Abstract
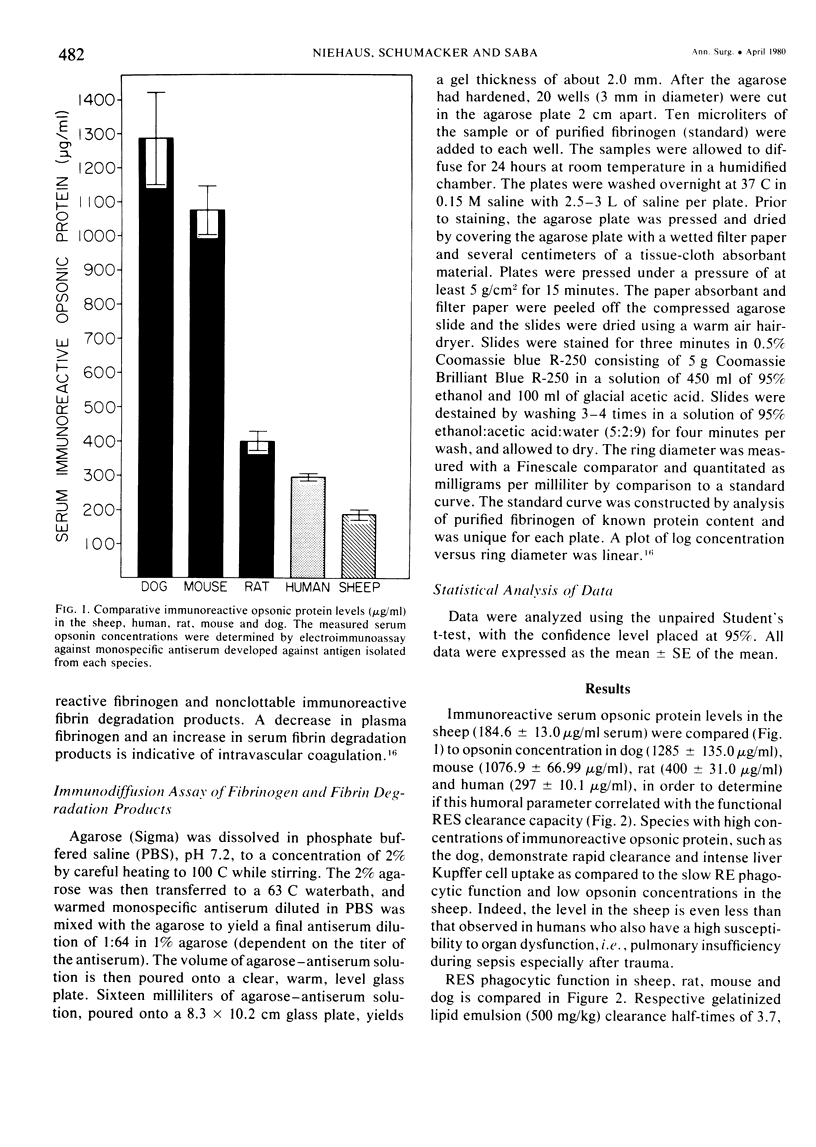
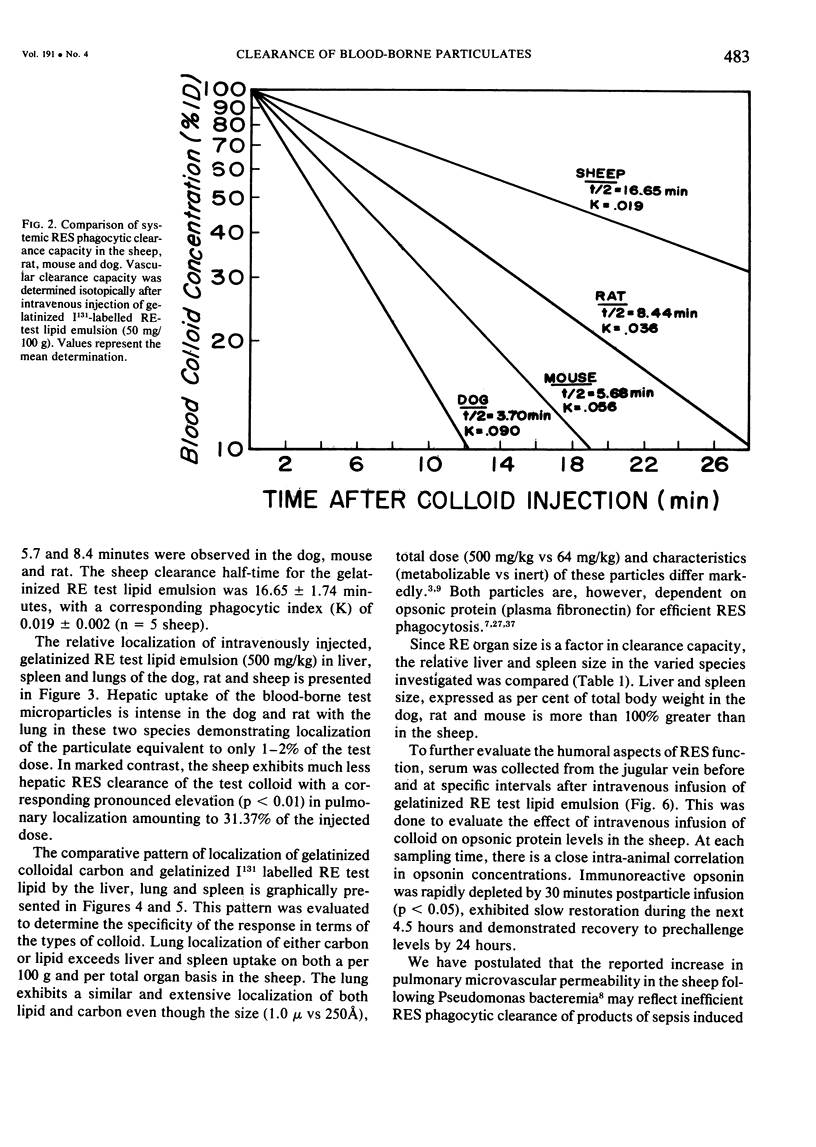
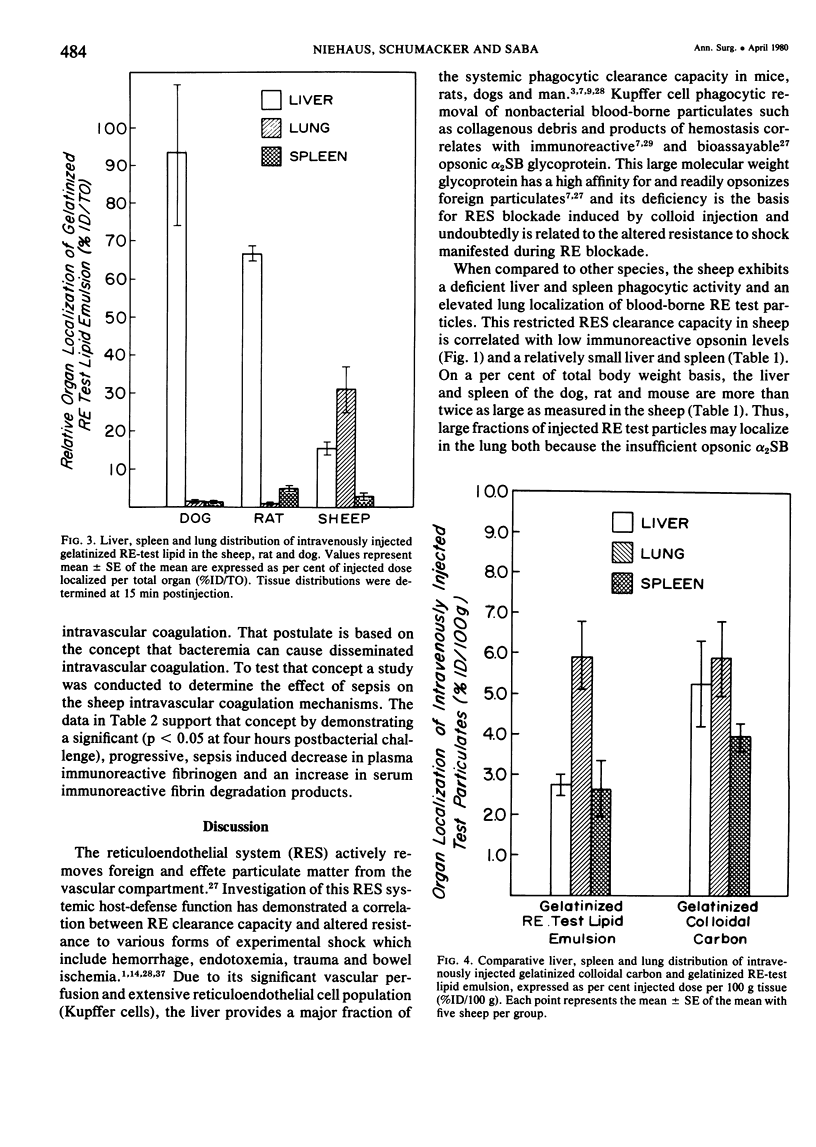
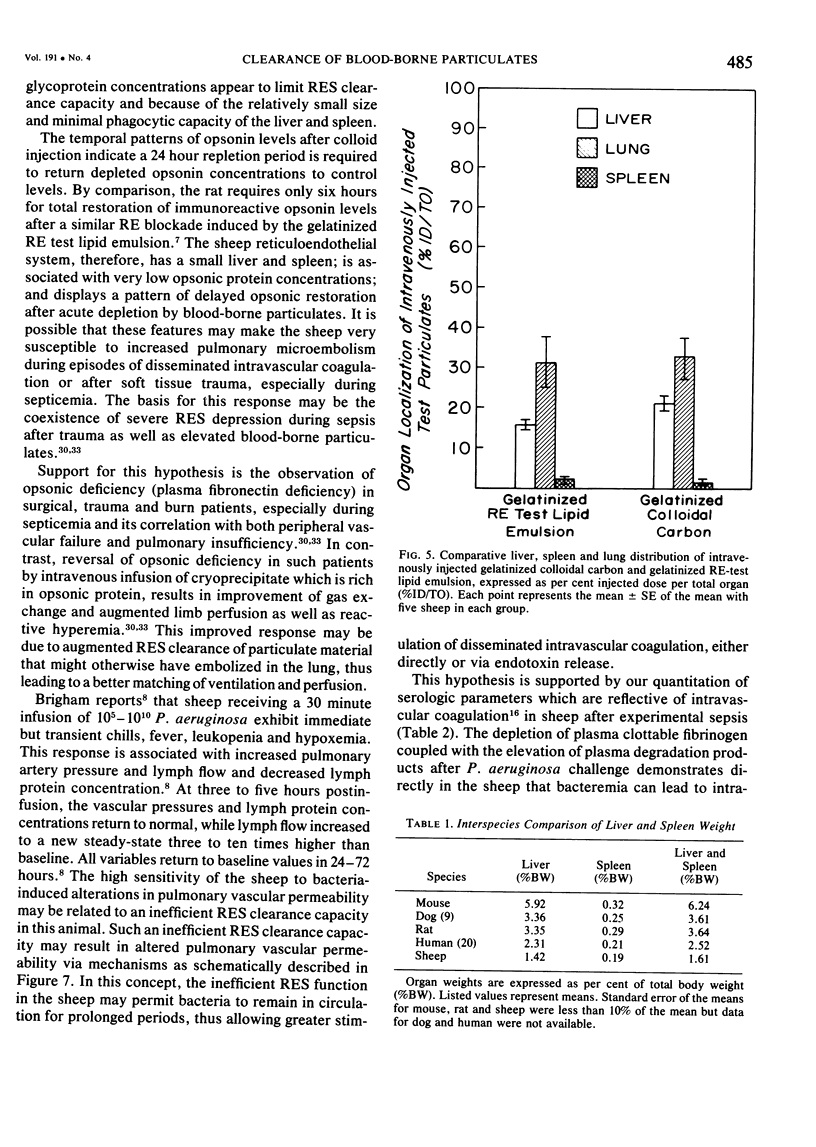
The rapid increase in sheep lung vascular permeability observed during Pseudomonas aeruginosa bacteremia may be due to embolization of the pulmonary microvasculature by bloodborne particulates. Since alterations in lung microvascular permeability during mild septicemia in sheep may reflect inefficient RES phagocytic clearance of bacteria as well as products of bacterial induced intravascular coagulation, the opsonic and phagocytic aspects of RES function in sheep (30-50 kg) were compared to other species. RES function was evaluated by both the clearance and relative organ uptake of gelatinized I131 RE test lipid emulsion and gelatinized colloidal carbon. Immunoreactive opsonic a2SB glycoprotein levels were determined by electroimmunoassay. The phagocytic index for RES clearance of the gelatinized (500 mg/kg) test lipid in sheep was 0.019 ± 0.002 corresponding to a half-time of 16.65 ± 1.74 minutes. With colloidal carbon (64 mg/kg), the phagocytic index in sheep was 0.080 ± 0.026, corresponding to a half-time of 6.16 ± 1.99 minutes. The per cent of injected lipid emulsion (%ID) in major RE organs, on a total organ basis (TO), was: liver = 15.69 ± 1.65%; spleen = 2.09 ± 0.78%. Localization in the lung = 31.39 ± 6.2%. The per cent of carbon localized in major RE organs (%ID/TO) was: liver = 21.37 ± 1.9%; spleen = 1.95 ± 0.55%. Localization in the lung = 32.70 ± 4.55%. In contrast, clearance and organ distribution of the blood-borne test microparticles in rats and dogs at the same relative challenging dose revealed a much more intense and rapid liver and spleen RES uptake with minimal lung localization (1-2%). Immunoreactive opsonic protein concentrations varied greatly with species and directly correlated with efficiency of RES function. Levels observed were: dog = 1285 ± 135 µg/ml; mouse = 1077 ± 67 µg/ml; rat = 400 ± 31 µg/ml; human = 297 ± 10 µg/ml; and sheep = 184 ± 13 µg/ml. After intravenous particulate challenge, circulating immunoreactive opsonic protein in the sheep was depleted (p < 0.05) rapidly with partial recovery at 24 hours and mild rebound hyperopsonemia at 48 hours. This pattern is in contrast to the rapid restoration seen in dog and rat within three to six hours postchallenge. Thus, in sheep, the extensive pulmonary localization of blood-borne microparticles appears related to inefficient RES clearance function mediated by a relative deficiency of circulating opsonic protein (plasma fibronectin).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altura B. M., Hershey S. G. Reticuloendothelial function in experimental injury and tolerance to shock. Adv Exp Med Biol. 1972;33(0):545–569. doi: 10.1007/978-1-4684-3228-2_57. [DOI] [PubMed] [Google Scholar]
- Attar S., Mansberger A. R., Jr, Irani B., Kirby W., Jr, Masaitis C., Cowley R. A. Coagulation changes in clinical shock. II. Effect of septic shock on clotting times and fibrinogen in humans. Ann Surg. 1966 Jul;164(1):41–50. [PMC free article] [PubMed] [Google Scholar]
- BIOZZI G., BENACERRAF B., HALPERN B. N. Quantitative study of the granulopectic activity of the reticulo-endothelial system. II. A study of the kinetics of the R. E. S. in relation to the dose of carbon injected; relationship between the weight of the organs and their activity. Br J Exp Pathol. 1953 Aug;34(4):441–457. [PMC free article] [PubMed] [Google Scholar]
- Blumenstock F. A., Saba T. M., Weber P., Laffin R. Biochemical and immunological characterization of human opsonic alpha2SB glycoprotein: its identity with cold-insoluble globulin. J Biol Chem. 1978 Jun 25;253(12):4287–4291. [PubMed] [Google Scholar]
- Blumenstock F. A., Saba T. M., Weber P. Purification of alpha-2-opsonic protein from human serum and its measurement by immunoassay. J Reticuloendothel Soc. 1978 Feb;23(2):119–134. [PubMed] [Google Scholar]
- Blumenstock F., Weber P., Saba T. M., Laffin R. Electroimmunoassay of alpha-2-opsonic protein during reticuloendothelial blockade. Am J Physiol. 1977 Mar;232(3):R80–R87. doi: 10.1152/ajpregu.1977.232.3.R80. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Owen P. J., Bowers R. E. Increased permeability of sheep lung vessels to proteins after Pseudomonas bacteremia. Microvasc Res. 1976 May;11(3):415–419. doi: 10.1016/0026-2862(76)90067-4. [DOI] [PubMed] [Google Scholar]
- Cornell R. P., Saba T. M. Bioassay of serum opsonin and its depletion after colloid clearance in dogs. Am J Physiol. 1972 Sep;223(3):569–574. doi: 10.1152/ajplegacy.1972.223.3.569. [DOI] [PubMed] [Google Scholar]
- Costabella P. M., Lindquist O., Kapanci Y., Saldeen T. Increased vascular permeability in the delayed microembolism syndrome. Experimental and human findings. Microvasc Res. 1978 May;15(3):275–286. doi: 10.1016/0026-2862(78)90028-6. [DOI] [PubMed] [Google Scholar]
- Demling R. H., Niehaus G., Perea A., Will J. A. Effect of burn-induced hypoproteinemia on pulmonary transvascular fluid filtration rate. Surgery. 1979 Mar;85(3):339–343. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Josephson S., Swedenborg J., Dahlgren S. E. Delayed lung lesion in dogs after thrombin-induced disseminated intravascular coagulation. Acta Chir Scand. 1974;140(6):431–435. [PubMed] [Google Scholar]
- Kaplan J. E., Blumenstock F. A., Saba T. M. A radial immunodiffusion method for the measurement of rat fibrinogen and fibrin degradation products. Vox Sang. 1979;36(2):65–71. doi: 10.1111/j.1423-0410.1979.tb04400.x. [DOI] [PubMed] [Google Scholar]
- Kaplan J. E., Saba T. M. Low-grade intravascular coagulation and reticuloendothelial function. Am J Physiol. 1978 Apr;234(4):H323–H329. doi: 10.1152/ajpheart.1978.234.4.H323. [DOI] [PubMed] [Google Scholar]
- Kaplan J. E., Scovill W. A., Bernard H., Saba T. M., Gray V. Reticuloendothelial phagocytic response to bacterial challenge after traumatic shock. Circ Shock. 1977;4(1):1–12. [PubMed] [Google Scholar]
- Lindquist O., Saldeen T., Sandler H. Pulmonary damage following pulmonary microembolism in the dog. Effect of various types of treatment. Acta Chir Scand. 1976;142(1):15–19. [PubMed] [Google Scholar]
- Luterman A., Manwaring D., Curreri P. W. The role of fibrinogen degradation products in the pathogenesis of the respiratory distress syndrome. Surgery. 1977 Nov;82(5):703–709. [PubMed] [Google Scholar]
- Malik A. B., van der Zee H. Thrombin induced pulmonary insufficiency. Thromb Res. 1977 Oct;11(4):497–506. doi: 10.1016/0049-3848(77)90203-1. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Williams E. M. Fibronectin concentration is decreased in plasma of severely ill patients with disseminated intravascular coagulation. J Lab Clin Med. 1978 May;91(5):729–735. [PubMed] [Google Scholar]
- Pardy B. J., Dudley H. A. Post-traumatic pulmonary insufficiency. Surg Gynecol Obstet. 1977 Feb;144(2):259–269. [PubMed] [Google Scholar]
- ROGERS D. E. Host mechanisms which act to remove bacteria from the blood stream. Bacteriol Rev. 1960 Mar;24(1):50–66. doi: 10.1128/br.24.1.50-66.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba T. M., Blumenstock F. A., Scovill W. A., Bernard H. Cryoprecipitate reversal of opsonic alpha2-surface binding glycoprotein deficiency in septic surgical and trauma patients. Science. 1978 Aug 18;201(4356):622–624. doi: 10.1126/science.675246. [DOI] [PubMed] [Google Scholar]
- Saba T. M., Cho E. Reticuloendothelial systemic response to operative trauma as influenced by cryoprecipitate or cold-insoluble globulin therapy. J Reticuloendothel Soc. 1979 Aug;26(2):171–186. [PubMed] [Google Scholar]
- Saba T. M., Di Luzio N. R. Reticuloendothelial blockade and recovery as a function of opsonic activity. Am J Physiol. 1969 Jan;216(1):197–205. doi: 10.1152/ajplegacy.1969.216.1.197. [DOI] [PubMed] [Google Scholar]
- Saba T. M. Prevention of liver reticuloendothelial systemic host defense failure after surgery by intravenous opsonic glycoprotein therapy. Ann Surg. 1978 Aug;188(2):142–152. doi: 10.1097/00000658-197808000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scovill W. A., Saba T. M., Blumenstock F. A., Bernard H., Powers S. R., Jr Opsonic alpha2 surface binding glycoprotein therapy during sepsis. Ann Surg. 1978 Oct;188(4):521–529. doi: 10.1097/00000658-197810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A., Harwig S., Lee J. In vitro formation and i vivo clearance of fibrinogen: fibrin complexes. J Lab Clin Med. 1975 Jul;86(1):100–111. [PubMed] [Google Scholar]
- Staub N. C., Bland R. D., Brigham K. L., Demling R., Erdmann A. J., 3rd, Woolverton W. C. Preparation of chronic lung lymph fistulas in sheep. J Surg Res. 1975 Nov;19(5):315–320. doi: 10.1016/0022-4804(75)90056-6. [DOI] [PubMed] [Google Scholar]
- Staub N. C. Pulmonary edema. Physiol Rev. 1974 Jul;54(3):678–811. doi: 10.1152/physrev.1974.54.3.678. [DOI] [PubMed] [Google Scholar]
- Wing D. A., Yamada T., Hawley H. B., Pettit G. W. Model for disseminated intravascular coagulation: bacterial sepsis in rhesus monkeys. J Lab Clin Med. 1978 Aug;92(2):239–251. [PubMed] [Google Scholar]


