Abstract
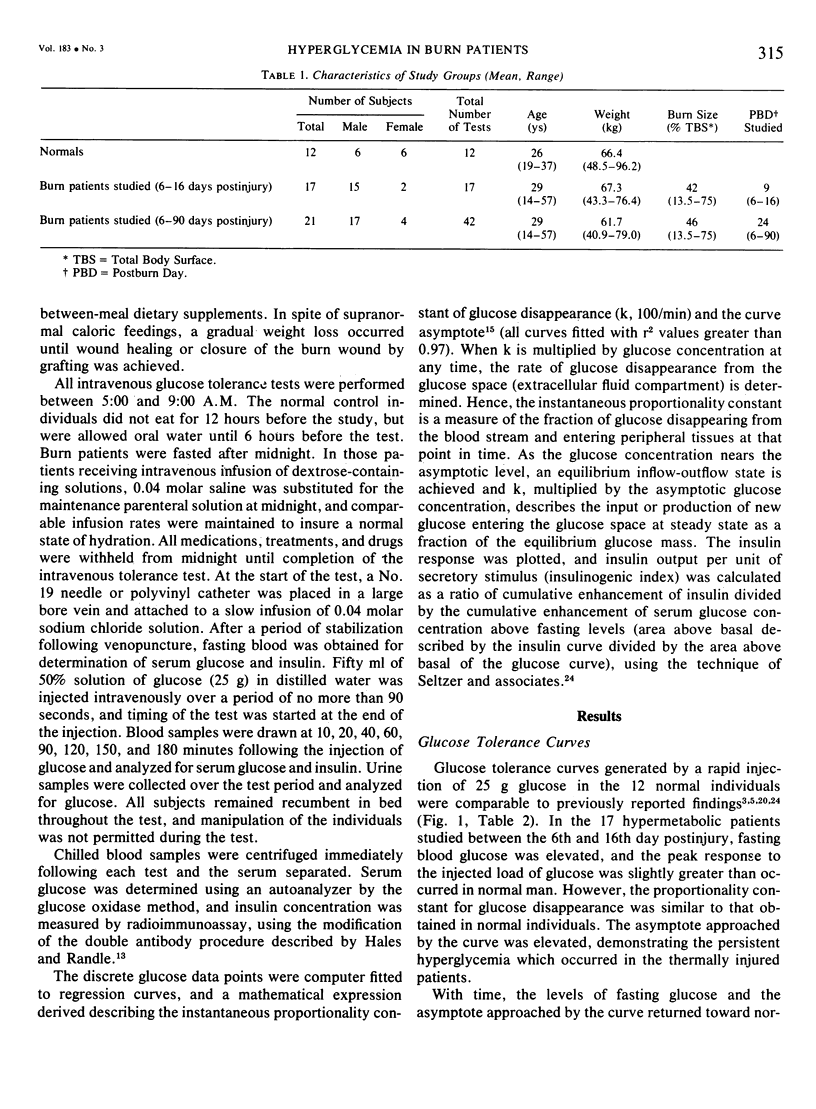
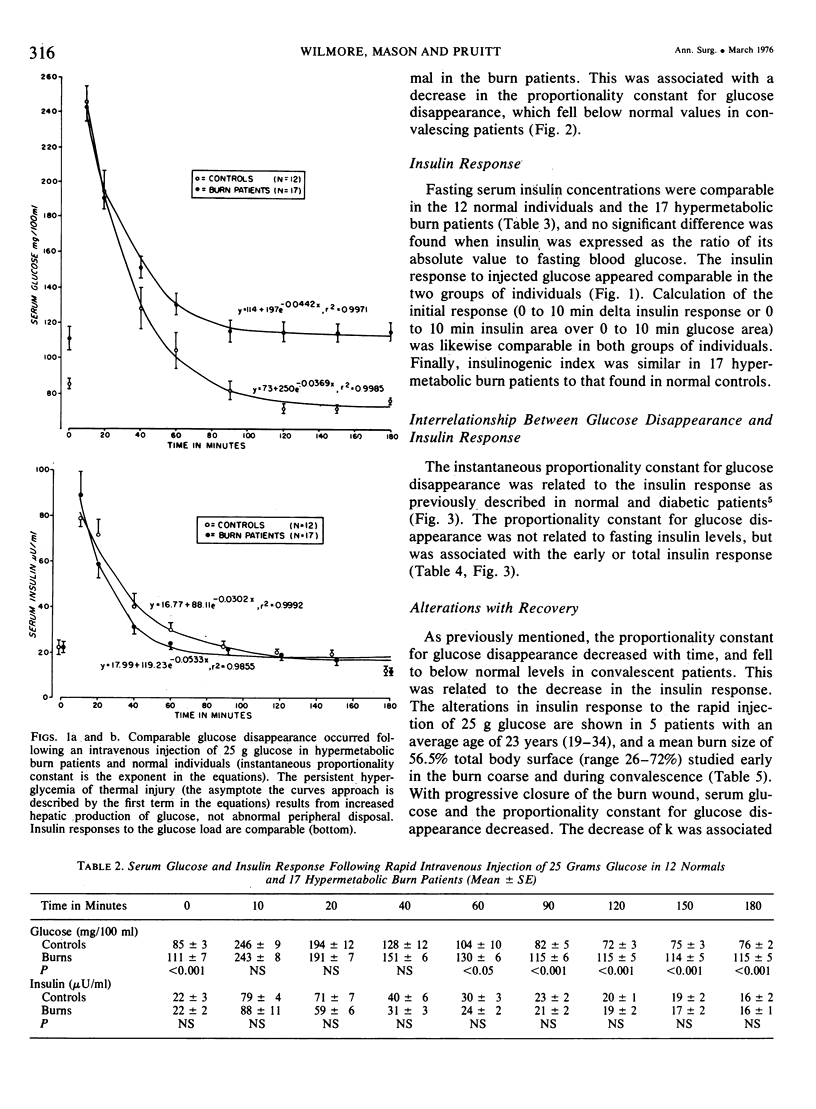
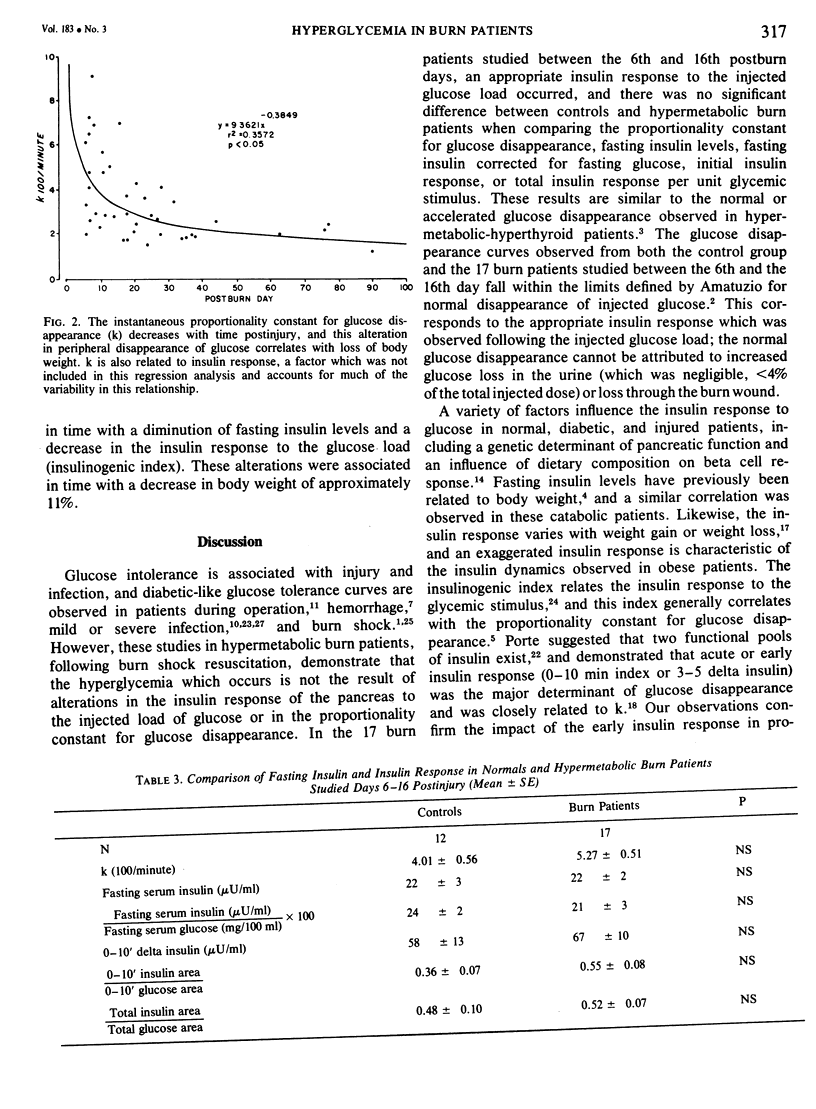
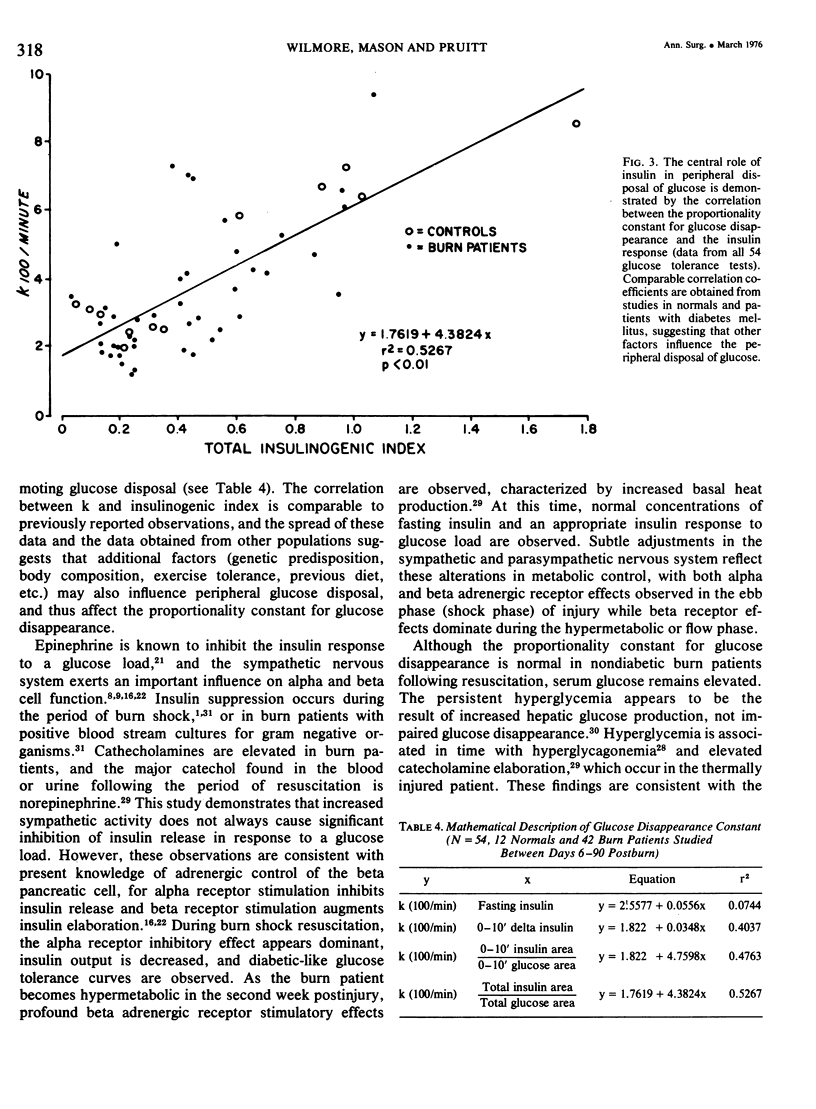
Fifty-four intravenous glucose tolerance tests were performed in 12 normal individuals and 21 thermally injured patients. In the 17 hypermetabolic burn patients studied between the 6th and 16th days postinjury, fasting blood glucose was elevated (111 +/- 7 mg/100 ml, mean +/- SE compared to 85 +/- 3 in controls, P less than 0.001), but the instantaneous proportionality constant for glucose disappearance (k) was similar to that obtained in normal individuals (5.27 +/- 0.51, 100/min vs 4.01 +/- 0.58 in normals, NS). Fasting serum insulin concentrations were comparable in the 12 normals and 17 hypermetabolic burn patients (22 +/- 3muU/ml in normals vs 22 +/- 2), as was fasting insulin corrected for fasting glucose (24 +/- 3 in normals vs 21 +/- 3, NS), initial insulin response (0-10 min delta insulin, 58 +/- 13 in normals vs 67 +/- 10, NS) or total insulin response corrected per unit glycemic stimulus (insulinogenic index, 0.48 +/- 0.10 in normals vs 0.52 +/- 0.07, NS). With time following injury, the proportionality constant for glucose disappearance and insulin response decreased, and these alterations were related to the posttraumatic weight loss. In the 5 convalescent patients studied between the 37th and 90th days postinjury, glucose and insulin dynamics appeared similar to those observed in starved man. In these burn patients, hypermetabolism and negative nitrogen balance occurred in association with a normal insulin response to glucose. Increased hepatic gluconeogenesis appears to be characteristic of the catabolic response to this stress, directed by increased glucagon and catecholamines, not a decrease in fasting insulin or dampened insulin response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMATUZIO D. S., NESBITT S., RAMES E. D. The practical application of the rapid intravenous glucose tolerance test in various disease states affecting glucose metabolism. J Lab Clin Med. 1956 Nov;48(5):714–720. [PubMed] [Google Scholar]
- AMATUZIO D. S., SCHULTZ A. L., VANDERBILT M. J., RAMES E. D., NESBITT S. The effect of epinephrine, insulin, and hyperthyroidism on the rapid intravenous glucose tolerance test. J Clin Invest. 1954 Jan;33(1):97–102. doi: 10.1172/JCI102876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison S. P., Hinton P., Chamberlain M. J. Intravenous glucose-tolerance, insulin, and free-fatty-acid levels in burned patients. Lancet. 1968 Nov 23;2(7578):1113–1116. doi: 10.1016/s0140-6736(68)91581-x. [DOI] [PubMed] [Google Scholar]
- Bagdade J. D., Bierman E. L., Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest. 1967 Oct;46(10):1549–1557. doi: 10.1172/JCI105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan K. D., McKiddie M. T. The insulin response to glucose: a comparison between oral and intravenous tolerance tests. J Endocrinol. 1967 Sep;39(1):13–20. doi: 10.1677/joe.0.0390013. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey L. C., Lowery B. D., Cloutier C. T. Blood sugar and insulin response of humans in shock. Ann Surg. 1970 Sep;172(3):342–350. doi: 10.1097/00000658-197009000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatonnet J., Minaire Y., Pernod A., Vincent-Falquet J. C. Inhibition of glucose uptake by epinephrine in dogs during cold exposure. J Appl Physiol. 1972 Feb;32(2):170–175. doi: 10.1152/jappl.1972.32.2.170. [DOI] [PubMed] [Google Scholar]
- Christensen N. J., Videbaek J. Plasma catecholamines and carbohydrate metabolism in patients with acute myocardial infarction. J Clin Invest. 1974 Aug;54(2):278–286. doi: 10.1172/JCI107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer P. E., Herman C. M., Sode J. Carbohydrate metabolism in the baboon subjected to gram-negative (E. coli) septicemia. I. Hyperglycemia with depressed plasma insulin concentrations. Ann Surg. 1971 Jul;174(1):91–100. doi: 10.1097/00000658-197107010-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELRICK H., HLAD C. J., Jr, WITTEN T. A. Studies on the kinetics of glucose utilization. J Clin Invest. 1956 Oct;35(10):1139–1149. doi: 10.1172/JCI103368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump F. E., Long C., Killian P., Kinney J. M. Studies of glucose intolerance in septic injured patients. J Trauma. 1974 May;14(5):378–388. doi: 10.1097/00005373-197405000-00004. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen J. Adrenergic receptors and the secretion of glucagon and insulin from the isolated, perfused canine pancreas. J Clin Invest. 1973 Sep;52(9):2102–2116. doi: 10.1172/JCI107395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K., Hagura R., Odagiri R., Saito F., Kuzuya T. Effect of weight changes on serum insulin response in subjects with normal oral glucose tolerance. J Clin Endocrinol Metab. 1972 Nov;35(5):655–658. doi: 10.1210/jcem-35-5-655. [DOI] [PubMed] [Google Scholar]
- Long C. L., Spencer J. L., Kinney J. M., Geiger J. W. Carbohydrate metabolism in man: effect of elective operations and major injury. J Appl Physiol. 1971 Jul;31(1):110–116. doi: 10.1152/jappl.1971.31.1.110. [DOI] [PubMed] [Google Scholar]
- Lozner E. L., Winkler A. W., Taylor F. H., Peters J. P. THE INTRAVENOUS GLUCOSE TOLERANCE TEST. J Clin Invest. 1941 Sep;20(5):507–515. doi: 10.1172/JCI101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte D., Jr, Graber A. L., Kuzuya T., Williams R. H. The effect of epinephrine on immunoreactive insulin levels in man. J Clin Invest. 1966 Feb;45(2):228–236. doi: 10.1172/JCI105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte D., Jr, Robertson R. P. Control of insulin secretion by catecholamines, stress, and the sympathetic nervous system. Fed Proc. 1973 Jul;32(7):1792–1796. [PubMed] [Google Scholar]
- Rayfield E. J., Curnow R. T., George D. T., Beisel W. R. Impaired carbohydrate metabolism during a mild viral illness. N Engl J Med. 1973 Sep 20;289(12):618–621. doi: 10.1056/NEJM197309202891207. [DOI] [PubMed] [Google Scholar]
- Seltzer H. S., Allen E. W., Herron A. L., Jr, Brennan M. T. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1967 Mar;46(3):323–335. doi: 10.1172/JCI105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975 Jan 4;1(7897):14–16. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W., Lindsey C. A., Moyland J. A., Faloona G. R., Pruitt B. A., Unger R. H. Hyperglucagonaemia after burns. Lancet. 1974 Jan 19;1(7847):73–75. doi: 10.1016/s0140-6736(74)92290-9. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W., Long J. M., Mason A. D., Jr, Skreen R. W., Pruitt B. A., Jr Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974 Oct;180(4):653–669. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore D. W., Mason A. D., Pruitt B. A., Jr Alterations in glucose kinetics following thermal injury. Surg Forum. 1975;26:81–83. [PubMed] [Google Scholar]


