Abstract
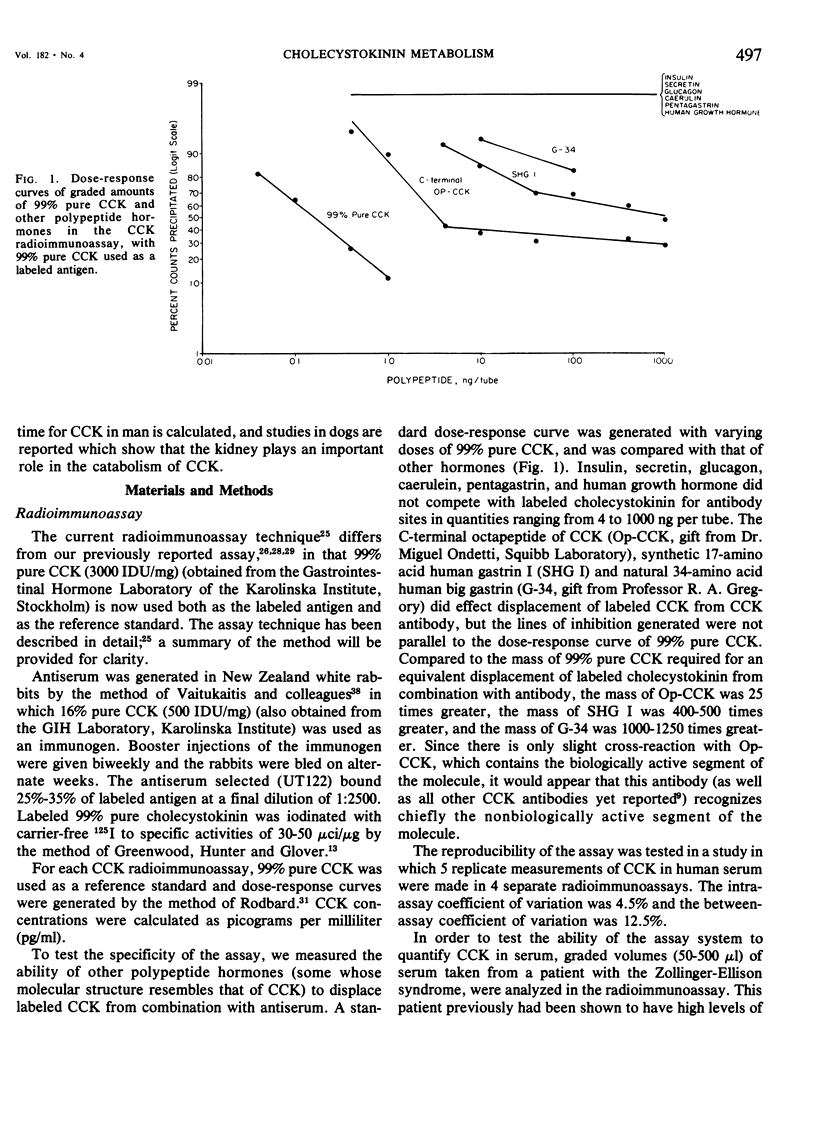
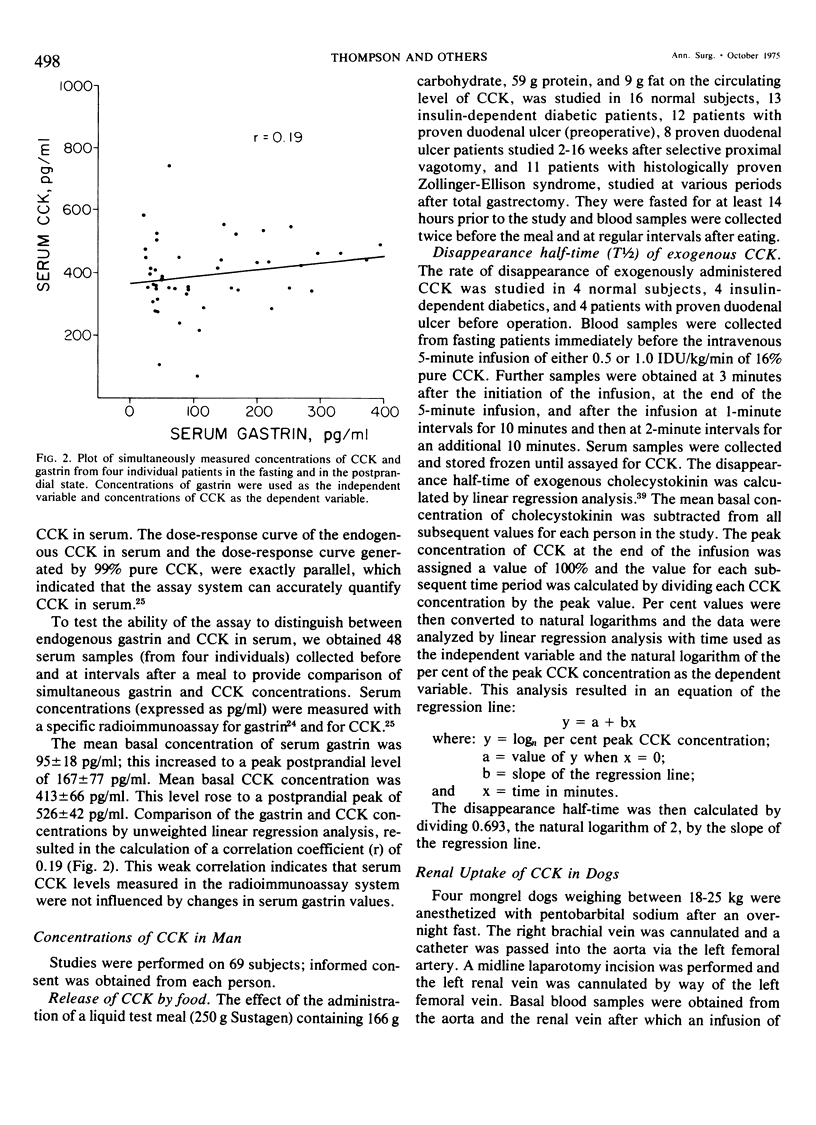
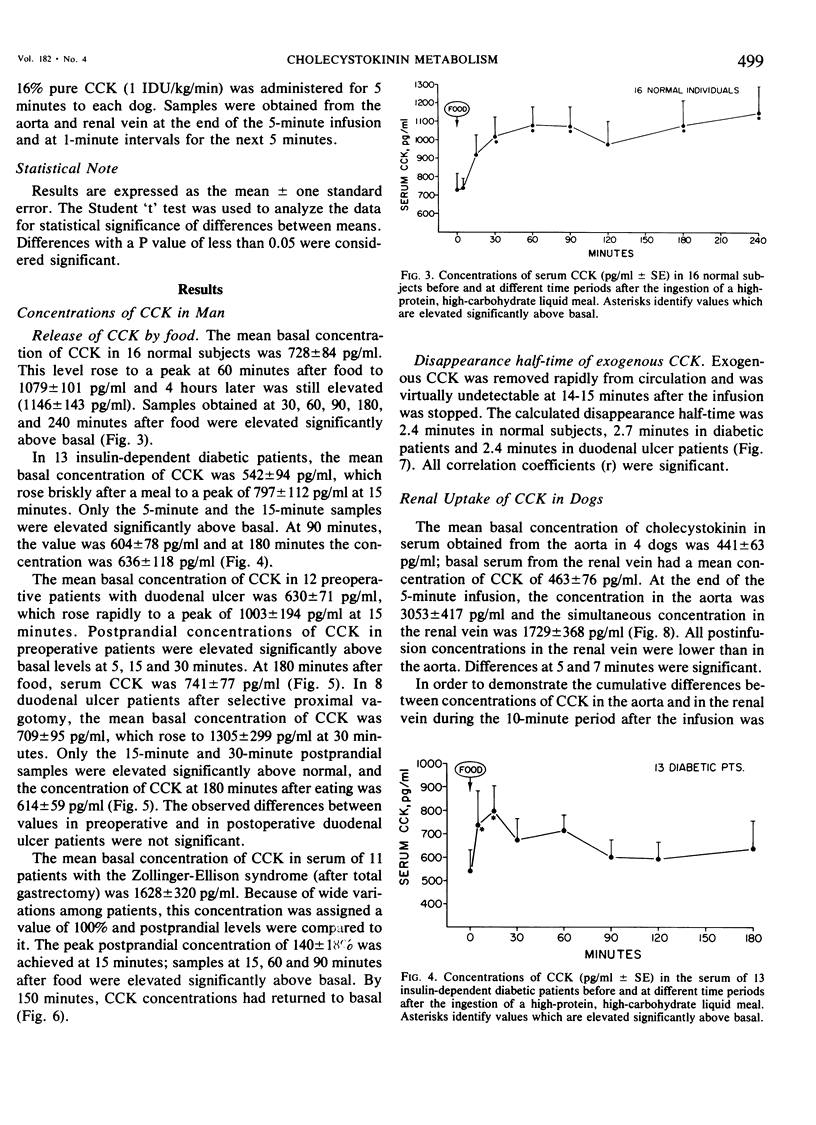
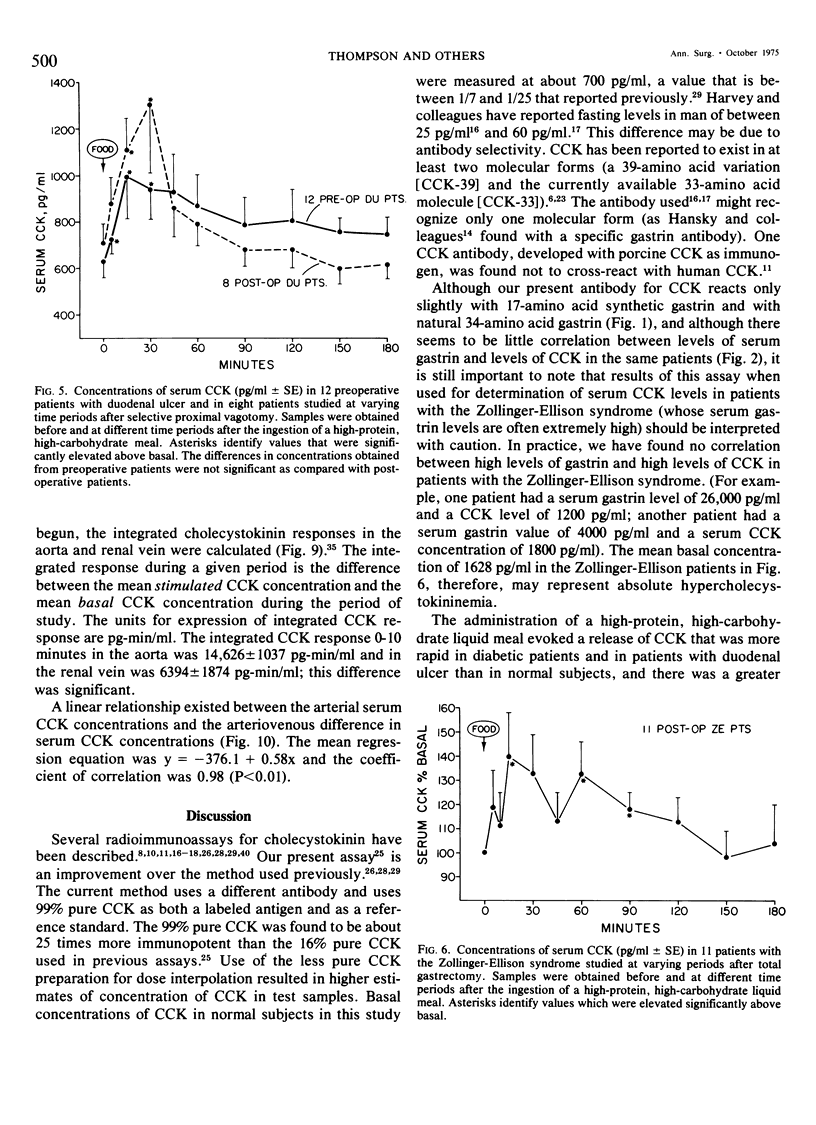
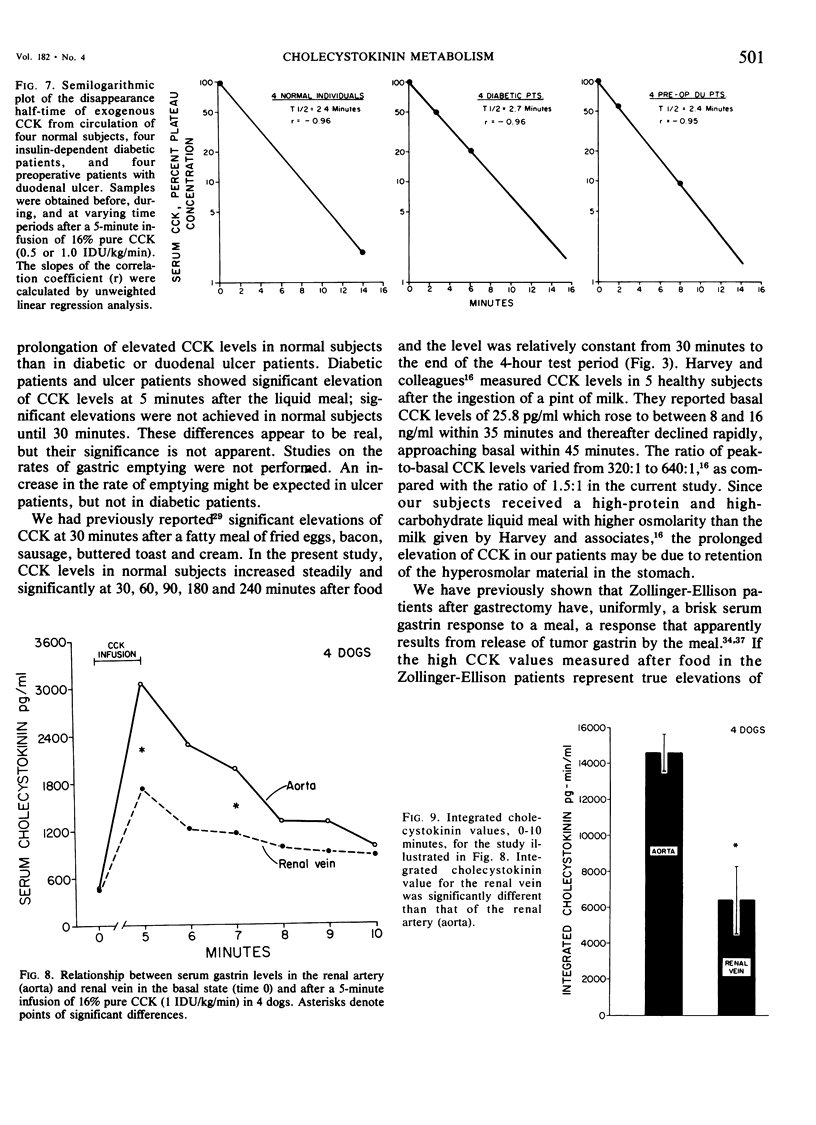
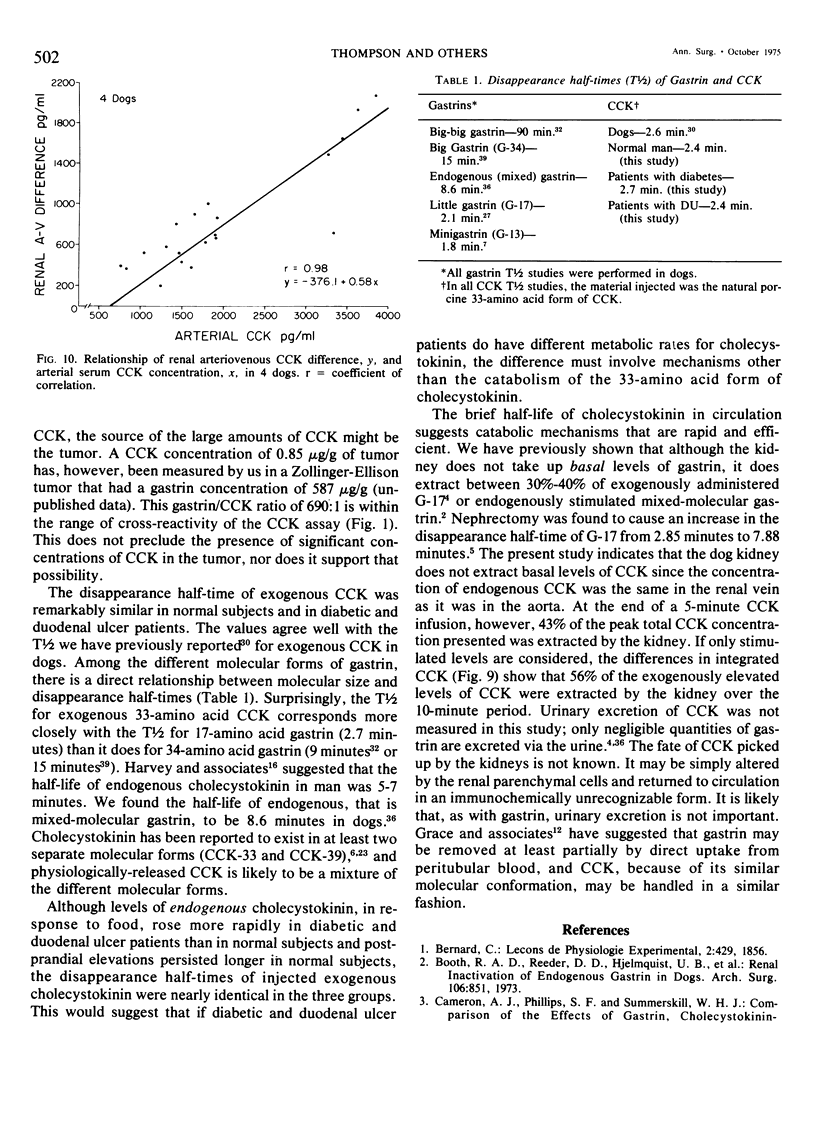
We have developed a sensitive, specific and reproducible radioimmunoassay for cholecystokinin (CCK) with which basal levels of CCK of between 400-800 pg/ml have been measured in normal man, in patients with diabetes and with duodenal ulcer disease, and in normal dogs. After a meal, circulating levels of CCK rose to 1000-1200 pg/ml in human subjects. Release of CCK was more rapid in diabetic and duodenal ulcer patients than in normal subjects, but elevated postprandial levels persisted much longer in normal subjects. Patients with the Zollinger-Ellison syndrome had elevated values of cholecystokinin which rose after a meal. Lack of correlation between elevated basal levels of gastrin and CCK in patients with the Zollinger-Ellison syndrome suggest that the hypercholecystokininemia may be absolute. The disappearance half-time of exogenous CCK was about 21/2 minutes in normal subjects as well as in diabetic and duodenal ulcer patients. Studies in dogs demonstrated no uptake of basal levels of cholecystokinin by the kidney; on infusion of exogenous CCK-33, the kidney extracted 43% of the total CCK presented and 56% of the integrated CCK. We conclude that: 1) circulating basal and postprandial levels of CCK may be measured in a reproducible fashion; 2) postprandial release of CCK is more rapid in diabetic and duodenal ulcer patients than in normal man; 3) the disappearance half-time of exogenous CCK in man and dogs is about 21/2 minutes; 4) the kidney is a major site for uptake of CCK.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth R. A., Reeder D. D., Hjelmquist U. B., Brandt E. N., Jr, Thompson J. C. Renal inactivation of endogenous gastrin in dogs. Arch Surg. 1973 Jun;106(6):851–854. doi: 10.1001/archsurg.1973.01350180085024. [DOI] [PubMed] [Google Scholar]
- Cameron A. J., Phillips S. F., Summerskill W. H. Comparison of effects of gastrin, cholecystokinin-pancreozymin, secretin, and glucagon on human stomach muscle in vitro. Gastroenterology. 1970 Oct;59(4):539–545. [PubMed] [Google Scholar]
- Clendinnen B. G., Davidson W. D., Reeder D. D., Jackson B. M., Thompson J. C. Renal uptake and excretion of gastrin in the dog. Surg Gynecol Obstet. 1971 Jun;132(6):1039–1043. [PubMed] [Google Scholar]
- Clendinnen B. G., Reeder D. D., Brandt E. N., Jr, Thompson J. C. Effect of nephrectomy on the rate and pattern of the disappearance of exogenous gastrin in dogs. Gut. 1973 Jun;14(6):462–467. doi: 10.1136/gut.14.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debas H. T., Grossman M. I. Pure cholecystokinin: pancreatic protein and bicarbonate response. Digestion. 1973;9(6):469–481. doi: 10.1159/000197476. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go V. L., Ryan R. J., Summerskill W. H. Radioimmunoassay of porcine cholecystokinin-pancreozymin. J Lab Clin Med. 1971 Apr;77(4):684–689. [PubMed] [Google Scholar]
- Grace S. G., Davidson W. D., State D. Renal mechanisms for removal of gastrin from the circulation. Surg Forum. 1974;25(0):323–325. [PubMed] [Google Scholar]
- Hansky J., Soveny C., Korman M. G. Studies with two gastrin antisera of different specificity for gastrins I and II. Digestion. 1974;10(2):97–107. doi: 10.1159/000197528. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Raper H. S. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol. 1943 Jun 30;102(1):115–125. doi: 10.1113/jphysiol.1943.sp004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. F., Dowsett L., Hartog M., Read A. E. A radioimmunoassay for cholecystokinin-pancreozymin. Lancet. 1973 Oct 13;2(7833):826–828. doi: 10.1016/s0140-6736(73)90863-5. [DOI] [PubMed] [Google Scholar]
- Harvey R. F., Dowsett L., Hartog M., Read A. E. Radioimmunoassay of cholecystokinin-pancreozymin. Gut. 1974 Sep;15(9):690–699. doi: 10.1136/gut.15.9.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorpes J. E. The isolation and chemistry of secretin and cholecystokinin. Gastroenterology. 1968 Aug;55(2):157–164. [PubMed] [Google Scholar]
- Mutt V., Jorpes J. E. Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur J Biochem. 1968 Oct 17;6(1):156–162. doi: 10.1111/j.1432-1033.1968.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Reeder D. D., Becker H. D., Smith N. J., Rayford P. L., Thompson J. C. Measurement of endogenous release of cholecystokinin by radioimmunoassay. Ann Surg. 1973 Sep;178(3):304–310. doi: 10.1097/00000658-197309000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder D. D., Becker H. D., Smith N. J., Rayford P. L., Thompson J. C. Radioimmunoassay of cholecystokinin. Surg Forum. 1972;23(0):361–362. [PubMed] [Google Scholar]
- Reeder D. D., Jackson B. M., Brandt E. N., Jr, Thompson J. C. Rate and pattern of disappearance of exogenous gastrin in dogs. Am J Physiol. 1972 Jun;222(6):1571–1574. doi: 10.1152/ajplegacy.1972.222.6.1571. [DOI] [PubMed] [Google Scholar]
- Straus E., Yalow R. S. Studies on the distribution and degradation of heptadecapeptide, big, and big big gastrin. Gastroenterology. 1974 May;66(5):936–943. [PubMed] [Google Scholar]
- Thompson J. C., Reeder D. D., Bunchman H. H., Becker H. D., Brandt E. N., Jr Effect of secretin on circulating gastrin. Ann Surg. 1972 Sep;176(3):384–393. doi: 10.1097/00000658-197209000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. C., Reeder D. D., Bunchman H. H. Clinical role of serum gastrin measurements in the Zollinger-Ellison syndrome. Am J Surg. 1972 Aug;124(2):250–261. doi: 10.1016/0002-9610(72)90022-0. [DOI] [PubMed] [Google Scholar]
- Thompson J. C., Reeder D. D., Villar H. V., Fender H. R. Natural history and experience with diagnosis and treatment of the Zollinger-Ellison syndrome. Surg Gynecol Obstet. 1975 May;140(5):721–739. [PubMed] [Google Scholar]
- Vaitukaitis J., Robbins J. B., Nieschlag E., Ross G. T. A method for producing specific antisera with small doses of immunogen. J Clin Endocrinol Metab. 1971 Dec;33(6):988–991. doi: 10.1210/jcem-33-6-988. [DOI] [PubMed] [Google Scholar]
- Walsh J. H., Debas H. T., Grossman M. I. Pure human big gastrin. Immunochemical properties, disappearance half time, and acid-stimulating action in dogs. J Clin Invest. 1974 Aug;54(2):477–485. doi: 10.1172/JCI107783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Lazarus L., Chisholm D. J. Radioimmunoassay of pancreozymin cholecystokinin in human serum. J Nucl Med. 1969 Dec;10(12):743–745. [PubMed] [Google Scholar]


