Abstract
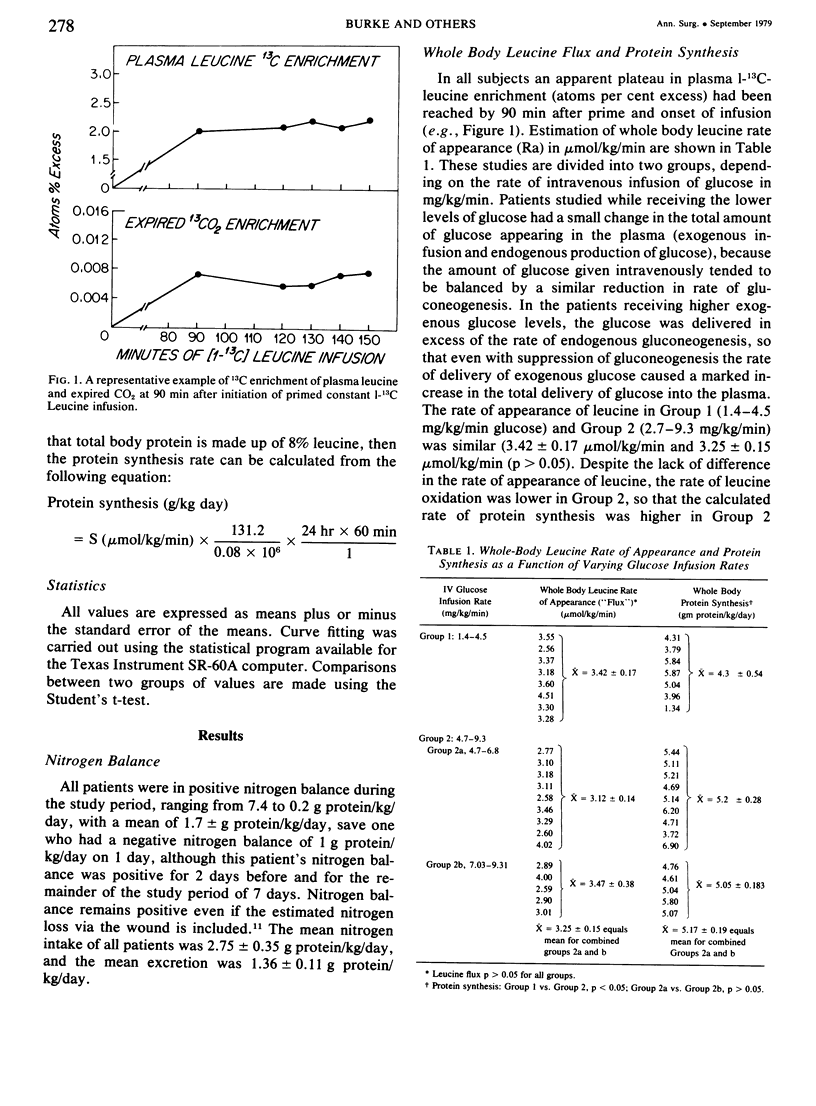
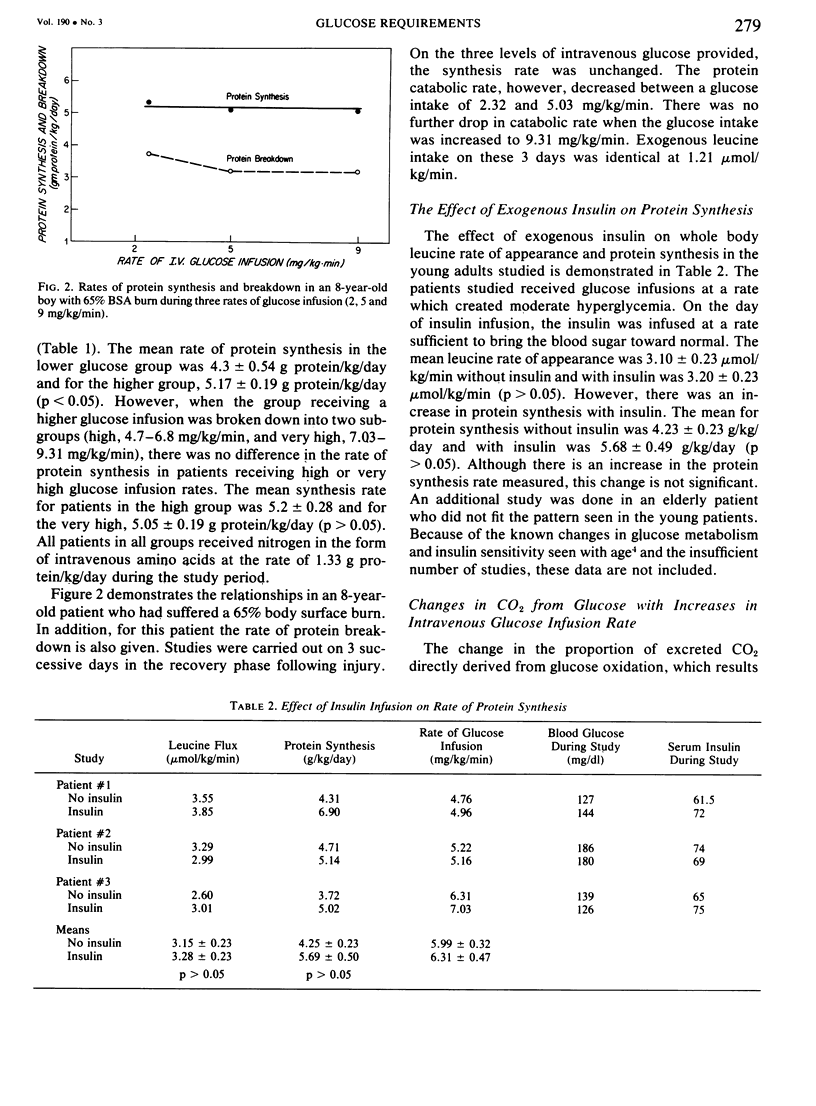
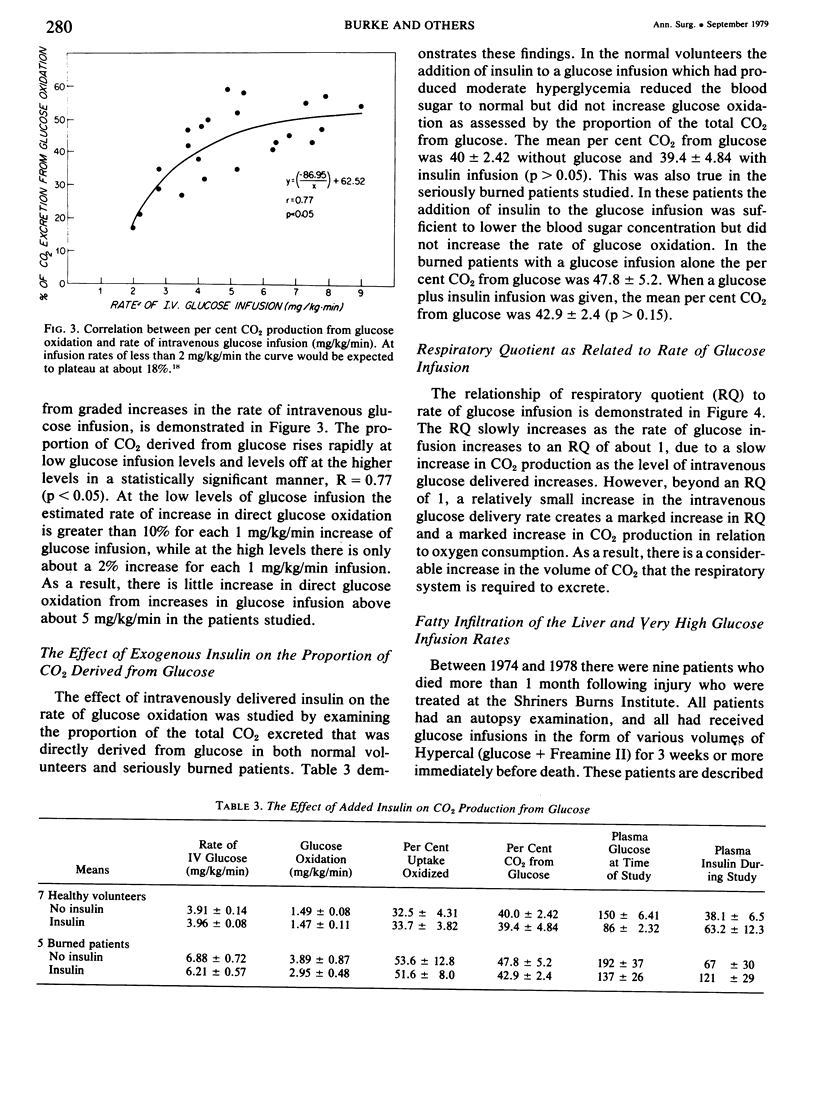
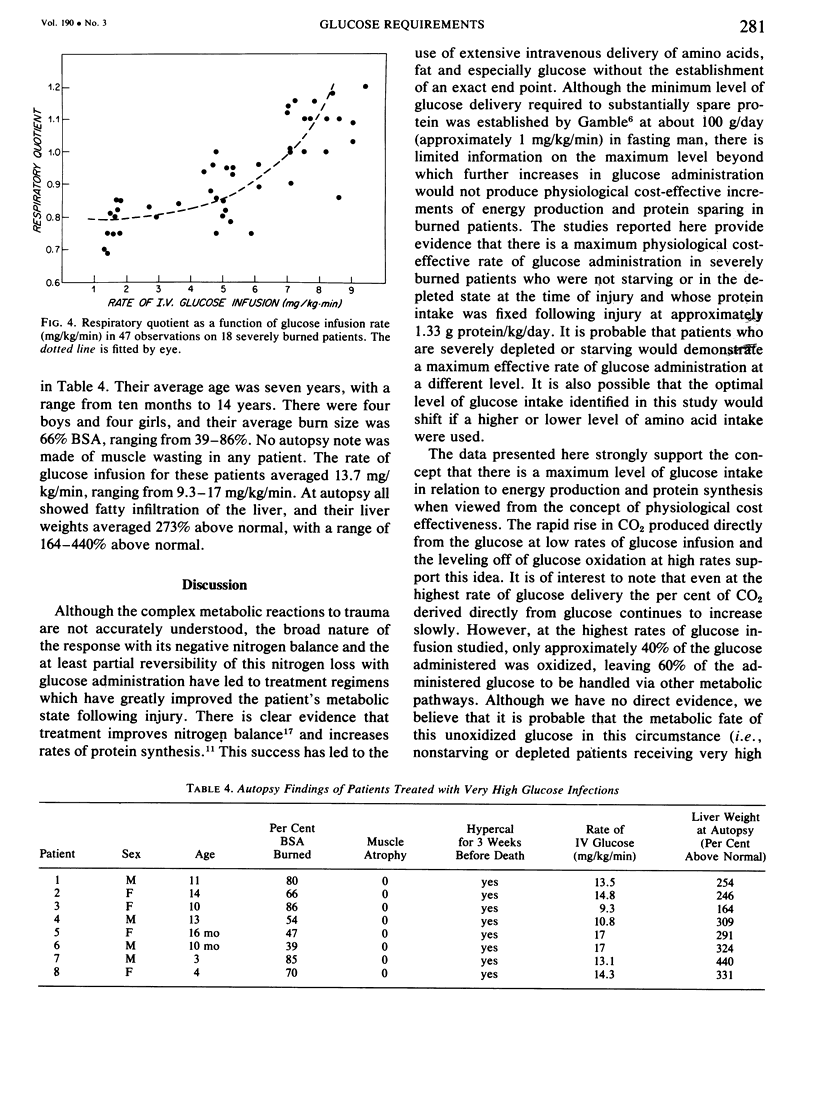
Glucose and leucine metabolism in 18 severely burned patients were studied using the primed constant infusion of U-13C-glucose and 1-13C-leucine, respectively. The leucine data were used to calculate rates of whole-body protein synthesis. In four additional burn patients and seven normal controls, the effects of exogenously infused insulin on the metabolism of infused glucose were evaluated. Also, the effect on leucine metabolism of adding insulin to infused glucose was tested and rates of protein synthesis were calculated. The protein studies were divided into two groups depending on the rate of glucose infusion. Protein synthesis was 4.3 + 0.54 g protein/kg/day during the lower infusion rates (1.4--4.5 mg/kg/min) and 5.17 + 0.19 g protein/kg/day during the higher infusion rates (4.7--9.3 mg/kg/min) (statistically different, p less than 0.05). However, when the high infusion rate group was divided into two subgroups (high, 4.7--6.8 mg/kg/min, and very high, 7.03--9.31 mg/kg/min), there was no difference in the rate of protein synthesis. When U-13C-glucose was infused during varying rates of unlabeled glucose infusion, we found that the per cent of CO2 coming from the direct oxidation of glucose rose rapidly at the lower infusion rates but reached a plateau at approximately 55% as the infusion rates exceeded 5 mg/kg/min. Addition of insulin did not affect the rate of glucose oxidation but did seem to exert a stimulatory effect on protein synthesis. It was concluded that there appears to be a maximal rate of glucose infusion, beyond which physiologically significant increases in protein synthesis and direct oxidation of glucose cannot be expected. Furthermore, there appears to be a physiological cost of exceeding the optimal glucose infusion rate, as indicated by increased rates of CO2 production during infusion as well as large fat deposits in the liver at autopsy in patients infused with large amounts of glucose.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Allsop J. R., Wolfe R. R., Burke J. F. The realiability of rates of glucose appearance in vivo calculated from constant tracer infusions. Biochem J. 1978 Jun 15;172(3):407–416. doi: 10.1042/bj1720407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop J. R., Wolfe R. R., Burke J. F. Tracer priming the bicarbonate pool. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):137–139. doi: 10.1152/jappl.1978.45.1.137. [DOI] [PubMed] [Google Scholar]
- Andres R. Aging and diabetes. Med Clin North Am. 1971 Jul;55(4):835–846. doi: 10.1016/s0025-7125(16)32479-8. [DOI] [PubMed] [Google Scholar]
- Bier D. M., Arnold K. J., Sherman W. R., Holland W. H., Holmes W. F., Kipnis D. M. In-vivo measurement of glucose and alanine metabolism with stable isotopic tracers. Diabetes. 1977 Nov;26(11):1005–1015. doi: 10.2337/diab.26.11.1005. [DOI] [PubMed] [Google Scholar]
- Golden M. H., Waterlow J. C. Total protein synthesis in elderly people: a comparison of results with [15N]glycine and [14C]leucine. Clin Sci Mol Med. 1977 Sep;53(3):277–288. doi: 10.1042/cs0530277. [DOI] [PubMed] [Google Scholar]
- Gruenke L. D., Craig J. C., Bier D. M. Multiple ion detection by accelerating voltage alternation in conjunction with voltage sweeping. Biomed Mass Spectrom. 1974 Dec;1(6):418–422. doi: 10.1002/bms.1200010610. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr, Paul P., Miller H. I. Metabolism in normal and pancreatectomized dogs during steady-state exercise. Am J Physiol. 1967 Oct;213(4):857–862. doi: 10.1152/ajplegacy.1967.213.4.857. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W. Nutrition and metabolism following thermal injury. Clin Plast Surg. 1974 Oct;1(4):603–619. [PubMed] [Google Scholar]
- Wolfe R. R., Allsop J. R., Burke J. F. Glucose metabolism in man: responses to intravenous glucose infusion. Metabolism. 1979 Mar;28(3):210–220. doi: 10.1016/0026-0495(79)90066-0. [DOI] [PubMed] [Google Scholar]


