 h, followed by frozen storage in a freezer at −30°C for 1 day, 10 volumes of YP containing 33 mM glucose were added to the control samples and the thawed samples. After incubation at 30°C for either 2.5 h (industrial strains) or 4 h (laboratory strains), the cell suspensions were centrifuged and the glucose concentration of 4 μl of supernatant was determined using 200 μl of Trinder reagent (Sigma Diagnostics). The residual glucose consumption (RGC) was calculated as the glucose consumption of the two frozen samples (FGC) compared to that of the two control samples (initial glucose consumption [IGC]) from both fermenting and nonfermenting cells.
Growth.
The length of the lag phase and the maximum growth rate of yeast strains in YPD and molasses medium were monitored automatically by OD600 measurement with a BioscreenC apparatus (Labsystems). The parameters were as follows: 250 μl of culture in each well, 30 s of shaking each min (medium intensity), an OD600 measurement every 30 min. Readings are saturated at OD600s above 1.5.
Frozen doughs.
A 100-μl volume of an overnight culture in 3 ml of YPD was spread out on molasses plates (25 ml) and grown at 30°C for 24 h. Molasses plates were washed with 6 ml of water, and for each strain the same amount of cells was added to 7.5 g of flour and 0.15 g of salt. The doughs were mixed and kneaded with a spatula, divided into 0.25-g amounts in screw-cap tubes, and fermented for 30 min at 30°C in an incubator. All doughs were put at −30°C in an ethanol bath except for two nonfrozen controls that were analyzed immediately. After 1 h, the samples were either stored in the freezer (−30°C) or subjected to freeze-thaw cycles in a computer-controlled cryostat (one cycle consists of 30°C, −30°C, and 30°C in 2 h). For each measuring point (x days in the freezer or y freeze-thaw cycles), two tubes for each strain were taken out of the freezer or cryostat. To analyze survival, 1 ml of TS (1 g of tryptone/liter and 9 g of NaCl/liter) and 0.5 ml of glass beads (diameter, 3 mm) were added to the dough and yeast cells were released from the dough by vortexing for 1 min. The suspension obtained was diluted and plated on YPD to determine the number of CFU.
Selection of aquaporin overexpression strains based on freeze resistance.
Strain AT25 was transformed with pYX012 KanMX AQY2-1, a recovery period of 1 h at 30°C in YPD was given, and the transformation mixture was aliquoted (25 aliquots of 15 μl, each containing about 4 × 107 cells). Two aliquots were diluted and plated on YPD plates immediately, and the remaining aliquots were enriched for the desired recombinants via freeze-thaw cycling in a computer-controlled cryostat (one cycle consists of 30°C, −30°C, and 30°C in 2 h). After six cycles, all aliquots were diluted and plated on YPD. The resulting colonies were subcultured three times to ensure removal of all nonintegrated plasmids. Subsequently, the surviving strains were tested for the presence of the overexpression construct via PCR analysis using primers complementary to the 5′ end of the TPI promoter and the 3′end of the AQY2-1 gene.
Reproducibility of the results.
All experiments were repeated at least three times with reproducible results. Representative results are shown. For glucose consumption experiments, the RGCs obtained for the control strains are variable between experiments; therefore, mean ratios of the RGCs of the studied strains to the RGCs of the control strains ± errors are reported.
RESULTS
Genomewide gene expression analyses at the onset of fermentation reveal upregulation of AQY2 in freeze-resistant strains.
Using microarrays containing 6,144 yeast ORFs on nylon membranes, genomewide gene expression analyses of different freeze-resistant and freeze-sensitive yeast strains were performed. The resistant strains HAT36, HAT43, and HAT44 (Fig. 1) are derived from the freeze-resistant mutant AT25 (41a), and the sensitive strain SS1 (Fig. 1) is derived from the freeze-sensitive industrial strain S47 (Lesaffre Développement). The global gene expression patterns of these strains were compared at the onset of fermentation, i.e., 30 min after addition of glucose to YPM-grown stationary-phase cells, so as to mimic the conditions under which the commercial yeast should maintain better freeze tolerance.
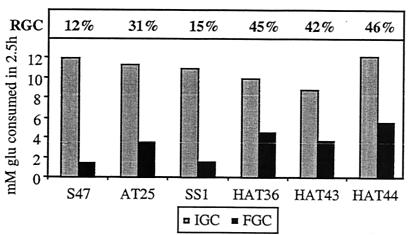
FIG. 1.
Freeze tolerance of freeze-resistant strains (AT25, HAT36, HAT43, HAT44) and freeze-sensitive strains (S47 and SS1) used for microarray analysis. IGC, FGC, and RGC were determined 30 min after the onset of fermentation by addition of 100 mM glucose. The cells were either frozen (for 1 day at −30°C) (FGC) or not frozen (i.e., cooled on ice) (IGC). After thawing, glucose consumption was measured for 2.5 h to assess residual yeast activity. RGC is calculated as (FGC/IGC) × 100. Representative results are shown. AT25 showed an RGC 2.0 (±0.3) times higher than that of S47. HAT36, HAT43, and HAT44 each showed an RGC 2.9 (±0.1) times higher than that of SS1.
Six genes showed at least a 2.5-fold-higher or -lower expression in all comparisons between a resistant and a sensitive strain (see Discussion). However, neither individual overexpression nor individual deletion of these genes in different strain backgrounds resulted in significant effects on freeze tolerance (data not shown). In addition, expression of the AQY2 (YLL052c-YLL053c) gene was higher in the freeze-resistant strains HAT36 (Fig. 2A), HAT43, and HAT44 than in the freeze-sensitive strain SS1. Although AQY2 was not among the genes with the most-pronounced differences in expression, a possible role of a water channel in freeze resistance was intriguing. Expression of the other water channel, AQY1 (YPR192w), was not monitored in the genomewide gene analysis, because it is not represented on the Yeast Genefilters Microarrays. The sequence identity between AQY1-2 and AQY2-2 is 75.5% at the DNA level, which should exclude cross-hybridization between the two genes (34). By use of probes designed to check specific expression of AQY1 and AQY2 by Northern analysis, the higher expression of AQY2 in the resistant strains HAT36 (Fig. 2B), HAT43, and HAT44 than in the sensitive strain SS1 was confirmed, whereas expression of AQY1 could not be detected 30 min after the onset of fermentation in either the freeze-resistant or the freeze-sensitive strains (data not shown).
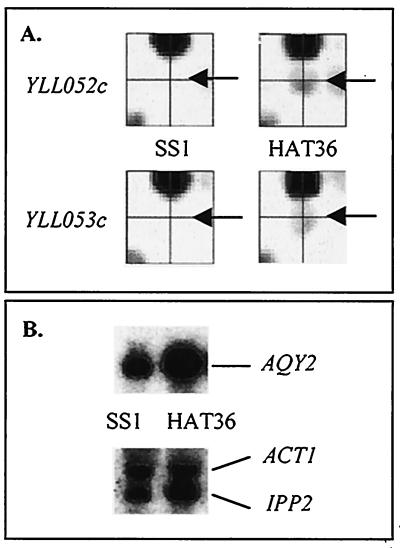
FIG. 2.
Differential expression of the AQY2 (YLL052c and YLL053c) gene in the freeze-resistant strain HAT36 and the freeze-sensitive strain SS1 at the onset of fermentation. (A) Microarray analysis. The YLL052c and YLL053c signals are situated at the center of the crosshair and are indicated by an arrow. (B) Northern blot analysis. ACT1 and IPP2 were used as loading controls. The HAT36/SS1 expression ratio was 3.5.
Overexpression of water channel proteins Aqy1-1 and Aqy2-1 improves freeze tolerance in laboratory and industrial yeast strains without affecting growth and fermentation rates.
Alleles AQY1-1 and AQY2-1, encoding functional Aqy1 and Aqy2, respectively, were overexpressed in laboratory strains BY4743 and 10560-6B as well as in industrial strains S47 and AT25. As determined by diagnostic restriction analysis of the PCR-amplified ORFs according to the work of Laizé et al. (20), BY4743 contains no functional endogenous aquaporin alleles, whereas 10560-6B contains functional endogenous alleles of both aquaporins. AT25, like S47, possibly contains a functional AQY2-1 allele, and it contains at least one functional AQY1-1 allele (data not shown). Freeze tolerance was determined as the difference in glucose consumption between frozen and nonfrozen cells (RGC, expressed as a percentage), which is the most meaningful assay for yeast activity in frozen doughs. Overexpression of AQY1-1 or AQY2-1 clearly improved the RGC after prefermentation and freezing for both laboratory strains (data not shown) and industrial strains (Fig. 3A). Overexpression of the aquaporin genes at the moment of freeze treatment, i.e., 30 min after addition of glucose, was confirmed by Northern and Western blot analyses (data not shown). For neither of the two aquaporin genes did overexpression affect the growth rate, the length of the lag phase in YPD (Fig. 3B) or molasses (data not shown) medium, or the initial fermentation capacity (IGC) (Fig. 3A). The improvement of stress resistance appeared to be specific for freeze stress, since no effect of aquaporin overexpression was observed on the resistance of AT25 to heat (56°C), cold (4°C), ethanol (7.5%), osmotic stress, or salt (1.5 M NaCl, KCl, sorbitol) (data not shown).
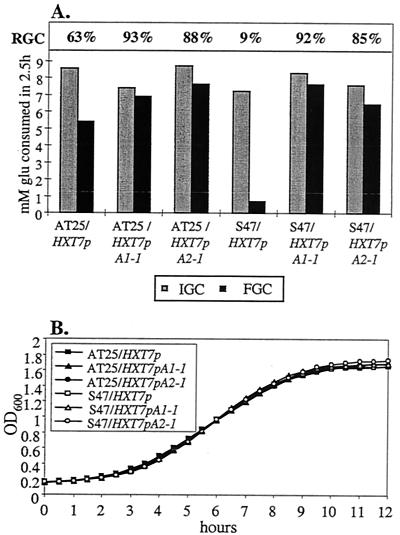
FIG. 3.
Overexpression of aquaporin-encoding genes improves freeze tolerance without affecting growth and initial fermentation rates. (A) IGC, FGC, and RGC were determined for S47 and AT25 overexpressing AQY1-1, S47 and AT25 overexpressing AQY2-1, and, as a control, S47 and AT25 with an integrated empty plasmid. The cells were either frozen (for 1 day at −30°C) (FGC) or not frozen (i.e., cooled on ice) (IGC) 30 min after the onset of fermentation by addition of 100 mM glucose. After thawing, glucose consumption was measured for 2.5 h to assess residual yeast activity. RGC is calculated as (FGC/IGC) × 100. Representative results are shown. Compared to AT25 containing an empty plasmid, AT25 AQY1-1 and AQY2-1 overexpression strains showed 1.5 (±0.1)- and 1.4 (±0.1)-times-higher RGCs, respectively. Compared to S47 containing an empty plasmid, S47 AQY1-1 and AQY2-1 overexpression strains showed 9.8 (±0.8)- and 9.0 (±1.2)-times-higher RGCs, respectively. (B) Growth of the same strains in YPD medium (Bioscreen measurements).
Deletion of AQY1-1 and AQY2-1 in a laboratory strain reduces freeze tolerance.
The freeze tolerances of AQY1-1 and AQY2-1 single- and double-deletion strains were determined in the laboratory strain background 10560-6B. Both nonfermenting and fermenting cells were tested. Freeze tolerance was measured as RGC in frozen versus nonfrozen cells. In nonfermenting cells (Fig. 4A), deletion of AQY1-1 reduced freeze tolerance, whereas this was not the case for AQY2-1. The double-deletion strain showed a freeze sensitivity similar to that of the AQY1-1 single-deletion strain. In fermenting cells (Fig. 4B), single deletion of either AQY1-1 or AQY2-1 reduced freeze tolerance, with the latter producing the largest effect. The double-deletion strain was more freeze sensitive than the single-deletion strains. These results appear to fit with the mRNA expression patterns of the aquaporin genes at the onset of fermentation (Fig. 4C). The AQY1-1 gene is highly expressed in nonfermenting cells and poorly expressed in glucose medium, while expression of AQY2-1 is very low in nonfermenting cells and increases after the addition of glucose. This is in accordance with the findings of recent expression studies of the two aquaporins using Northern blot analysis (18).
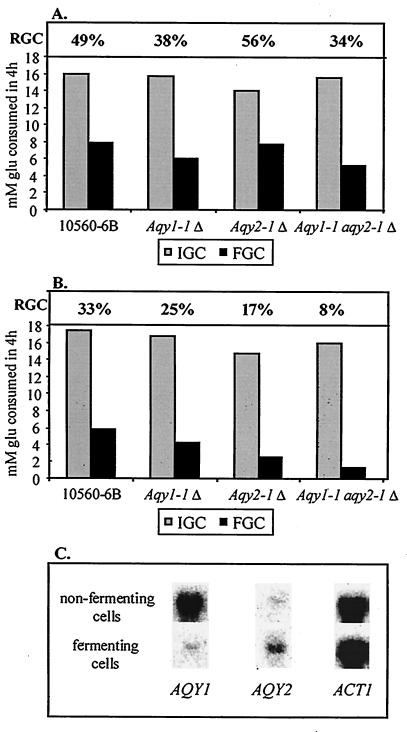
FIG. 4.
Deletion of aquaporin-encoding genes reduces freeze tolerance. (A and B) The effects of freezing on glucose consumption were measured in nonfermenting and fermenting cells of aquaporin single- and double-deletion mutants in the 10560-6B background. IGC, FGC, and RGC were determined for the wild-type strain, the aqy1Δ strain, the aqy2Δ strain, and the aqy1Δ aqy2Δ strain. The cells were either frozen (for 1 day at −30°C) (FGC) or not frozen (i.e., cooled on ice) (IGC) 30 min after resuspension of stationary-phase cells in YP (nonfermenting cells) (A) or 40 min after the subsequent addition of 200 mM glucose (fermenting cells) (B). After thawing, glucose consumption was measured for 4 h to assess residual yeast activity. RGC is calculated as (FGC/IGC) × 100. Representative results are shown. Compared to the wild-type strain 10560-6B, Aqy1-1, Aqy2-1, and double-deletion strains showed RGCs that were 0.7 (±0.1), 1.1 (±0.2), and 0.3 (±0.1) times higher, respectively, for nonfermenting cells and 0.6 (±0.1), 0.4 (±0.1), and 0.2 (±0.1) times higher, respectively, for fermenting cells. (C) Northern analysis of AQY1 and AQY2 expression in nonfermenting and fermenting wild-type 10560-6B cells. ACT1 was used as a loading control.
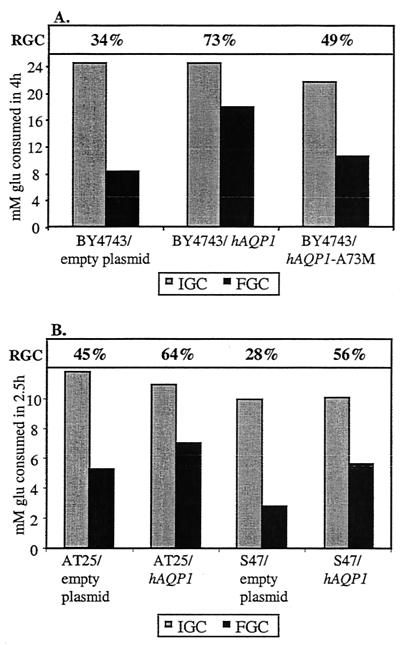
Overexpression of human aquaporin hAQP1 also enhances freeze tolerance in yeast, which is only partly the case for the poorly functional hAQP1-A73M allele.
To gain further evidence that the water transport capacity of cells is the true determinant of freeze resistance, the human aquaporin gene hAQP1 was overexpressed in yeast, as was a mutant allele encoding a water channel with impaired function. Laizé et al. have shown that hAQP1 was highly expressed, correctly localized, and active upon heterologous expression in yeast under the control of the inducible GAL10-CYC1 hybrid promoter (19). Essentially the same construct has been made with a mutant allele, hAQP1-A73M; this construct is localized in the membrane but is poorly functional (R. Bill and S. Hohmann, unpublished data). hAQP1 and its mutant allele hAQP1-A73M were expressed in strain BY4743, and freeze tolerance was determined for cells grown in YPGal, to obtain full induction of hAQP1, and also in YPD, where the GAL10-CYC1 promoter is repressed. Freeze tolerance, as determined by RGC in frozen versus nonfrozen cells, was significantly improved in galactose-grown cells expressing hAQP1 compared to that in cells transformed with an empty plasmid (Fig. 5A). In cells expressing the poorly functional hAQP1-A73M allele, only a partial effect was observed (Fig. 5A). In cells grown on glucose, there was no difference among the strains (data not shown). Also, overexpression of hAQP1 in the industrial strains S47 and AT25 improved freeze tolerance in comparison with that of strains transformed with an empty plasmid (Fig. 5B). Similarly, overexpression of the nonfunctional yeast AQY2-2 allele in several strain backgrounds failed to improve freeze tolerance (data not shown).
FIG. 5.
Heterologous overexpression of the human aquaporin gene hAQP1 improves freeze tolerance. (A) Overexpression of the wild-type gene, but not of the mutant allele hAQP1-A73M, improves freeze tolerance in a laboratory strain. IGC, FGC, and RGC were determined for strain BY4743 overexpressing either wild-type hAQP1 or the poorly active mutant hAQP1-A73M versus strain BY4743 transformed with an empty plasmid. Cells were either frozen (for 1 day at −30°C) (FGC) or not frozen (i.e., cooled on ice) (IGC) 40 min after the onset of fermentation by addition of 100 mM glucose. After thawing, glucose consumption was measured for 4 h to assess residual yeast activity. RGC is calculated as (FGC/IGC) × 100. Representative results are shown. Compared to that of BY4743 containing an empty plasmid, hAQP1 and hAQP1-A73M expression strains showed RGCs that were 2.3 (±0.2) and 1.5 (±0.1) times higher, respectively. (B) Overexpression of the human aquaporin gene hAQP1 improves freeze tolerance in industrial strains. IGC, FGC, and RGC were determined for strains AT25 and S47 overexpressing wild-type hAQP1 versus the respective strains transformed with an empty plasmid. The procedure described for panel A was used, except that cells were frozen or cooled on ice 30 min after the onset of fermentation. Representative results are shown. Compared to those of AT25 and S47 containing empty plasmids, hAQP1 expression strains showed RGCs that were 1.5 (±0.1) and 2.0 (±0.0) times higher, respectively.
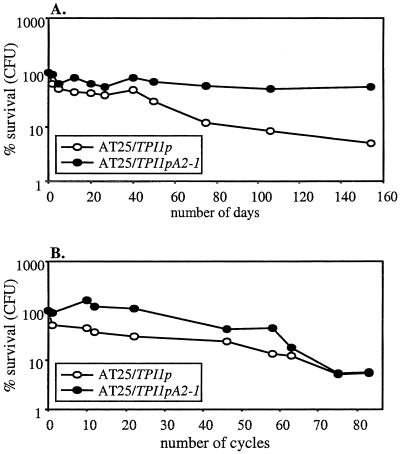
Overexpression of Aqy2-1 also provides protection to yeast in frozen doughs or in doughs submitted to multiple freeze-thaw cycles.
Routinely, yeast cell suspensions were used to determine freeze tolerance. However, to test whether the observed improvement of freeze tolerance by overexpression of aquaporins also applies to yeast in frozen dough conditions, small doughs were prepared with strain AT25 and with strain AT25 overexpressing the AQY2-1 gene and were either stored in frozen form or submitted to freeze-thaw cycles. Freeze tolerance was determined as the number of CFU with and without freezing of the doughs. The results clearly show that the strain overexpressing aquaporins survives better during storage in frozen doughs (Fig. 6A) as well as during most of the freeze-thaw cycling of the doughs (Fig. 6B).
FIG. 6.
Overexpression of functional aquaporins improves the freeze tolerance of yeast in dough. Shown is the survival of strain AT25 (open symbols) and that of strain AT25 overexpressing AQY2-1 (solid symbols) in small doughs during frozen storage (−30°C) (A) or in small doughs subjected to multiple freeze-thaw cycles (between −30°C and 30°C) (B). Survival was determined as the number of CFU isolated from the doughs relative to those from nonfrozen controls.
Improvement of freeze tolerance as a selection tool for isolation of aquaporin transformants.
An AT25 transformant overexpressing AQY2-1 could be isolated directly on the basis of better freeze-thaw survival by using six freeze-thaw cycles and PCR analysis of the surviving strains. Freeze-thaw selection on 23 aliquots, each containing about 4 × 107 cells, resulted in 23 surviving colonies (representing 2.5 × 10−6% survival), among which 1 strain contained the overexpression construct. The freeze resistance of this strain was similar to the freeze resistance of strain AT25/HXT7pA2-1 shown in Fig. 3A (data not shown). This implies that usage of an antibiotic selection marker is not required for the construction of freeze-resistant commercial yeast strains overexpressing aquaporins.
DISCUSSION
By use of genomewide gene expression analysis of different freeze-resistant and freeze-sensitive yeast strains, many genes were identified as differentially expressed (ratio, 2.5 or more) in at least two comparisons of a resistant and a sensitive strain: 67 genes were found to be expressed at higher levels, and 15 genes were found to be expressed at lower levels, in the resistant strains (data not shown). However, only six genes showed at least a 2.5-fold differential expression in all comparisons between a resistant and a sensitive strain (data not shown). Three of these genes were expressed at lower levels in resistant strains (ERG5, YHB1, and YGR154C), and three were expressed at higher levels in resistant strains (PLB2, CRH1, and CSI2). These differences in expression were confirmed by Northern analysis (data not shown). Intriguingly, five of the six genes are related to the cell membrane or cell wall, organelles that have always been regarded as the primary targets of freeze stress (38). ERG5 encodes a protein that catalyzes an intermediate step in the biosynthesis of ergosterol (37); PLB2 encodes a lysophospholipase for which it has been shown that overproduction causes a modest increase in total phospholipid content in late growth phase (6); YGR154C is an orphan gene, related to ECM4, encoding a protein possibly involved in cell wall structure or biosynthesis; CRH1 encodes a protein important for cell wall maintenance; and CSI2 encodes a protein involved in chitin synthesis. The other gene, YHB1, encodes a yeast flavohemoglobin. Expression of YHB1 seems to be repressed by a shift to high osmolarity (34), a stress that is inherent to the freezing process. However, neither individual overexpression (in the industrial strains S47 and AT25) nor individual deletion (in the laboratory strain BY4743) of the six genes resulted in significant effects on freeze tolerance (data not shown). In spite of this, the possibility that a particular combination of deletion and/or overexpression of several of these genes would affect freeze tolerance cannot be excluded. When aquaporin (AQY2-1) was overexpressed in strains with deletions of one of the genes YHB1, ERG5, and YGR154C, which were determined to be expressed at lower levels in the resistant strains examined, the effect of aquaporin overexpression was slightly more pronounced (data not shown). This supports the notion that freeze tolerance is a multifactorial property and that the presence or absence of certain gene products influences the effects of other gene products on freeze tolerance.
Expression of AQY2 was higher in most of the freeze-resistant strains. However, microarray analysis of the freeze-resistant mutant AT25 and its freeze-sensitive parent, S47, revealed lower expression of AQY2 in the resistant strain at the onset of fermentation. Also, when pools of total RNA from several other freeze-resistant versus freeze-sensitive strains were previously compared for AQY2 expression at the onset of fermentation, no clear differential expression was observed (41). Moreover, according to restriction analysis, the AQY2 gene appears to be a nonfunctional gene in the AT25 background (data not shown). This would indicate that the higher freeze tolerance of AT25 than of S47 is probably not primarily due to differential AQY2 expression. However, from the restriction analysis, the possibility that a particular AQY2-allele(s) in these strains encodes a functional water channel cannot be excluded. Only the cloning of all of the AQY2 alleles present and a subsequent test in X. laevis oocytes for water transport capacity could answer this question. Expression of AQY1 before the addition of glucose (Fig. 4C) could perhaps still influence resistance 30 min after the onset of fermentation, but no differential expression between AT25 and S47 could be detected under nonfermenting conditions (data not shown). Altogether, the possibility that the aquaporin genes have been identified “by accident” in the screening for genes with importance in freeze resistance cannot be excluded. It is very likely that other factors in addition to aquaporins also influence freeze tolerance in yeast at the onset of fermentation.
The aquaporin genes AQY1 and AQY2 were found to be important determinants of freeze resistance: overexpression improved freeze tolerance in laboratory (data not shown) and industrial (Fig. 3A) yeast strains, whereas deletion reduced freeze tolerance in a laboratory strain (Fig. 4A and B). Although it has been shown that both Aqy1-1 and Aqy2-1 mediate water transport (3, 26), it could in principle not be excluded that the yeast aquaporins influenced freeze tolerance in a manner unrelated to their water transport activity, for instance, by affecting membrane properties, such as membrane fluidity, that could affect water permeability (21). The fact that overexpression of the nonfunctional yeast AQY2-2 allele in several strain backgrounds did not improve freeze tolerance (data not shown) did not reliably exclude this possibility, since proper membrane localization has never been shown for this particular protein. Therefore, the human aquaporin gene hAQP1 was overexpressed in yeast, as was a mutant allele encoding a water channel with impaired function. Overexpression of the human aquaporin gene hAQP1 enhanced freeze tolerance in yeast, which was only partly the case for the poorly functional hAQP1-A73M allele (Fig. 5). These results support the notion that a rapid, osmotically driven water efflux from the cells during the initial freezing process lowers the intracellular water content and as a result reduces subsequent ice crystal formation upon freezing of the protoplasm (25). Higher levels of aquaporins in the plasma membrane would allow faster water efflux, especially at freezing temperatures, at which water diffusion through the phospholipid layer of the membrane is much slower than at higher temperatures. Because reduction of ice crystal formation results in reduced destruction of cellular membranes and other components, it allows the cells to maintain higher activity and viability. This explanation is in accordance with previous observations that the protective effects of ethanol and methanol against freeze damage correlate with their stimulating effects on membrane permeability, presumably allowing faster water efflux during freezing (22). Since aquaporin-mediated protection was specific for freeze stress (data not shown), the effect can apparently not be attributed to an improvement in general stress tolerance of the cells but appears to be due to a more specific mechanism, such as the stimulation of rapid water efflux from the cells.
The passive diffusion rate of water through membranes is in general relatively rapid (compared to those of other small hydrophilic molecules), and because of the high surface-to-volume ratios of microorganisms, one would not expect the water permeability of the plasma membrane to be rate limiting under most conditions. However, it has already been suggested that in microorganisms particular conditions might exist where water permeability would be limiting and therefore the presence of water channels would be advantageous (13). No such condition has yet been identified, and no well-defined phenotype indicative of a physiological function of any microbial aquaporin has yet been described. Our results indicate a possible novel function for water channels in microorganisms: aquaporins apparently help to increase the freeze tolerance of the cells by supporting rapid water efflux during initial freezing. Such a function would also fit with the apparently low selective advantage of functional aquaporins in yeast under laboratory conditions (3). Whereas nowadays yeast strains are routinely stored at −80°C in glycerol, in the past yeast strains were stored on agar slants and from time to time were reinoculated on fresh slants. Hence, laboratory strains normally never experience freeze stress, as strains in nature do under freezing conditions. This might explain why so few laboratory strains have maintained functional aquaporin alleles. The same applies to industrial yeast strains and even to some natural isolates which appear to have lost functional AQY2 alleles (20). There are probably other functions in yeast cells as well that confer a selective advantage only under highly specific natural conditions but not under other conditions, in particular those used for laboratory cultivation of yeast. Many laboratory strains, for instance, carry the same FLO8 mutation causing a defect in flocculation, and the capacity for pseudohyphal growth is also known to be deficient in most laboratory strains (23).
Since overexpression of Aqy2-1 also provides protection to yeast in frozen doughs or in doughs submitted to multiple freeze-thaw cycles (Fig. 6), this modification could be a convenient way to improve the freeze tolerance of commercial baker's yeast strains for use in frozen dough applications. In this context it is important that other commercially important properties such as the growth rate (Fig. 3B) and initial fermentation capacity (Fig. 3A) of the aquaporin overexpression strains were not affected. Construction of commercial baker's yeast strains overexpressing aquaporins normally requires the use of a dominant selection marker to identify the transformants. Generally, antibiotic resistance markers are used for that purpose. However, the use of antibiotic resistance markers in foodstuffs is controversial (9). We succeeded in isolating an AT25 transformant overexpressing AQY2-1 directly on the basis of better freeze-thaw survival, implying that usage of an antibiotic selection marker is not required for the construction of commercial yeast strains overexpressing aquaporins. This could facilitate the introduction of such strains on the market. Moreover, our results also imply that overexpression of a yeast aquaporin gene can be used as a selection marker for the construction of transformants of industrial yeast strains. Up to now no phenotype clearly indicative of a physiological function could be detected in yeast strains with aquaporin overexpression, except for the improvement of freeze tolerance as reported in this paper. Hence, it appears that aquaporin overexpression is unlikely to interfere with commercially important properties of industrial yeast strains.
In conclusion, our results show that genomewide microarray expression analysis can be used for the identification of genes relevant for a specific phenotype. They show that aquaporin expression influences the freeze tolerance of yeast cells, which appears to be the first clear physiological function identified for microbial aquaporins. Since aquaporin overexpression significantly improved the maintenance of viability of industrial yeast strains upon freezing and seems to have little effect on other yeast properties, it appears to be a promising tool for improvement of freeze tolerance in commercial baker's yeast strains.
Acknowledgments
This work was supported by a fellowship from the Institute for Scientific and Technological research (IWT) to An Tanghe and by grants from the Flemish Interuniversity Institute of Biotechnology (VIB/PRJ2), the Fund for Scientific Research—Flanders, and the Research Fund of the Katholieke Universiteit Leuven (Concerted Research Actions) to J.M.T. S.H. is a special researcher supported by Vetenskapsrådet, Stockholm, Sweden. Aquaporin research in S.H.'s laboratory is supported by the European Commission via grants BIO4-CT98-0024, FMRX-CT97-0128, and QLK3-2000-00778.
We are grateful to Renata Wicik for excellent technical assistance. We also thank Vincent Laizé, Roslyn Bill, and Frederic Sidoux-Walter for kindly providing strains, plasmids, and information, and we thank Markus Tamás for critical reading of the manuscript.
REFERENCES
- 1.Almeida, M. J., and C. Pais. 1996. Leavening ability and freeze tolerance of yeasts isolated from traditional corn and rye bread doughs. Appl. Environ. Microbiol. 62:4401-4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attfield, P. V. 1997. Stress tolerance: the key to effective strains of industrial baker's yeast. Nat. Biotechnol. 15:1351-1357. [DOI] [PubMed] [Google Scholar]
- 3.Bonhivers, M., J. M. Carbrey, S. J. Gould, and P. Agre. 1998. Aquaporins in Saccharomyces—genetic and functional distinctions between laboratory and wild-type strains. J. Biol. Chem. 273:27565-27572. [DOI] [PubMed] [Google Scholar]
- 4.Brachmann, C. B., A. Davies, C. J. Cost, E. Caputo, J. Li, P. Hieter, and J. D. Boeke. 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115-132. [DOI] [PubMed] [Google Scholar]
- 5.Calamita, G., B. Kempf, M. Bonhivers, W. R. Bishai, E. Bremer, and P. Agre. 1998. Regulation of the Escherichia coli water channel gene AqpZ. Proc. Natl. Acad. Sci. USA 95:3627-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fyrst, H., B. Oskouian, F. A. Kuypers, and J. D. Saba. 1999. The PLB2 gene of Saccharomyces cerevisiae confers resistance to lysophosphatidylcholine and encodes a phospholipase B/lysophospholipase. Biochemistry 38:5864-5871. [DOI] [PubMed] [Google Scholar]
- 7.Güldener, U., S. Heck, T. Fiedler, J. Beinhauer, and J. H. Hegemann. 1996. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24:2519-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn, Y. S., and H. Kawai. 1990. Isolation and characterization of freeze-tolerant yeasts from nature available for the frozen-dough method. Agric. Biol. Chem. 54:829-831. [Google Scholar]
- 9.Halford, N. G., and P. R. Shewry. 2000. Genetically modified crops: methodology, benefits, regulation and public concerns. Br. Med. Bull. 56:62-73. [DOI] [PubMed] [Google Scholar]
- 10.Hauf, J., F. K. Zimmermann, and S. Müller. 2000. Simultaneous genomic overexpression of seven glycolytic enzymes in the yeast Saccharomyces cerevisiae. Enzyme Microb. Technol. 26:688-698. [DOI] [PubMed] [Google Scholar]
- 11.Hino, A., K. Mihara, K. Nakashima, and H. Takano. 1990. Trehalose levels and survival ratio of freeze-tolerant versus freeze-sensitive yeasts. Appl. Environ. Microbiol. 56:1386-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hino, A., H. Takano, and Y. Tanaka. 1987. New freeze-tolerant yeast for frozen dough preparations. Cereal Chem. 64:269-275. [Google Scholar]
- 13.Hohmann, S., R. M. Bill, G. Kayingo, and B. A. Prior. 2000. Microbial MIP channels. Trends Microbiol. 8:33-38. [DOI] [PubMed] [Google Scholar]
- 14.Kaul, S. C., K. Obuchi, H. Iwahashi, and Y. Komatsu. 1992. Cryoprotection provided by heat shock treatment in Saccharomyces cerevisiae cells: induction of a 33 kDa protein and protection against freezing injury. Cell. Mol. Biol. 38:135-143. [PubMed] [Google Scholar]
- 15.Kayingo, G., R. M. Bill, G. Calamita, S. Hohmann, and B. A. Prior. 2001. Microbial water channels and glycerol facilitators. Curr. Top. Membr. 51:335-370. [Google Scholar]
- 16.Kim, J., P. Alizadeh, T. Harding, A. Hefnergravink, and D. J. Klionsky. 1996. Disruption of the yeast ATH1 gene confers better survival after dehydration, freezing, and ethanol shock: potential commercial applications. Appl. Environ. Microbiol. 62:1563-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraakman, L., K. Lemaire, P. Ma, A. W. R. H. Teunissen, M. C. V. Donaton, P. Van Dijck, J. Winderickx, J. H. de Winde, and J. M. Thevelein. 1999. A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol. Microbiol. 32:1002-1012. [DOI] [PubMed] [Google Scholar]
- 18.Laizé, V., M. C. De Jesus Ferreira, and S. Hohmann. 2000. Aquaporin water channels in Saccharomyces cerevisiae. Function and expression studies, p. 415-421. In S. Hohmann and S. Nielsen (ed.), Molecular biology and physiology of water and solute transport. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 19.Laizé, V., G. Rousselet, J. M. Verbavatz, V. Berthonaud, R. Gobin, N. Roudier, L. Abrami, P. Ripoche, and F. Tacnet. 1995. Functional expression of the human CHIP28 water channel in a yeast secretory mutant. FEBS Lett. 373:269-274. [DOI] [PubMed] [Google Scholar]
- 20.Laizé, V., F. Tacnet, P. Ripoche, and S. Hohmann. 2000. Polymorphism of Saccharomyces cerevisiae aquaporins. Yeast 16:897-903. [DOI] [PubMed] [Google Scholar]
- 21.Lande, M. B., J. M. Donovan, and M. L. Zeidel. 1995. The relationship between membrane fluidity and permeabilities to water, solutes, ammonia and protons. J. Gen. Physiol. 106:67-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis, J. G., R. P. Learmonth, and K. Watson. 1994. Cryoprotection of yeast by alcohols during rapid freezing. Cryobiology 31:193-198. [DOI] [PubMed] [Google Scholar]
- 23.Liu, H., C. A. Styles, and G. R. Fink. 1996. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics 144:967-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luyten, K., J. Albertyn, W. F. Skibbe, B. A. Prior, J. Ramos, J. M. Thevelein, and S. Hohmann. 1995. Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J. 14:1360-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazur, P. 1970. Cryobiology: the freezing of biological systems. Science 168:939-949. [DOI] [PubMed] [Google Scholar]
- 26.Meyrial, V., V. Laizé, R. Gobin, P. Ripoche, S. Hohmann, and F. Tacnet. 2001. Existence of a tightly regulated water channel in Saccharomyces cerevisiae. Eur. J. Biochem. 268:334-343. [DOI] [PubMed] [Google Scholar]
- 27.Murakami, Y., K. Yokoigawa, F. Kawai, and H. Kawai. 1996. Lipid composition of commercial bakers' yeasts having different freeze-tolerance in frozen dough. Biosci. Biotechnol. Biochem. 60:1874-1876. [DOI] [PubMed] [Google Scholar]
- 28.Nagodawithana, T. W., and N. B. Trivedi. 1990. Yeast selection for baking, p. 139-184. In C. J. Panchal (ed.), Yeast strain selection. Marcel Dekker, Inc., New York, N.Y.
- 29.Nakagawa, S., and K. Ouchi. 1994. Construction from a single parent of baker's yeast strains with high freeze tolerance and fermentative activity in both lean and sweet doughs. Appl. Environ. Microbiol. 60:3499-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park, J. H., and M. H. Saier, Jr. 1996. Phylogenetic characterization of the MIP family of transmembrane channel proteins. J. Membr. Biol. 153:171-180. [DOI] [PubMed] [Google Scholar]
- 31.Park, J. I., C. M. Grant, P. V. Attfield, and I. W. Dawes. 1997. The freeze-thaw stress response of the yeast Saccharomyces cerevisiae is growth phase specific and is controlled by nutritional state via the RAS-cyclic AMP signal. Appl. Environ. Microbiol. 63:3818-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pompon, D. 1988. cDNA cloning and functional expression in Saccharomyces cerevisiae of beta-naphtoflavone-induced rabbit liver P-450 LM4 and LM6. Eur. J. Biochem. 177:285-293. [DOI] [PubMed] [Google Scholar]
- 33.Randez-Gil, F., P. Sanz, and J. A. Prieto. 1999. Engineering baker's yeast: room for improvement. Trends Biotechnol. 17:237-244. [DOI] [PubMed] [Google Scholar]
- 34.Rep, M., M. Krantz, J. M. Thevelein, and S. Hohmann. 2000. The transcriptional response of Saccharomyces cerevisiae to osmotic shock—Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem. 275:8290-8300. [DOI] [PubMed] [Google Scholar]
- 35.Rose, A. H., and G. Vijayalakshmi. 1993. Baker's yeasts, p. 357-397. In A. H. Rose and J. S. Harrison (ed.), Yeast technology, vol. 5. Academic Press, New York, N.Y..
- 36.Shima, J., A. Hino, C. Yamada-Iyo, Y. Suzuki, R. Nakajima, H. Watanabe, K. Mori, and H. Takano. 1999. Stress tolerance in doughs of Saccharomyces cerevisiae trehalase mutants derived from commercial baker's yeast. Appl. Environ. Microbiol. 65:2841-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skaggs, B. A., J. F. Alexander, C. A. Pierson, K. S. Schweitzer, K. T. Chun, C. Koegel, R. Barbuch, and M. Bard. 1996. Cloning and characterization of the Saccharomyces cerevisiae C-22 sterol desaturase gene, encoding a second cytochrome P-450 involved in ergosterol biosynthesis. Gene. 169:105-109. [DOI] [PubMed] [Google Scholar]
- 38.Steponkus, P. L. 1984. Role of the plasma membrane in freezing injury and cold acclimation. Annu. Rev. Plant Physiol. 35:543-584. [Google Scholar]
- 39.Takagi, H., F. Iwamoto, and S. Nakamori. 1997. Isolation of freeze-tolerant laboratory strains of Saccharomyces cerevisiae from proline-analogue-resistant mutants. Appl. Microbiol. Biotechnol. 47:405-411. [DOI] [PubMed] [Google Scholar]
- 40.Tamás, M. J., K. Luyten, F. C. W. Sutherland, A. Hernandez, J. Albertyn, H. Valadi, H. Li, B. A. Prior, S. G. Killan, J. Ramos, L. Gustafsson, J. M. Thevelein, and S. Hohmann. 1999. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol. Microbiol. 31:1087-1104. [DOI] [PubMed] [Google Scholar]
- 41.Tanghe, A., A. Teunissen, P. Van Dijck, and J. M. Thevelein. 2000. Identification of genes responsible for improved cryoresistance in fermenting yeast cells. Int. J. Food Microbiol. 55:259-262. [DOI] [PubMed] [Google Scholar]
- 41a.Teunissen, A., F. Dumortier, M.-F. Gorwa, J. Bauer, A. Tanghe, A. Loïez, P. Smet, P. Van Dijck, and J. M. Thevelein. 2000. Isolation and characterization of a freeze-tolerant diploid derivative of an industrial baker’s yeast strain and its use in frozen doughs. Appl. Environ. Microbiol. 68:4780-4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thevelein, J. M., L. Cauwenberg, S. Colombo, J. H. de Winde, M. Donaton, F. Dumortier, L. Kraakman, K. Lemaire, P. Ma, D. Nauwelaers, F. Rolland, A. Teunissen, P. Van Dijck, M. Versele, S. Wera, and J. Winderickx. 2000. Nutrient-induced signal transduction through the protein kinase A pathway and its role in the control of metabolism, stress resistance and growth in yeast. Enzyme Microb. Technol. 26:819-825. [DOI] [PubMed] [Google Scholar]
- 43.Thevelein, J. M., and J. H. de Winde. 1999. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 33:904-918. [DOI] [PubMed] [Google Scholar]
- 44.Thomas, B. J., and R. J. Rothstein. 1989. Elevated recombination rates in transcriptionally active DNA. Cell 56:619-630. [DOI] [PubMed] [Google Scholar]
- 45.Van Dijck, P., D. Colavizza, P. Smet, and J. M. Thevelein. 1995. Differential importance of trehalose in stress resistance in fermenting and nonfermenting Saccharomyces cerevisiae cells. Appl. Environ. Microbiol. 61:109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Dijck, P., P. Ma, M. Versele, M.-F. Gorwa, S. Colombo, K. Lemaire, D. Bossi, A. Loïez, and J. M. Thevelein. 2000. A baker's yeast mutant (fil1) with a specific, partially inactivating mutation in adenylate cyclase maintains a high stress resistance during active fermentation and growth. J. Mol. Microbiol. Biotechnol. 2:521-530. [PubMed] [Google Scholar]