Abstract
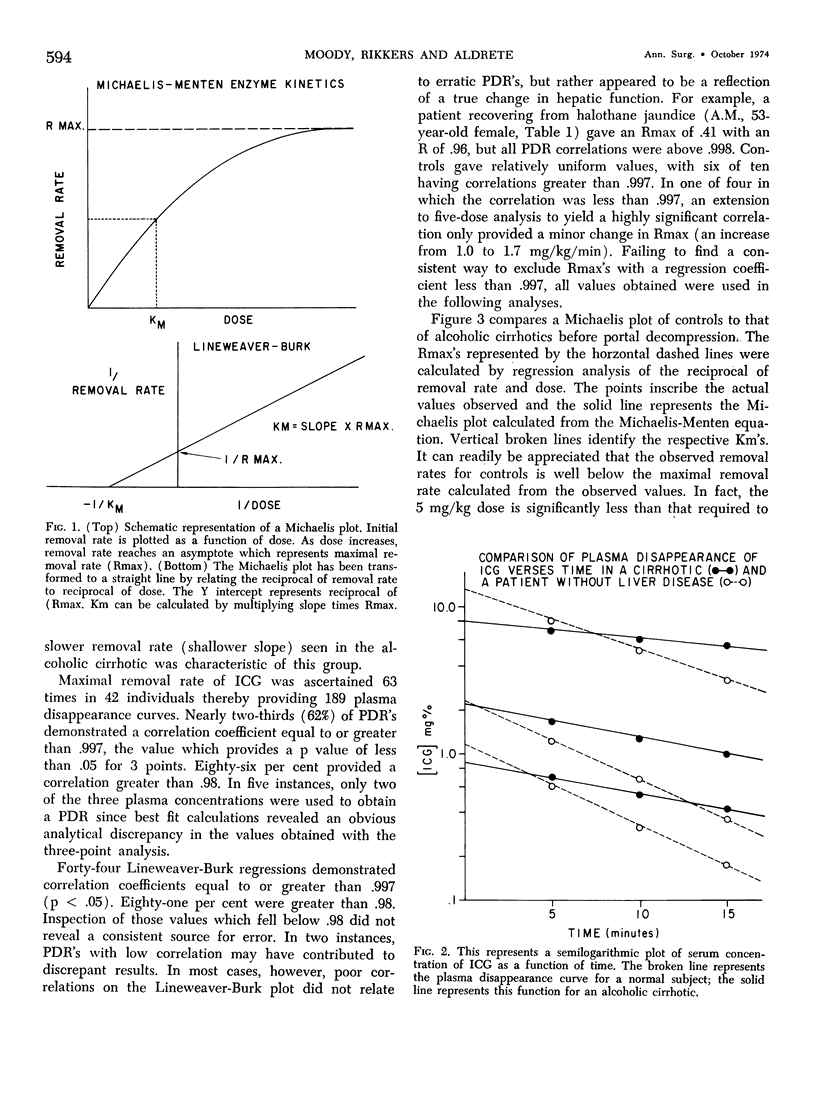
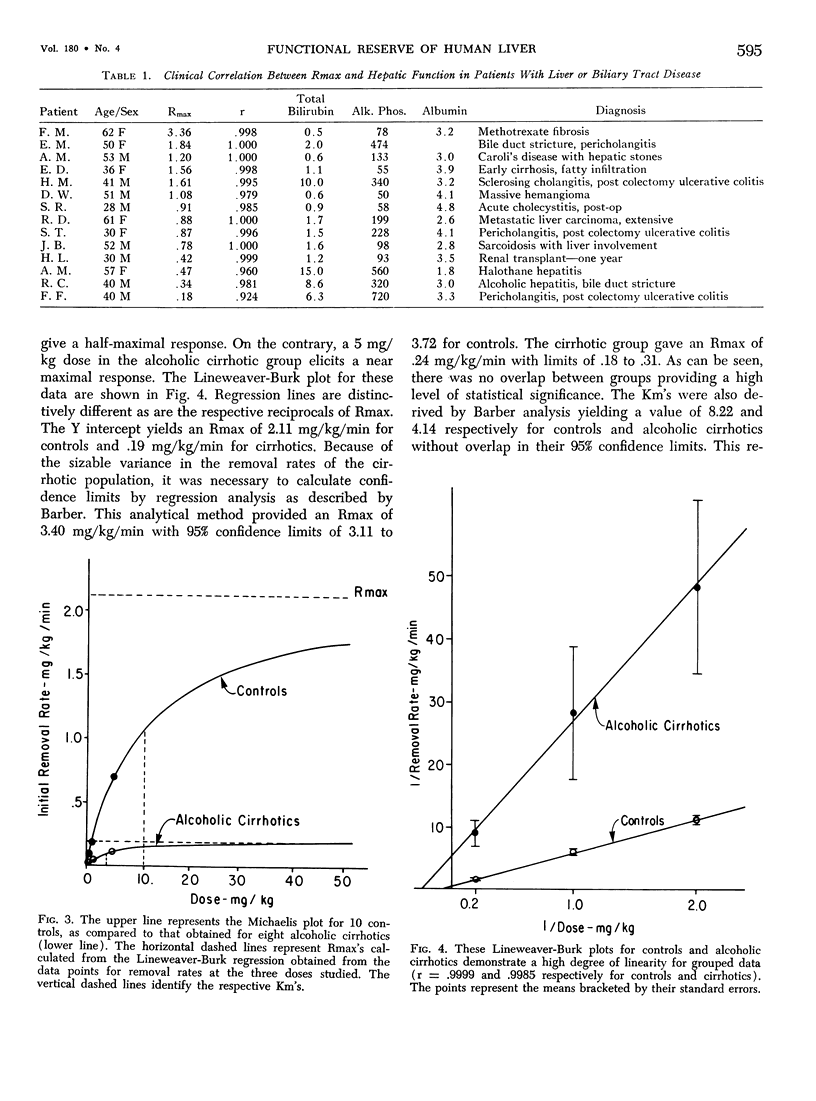
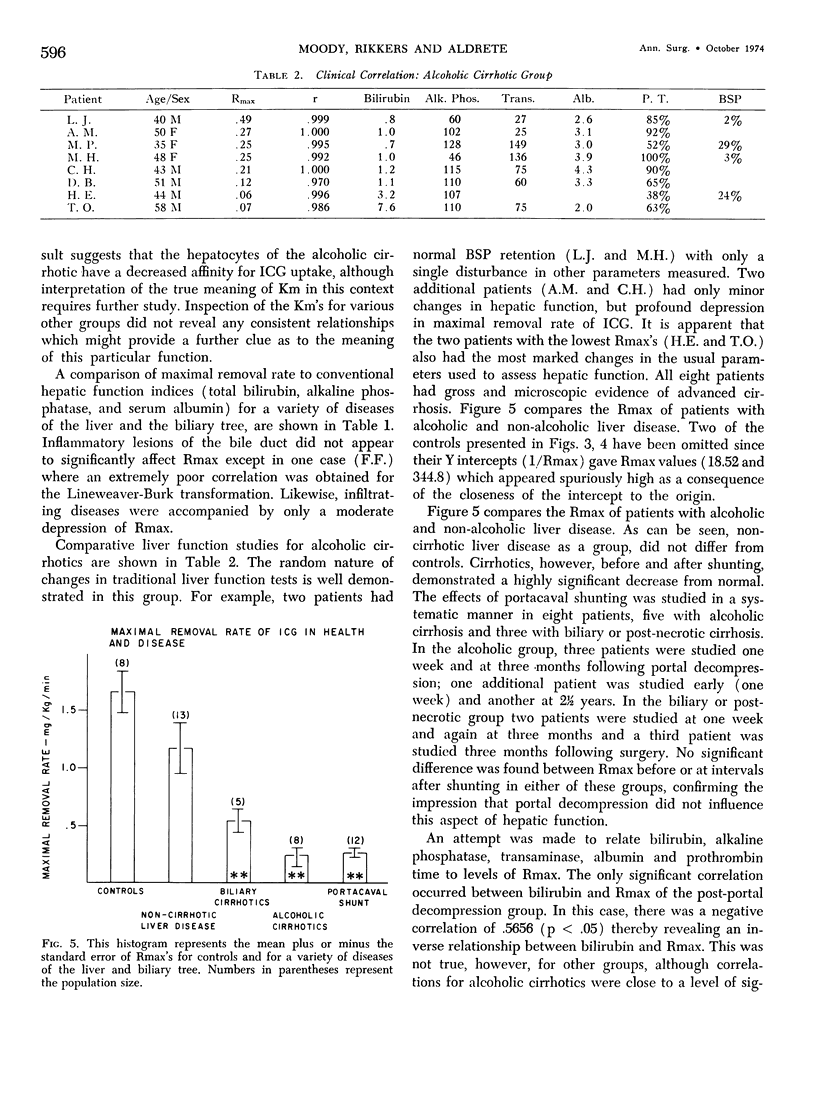
Functional hepatic reserve was determined in 32 patients with known liver or biliary tract disease employing kinetic analysis of hepatic removal of indocyanine green (ICG). The initial removal rates of incremental doses of ICG (0.5, 1.0 and 5.0 mg/kg body weight) were plotted as a reciprocal against the inverse of dose (Lineweaver-Burk plot) to provide a means of determining maximal removal rate from submaximal doses (Rmax). This function equalled 3.40 mg/kg/min in ten patients with normal livers, but was only .24 mg/kg/min in eight patients with alcoholic cirrhosis. Portasystemic shunting did not further influence Rmax. Infiltrative liver disease had only a mild depressive effect on this function. The results show that hepatic function can be precisely quantitated by classical enzyme kinetics (Michaelis-Menten). If Rmax is an estimate of protein receptor mass for organic anions, then the technique may allow an indirect means for quantitating hepatocytes even in the presence of changes in blood flow or hepatic function. The profound depression in Rmax observed in patients with alcoholic cirrhosis is consistent with the progressive loss in hepatic mass associated with this disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almersjö O., Bengmark S., Hafström L. O., Olsson R. Enzyme and function changes after extensive liver resection in man. Ann Surg. 1969 Jan;169(1):111–119. doi: 10.1097/00000658-196901000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber H. E., Welch B. L., Mackay D. The use of the logarithmic transformation in the calculation of the transport parameters of a system that obeys Michaelis-Menten kinetics. Biochem J. 1967 Apr;103(1):251–255. doi: 10.1042/bj1030251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAESAR J., SHALDON S., CHIANDUSSI L., GUEVARA L., SHERLOCK S. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci. 1961 Aug;21:43–57. [PubMed] [Google Scholar]
- CHERRICK G. R., STEIN S. W., LEEVY C. M., DAVIDSON C. S. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960 Apr;39:592–600. doi: 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTON D. B., BOLLMAN J. L., HOFFMAN H. N., 2nd The plasma removal on indocyanine green and sulfobromophthalein: effect of dosage and blocking agents. J Clin Invest. 1961 Sep;40:1648–1655. doi: 10.1172/JCI104387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTON D. B., BOLLMAN J. L., HOFFMAN H. N. Studies of hepatic function with indocyanine green. Gastroenterology. 1960 Dec;39:713–724. [PubMed] [Google Scholar]
- Klaassen C. D., Plaa G. L. Plasma disappearance and biliary excretion of indocyanine green in rats, rabbits, and dogs. Toxicol Appl Pharmacol. 1969 Sep;15(2):374–384. doi: 10.1016/0041-008x(69)90035-0. [DOI] [PubMed] [Google Scholar]
- LEEVY C. M., BENDER J. PHYSIOLOGY OF DYE EXTRACTION BY THE LIVER: COMPARATIVE STUDIES OF SULFOBROMOPHTHALEIN AND INDOCYANINE GREEN. Ann N Y Acad Sci. 1963 Dec 30;111:161–176. doi: 10.1111/j.1749-6632.1963.tb36956.x. [DOI] [PubMed] [Google Scholar]
- Rikkers L. F., Moody F. G. Estimation of functional reserve of normal and regenerating dog livers. Surgery. 1974 Mar;75(3):421–429. [PubMed] [Google Scholar]


